





Preview text:
CHE 1084
Bài tập Chương 1/ Homework Set#1 (Chap. 1) Bài 1. Sử d .
ụng phương trình van der Waals a P + ( V - b)= RT 2 V
Tính áp suất (theo bar) gây ra bởi 1,00 mol khí CH o
4 trong bình có thể tích 250 mL tại 0 C.
Biết a = 2,3026 dm6.bar.mol-2 và b = 0,043067 dm3.mol-1.
Use van der Waals equation to calculate the pressure (in bars) exerted by 1.00 mol of CH4
(g) that occupies a 250 mL container at 0oC. Take a = 2.3026 dm6.bar.mol-2 and b = 0.043067 dm3.mol-1. a P + ( V - b)= RT 2 V Bài 2.
Sử dụng phương trình van der Waals (dạng bậc 3 theo V) để tính thể tích mol phân tử của
etan tại 300 K và 200 atm. Biết = 5,5088 dm a
6.at.mol-2 và b = 0,065144 dm3.mol-1.
Use the van der Waals equation to calculate the molar volume of ethane at 300 K and 200
atm. Take a = 5.5088 dm6.at.mol-2 and b = 0.065144 dm3.mol-1. Bài 3.
Sử dụng phương trình Redlich-Kwong để tính thể tích mol phân t ử c a ủ etan tại 300 K và 200 atm. 3 2 RT RRT A AB 2 V - V - B + − V - = 0 1/ 2 P P T P 1/ 2 T P
Biết A = 97,539 dm6.atm.mol-1.K1/2, và B = 0,045153 dm3.mol-1
Use the Redlich-Kwong equation to calculate the molar volume of ethane at 300 K and 200 atm. 3 2 RT RRT A AB 2 V - V - B + − V - = 0 1/ 2 P P T P 1/ 2 T P
Take A = 97.539 dm6.atm.mol-1.K1/2, and B = 0.045153 dm3.mol-1 Bài 4. Tính tỉ s
ố P V / RT với phương trình van der Waals và phương trình Redlich-Kwong. c c c
Calculate the ratio P V / RT for the van der Waals equation and the Redlich-Kwong c c c equation. 1 Bài 5.
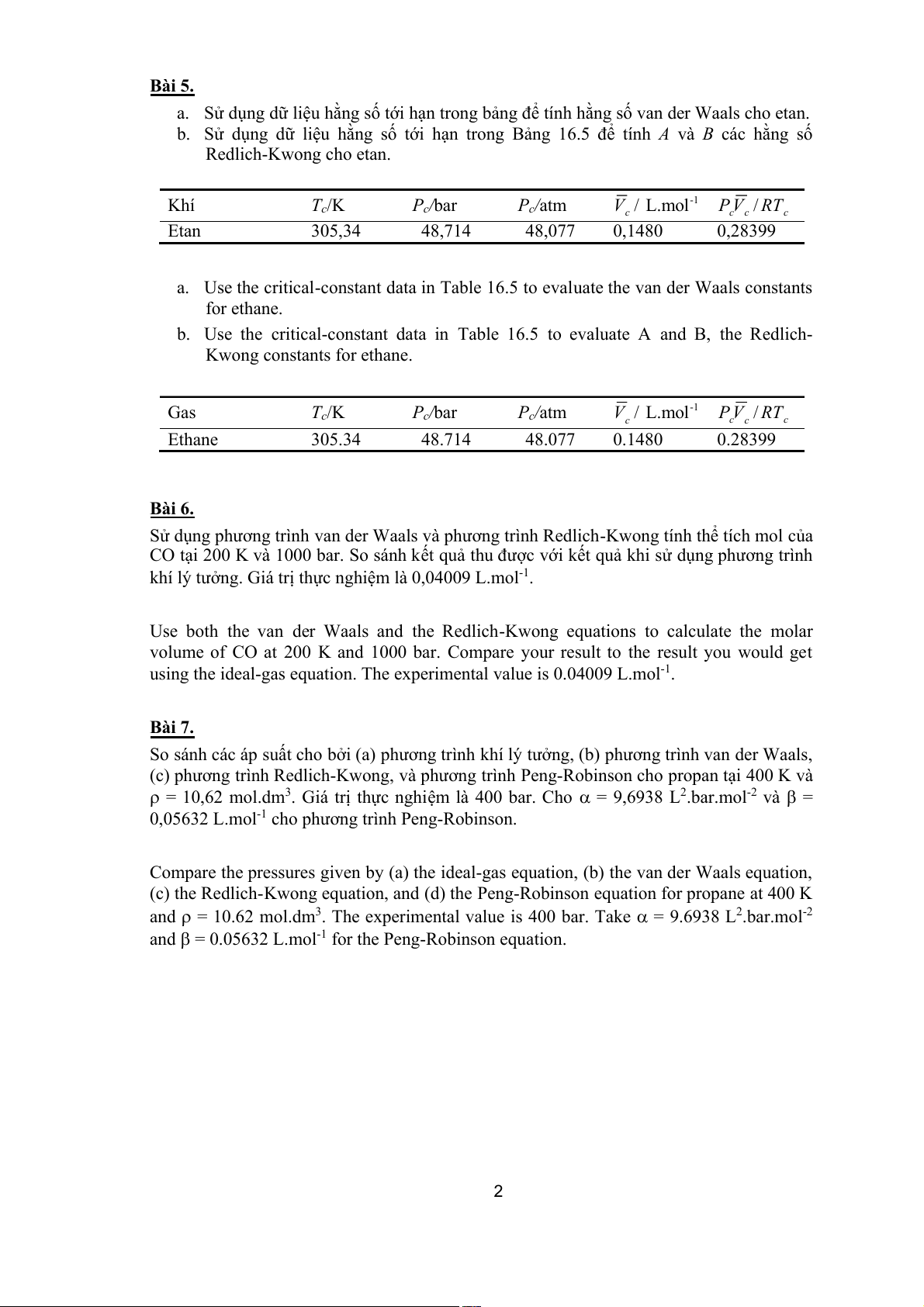
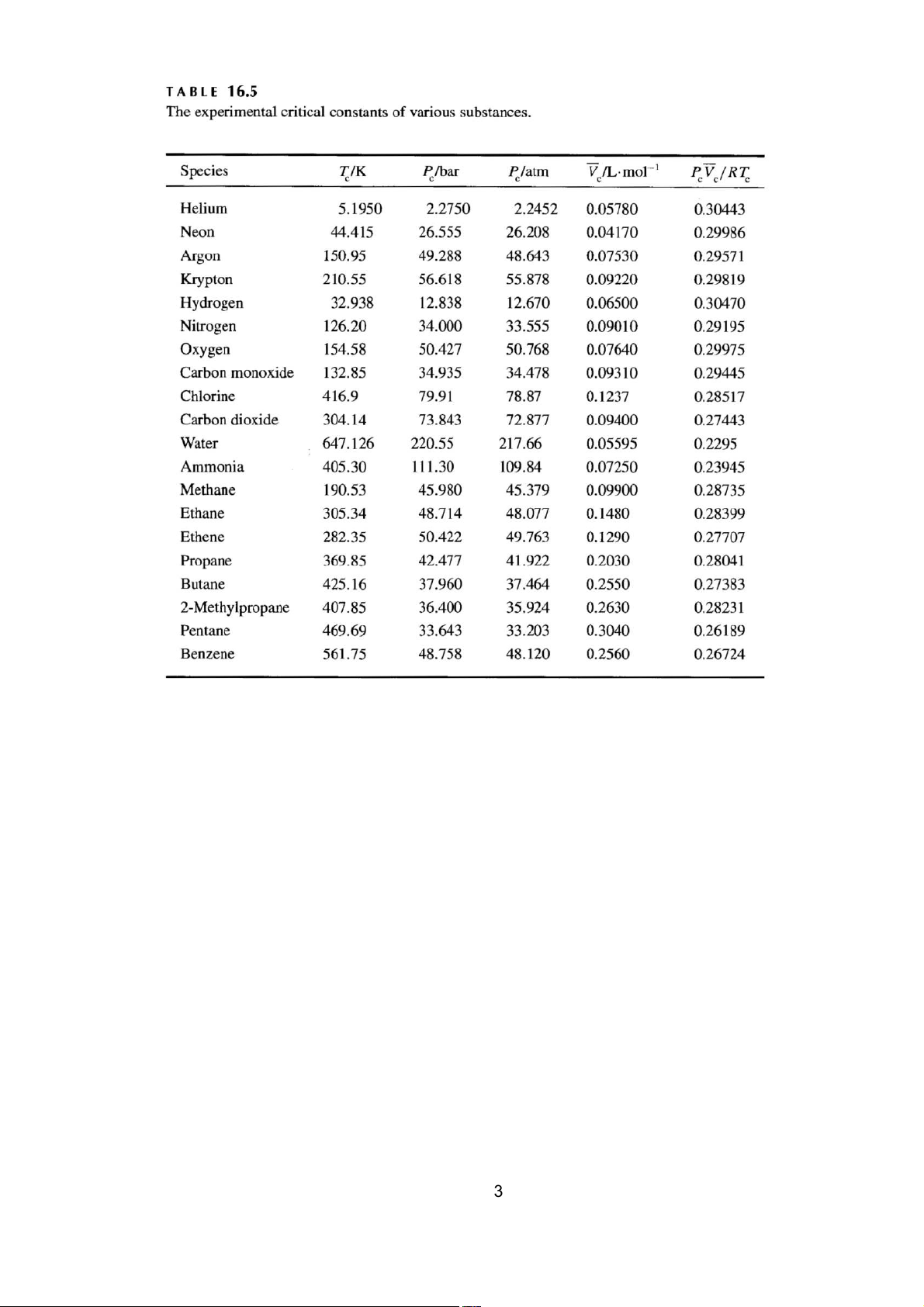
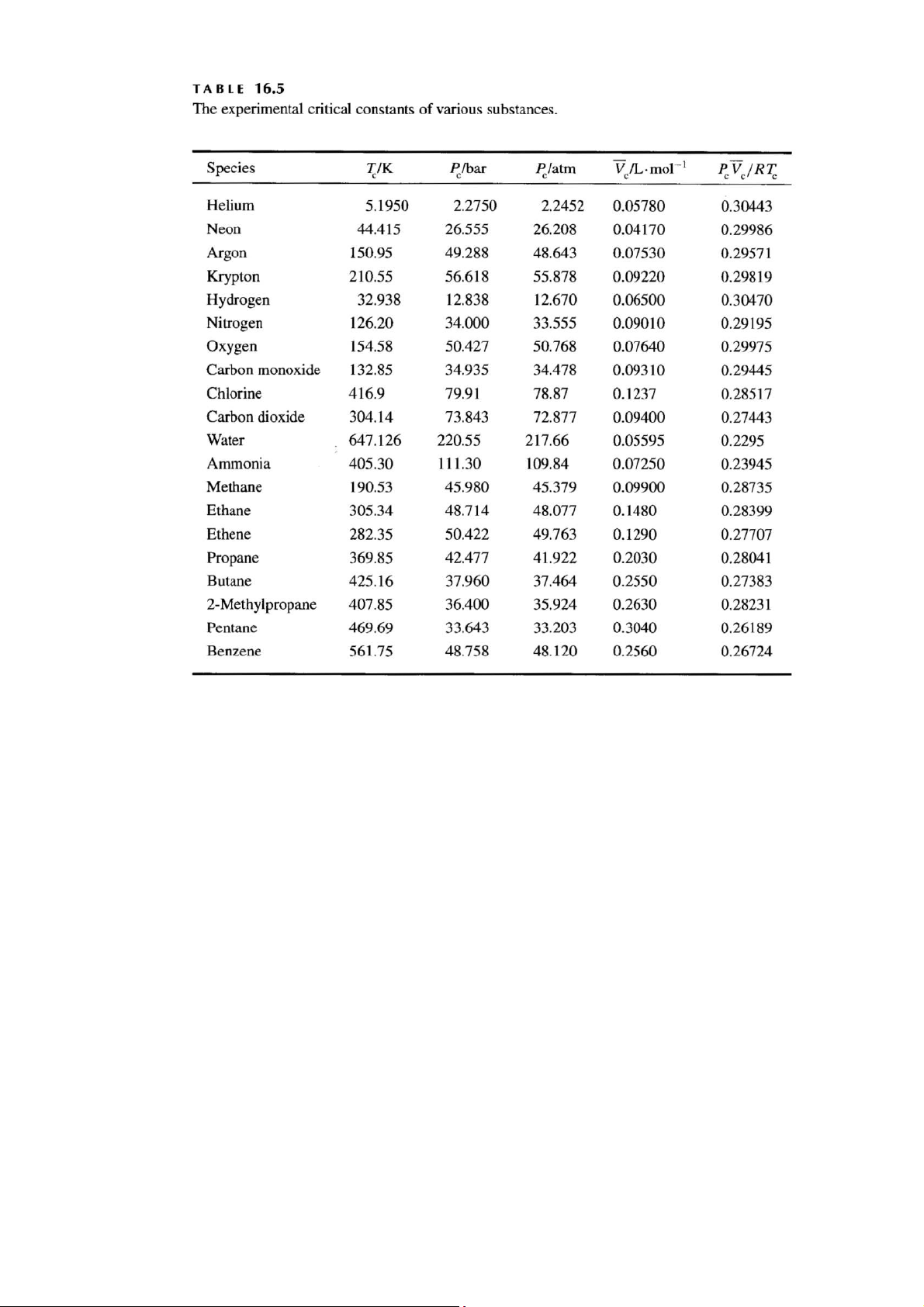
a. Sử dụng dữ liệu hằng số tới hạn trong b tính h ảng để
ằng số van der Waals cho etan.
b. Sử dụng dữ liệu hằng số tới hạn trong Bảng 16.5 để tính A và B các hằng số Redlich-Kwong cho etan. Khí Tc/K Pc/bar Pc/atm -1
V / L.mol P V / RT c c c c Etan 305,34 48,714 48,077 0,1480 0,28399
a. Use the critical-constant data in Table 16.5 to evaluate the van der Waals constants for ethane.
b. Use the critical-constant data in Table 16.5 to evaluate A and B, the Redlich- Kwong constants for ethane. Gas Tc/K Pc/bar Pc/atm -1
V / L.mol P V / RT c c c c Ethane 305.34 48.714 48.077 0.1480 0.28399 Bài 6.
Sử dụng phương trình van der Waals và phương trình Redlich-Kwong tính thể tích mol c a ủ
CO tại 200 K và 1000 bar. So sánh kết quả c
thu đượ với kết quả khi s ử dụng phương trình
khí lý tưởng. Giá trị thực nghiệm là 0,04009 L.mol-1.
Use both the van der Waals and the Redlich-Kwong equations to calculate the molar
volume of CO at 200 K and 1000 bar. Compare your result to the result you would get
using the ideal-gas equation. The experimental value is 0.04009 L.mol-1. Bài 7.
So sánh các áp suất cho bởi (a) phương trình khí lý tưởng, (b) phương trình van der Waals,
(c) phương trình Redlich Kwong, -
và phương trình Peng-Robinson cho propan tại 400 K và
= 10,62 mol.dm3. Giá trị thực nghiệm là 400 bar. Cho = 9,6938 L2.bar.mol-2 và = 0,05632 L.mol-1 -Robinson. cho phương trình Peng
Compare the pressures given by (a) the ideal-gas equation, (b) the van der Waals equation,
(c) the Redlich-Kwong equation, and (d) the Peng-Robinson equation for propane at 400 K
and = 10.62 mol.dm3. The experimental value is 400 bar. Take = 9.6938 L2.bar.mol-2
and = 0.05632 L.mol-1 for the Peng-Robinson equation. 2 3 Chem 1084
Homework Set #2 (Chap. 3) 1)
a) A sample of 4.50 g of methane occupies 12.7 L at 310K. Calculate the
work (in J) done when the gas expands isothermally against a constant
external pressure of 7.7 kPa until its volume has increased by 3.3 L.
b) Calculate the work done if the same expansion had occurred reversibly. 2)
a) What would be the final volume (in L) occupied by 1.00 mol of an ideal
gas initially at 0°C and 1.00 bar if q = 1,000 J during a reversible isothermal expansion?
b) Repeat the problem assuming an isothermal expansion against a constant pressure of 1.00 bar 3)
One mole of ethane at 25oC and one atm is heated to 1200oC at constant
pressure. Assuming ideal behavior, calculate the values of w, q, ∆U, and
∆H given that the molar heat capacity of ethane is given by
over the above temperature range. Repeat the calculation for a constant- volume process. (McQ&S, 19-22) 4)
A 10.0 kg sample of liquid water is used to cool an engine. Calculate the
heat removed (in joules) from the engine when the temperature of the water
is raised from 293 K to 373 K. Take C -1
P = 75.2 J·K ·mol-1 for H2O(l). (McQ&S, 19-26) Problem Set #2 - 1 - 5)
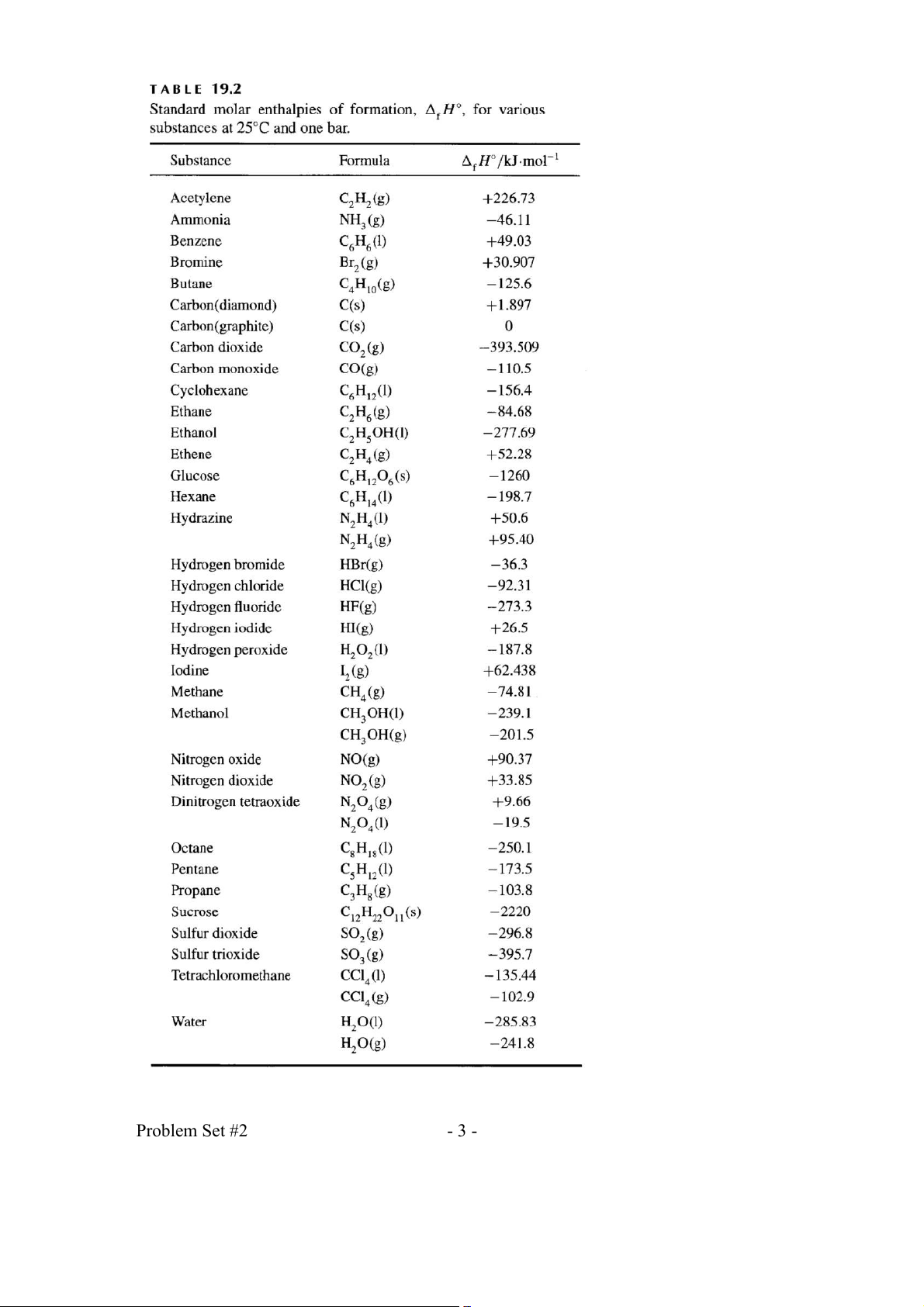
Combine the following thermochemical equations to predict ΔrH° at 298 K 3 3
for CH (g ) + Cl (g ) → CHCl (l ) + H (g ) . 4 2 3 2 2 2 5 1 3
(1) CHCl (l) + O ( g) → CO ( g) + H (
O l) + Cl ( g) H = 4 − 01.95 kJ 3 2 2 2 2 r 298 4 2 2 (2) C( grap )
h + 2H (g ) → CH ( g) H = 7 − 4.81 kJ 2 4 r 298 (3) ( C grap ) h + O ( )
g → CO ( g) H = 3 − 93.509 kJ 2 2 r 298 1
(4) H (g) + O (g) → H O(l) H = 2 − 85.830 kJ 2 2 2 r 298 2 6) (McQ&S, 19-38) 7)
Using Table 19.2 (below), calculate the heat required to vaporize 1.00 mol of CC14 (1) at 298 K. (McQ&S, 19-40) 8) For the gaseous reaction 1 C (
O g) + O (g) → CO (g) 2 2 2
ΔrH° = -282.987 kJ/mol at 298 K. Find Δ -1 -1
rH° at 1000 K if the heat capacities, in J K mol , are: − 3 − 7 2
C = 26.00 + 43.49710 T −148.3210 T for CO (g) P 2 −3 −7 2
C = 26.86 + 6.96610 T − 8.2010 T for CO(g) P − 3 − 7 2
C = 25.72 +12.9810 T − 38.610 T for O (g) P 2 Problem Set #2 - 2 - Problem Set #2 - 3 - Chem 1084 Homework Set # 3 1)
Calculate the change in entropy of the system and of the surroundings and the
total change in entropy if one mole of an ideal gas is expanded isothermally and
reversibly from a pressure of 10.0 bar to 2.00 bar at 300 K. (McQ&S, 20-25) 2)
Calculate the increase in entropy (J/K) when 2.00 mol of a monatomic ideal gas
(Cv = 3/2 nR) are heated from 300 K to 600 K and simultaneously expanded from 30.0 L to 50.0 L. 3)
1.00 mol of a monatomic ideal gas has an initial state of 10.0 atm and 600 K.
You can assume that all processes in this problem are reversible.
a) Calculate q, w, ΔU, ΔH (all in kJ) and ΔS (J/K) for an isothermal expansion
to a final pressure of 1.00 atm.
b) Calculate q, w, ΔU, ΔH (all in kJ) and ΔS (J/K) for a subsequent adiabatic
expansion to a final temperature of 300 K. 4)
Calculate the molar entropy (J K-1 mol-1) of a constant-volume sample of Ne at
500 K given that it is 146.22 J K-1 mol-1 at 298K. 5)
Calculate the entropy of mixing if two moles of N2 (g) are mixed with one mole
O2 (g) at the same temperature and pressure. Assume ideal behavior. (McQ&S, 20-29) Chem 1084 Homework Set # 4
1) The molar heat capacity of C2H4(g) can be expressed by
over the temperature range 300 K < T < 1000 K. Calculate ∆S if one mole of ethene
is heated from 300 K to 600 K at constant volume. (McQ&S, 21-4)
2) Use the data in Problem 1 to calculate ∆S if one mole of ethene is heated from
300 K to 600 K at constant pressure. Assume ethene behaves ideally. (McQ&S, 21-5)
3) Use the following data to calculate the standard molar entropy of N2(g) at 298.15 K. (McQ&S, 21-14)
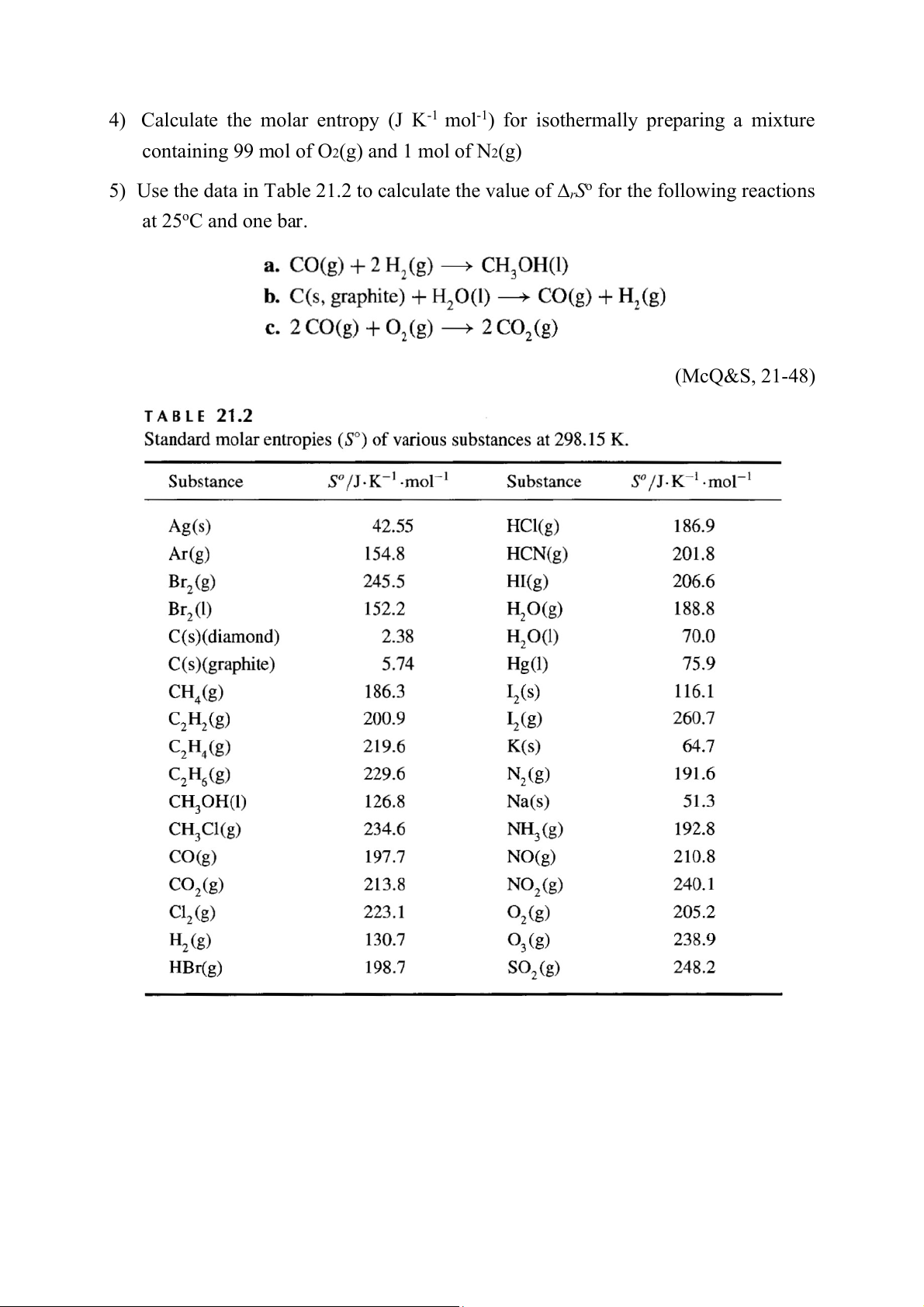
4) Calculate the molar entropy (J K-1 mol-1) for isothermally preparing a mixture
containing 99 mol of O2(g) and 1 mol of N2(g)
5) Use the data in Table 21.2 to calculate the value of ∆rSo for the following reactions at 25oC and one bar. (McQ&S, 21-48) Chem 1084 Homework Set #6 1)
Beginning with dU = TdS − PdV and H = U + PV …
a) Derive the expression dH = TdS +VdP T V
b) Derive the Maxwell relation = P S S P RT 2) Evaluate =
C − C for a gas that obeys the equation of state P P V V − b 3)
Calculate G (T P ) 0 ,
− G (T ) (in kJ/mol) for an ideal gas at 0.100 Pa and 25°C 4)
The compressibility factor for ethane at 600 K can be fit to the expression.
for 0 ≤ P /bar ≤ 600. Use this expression to determine the fugacity
coefficient of ethane as a function of pressure at 600 K. (McQ&S, 22-29) 5)
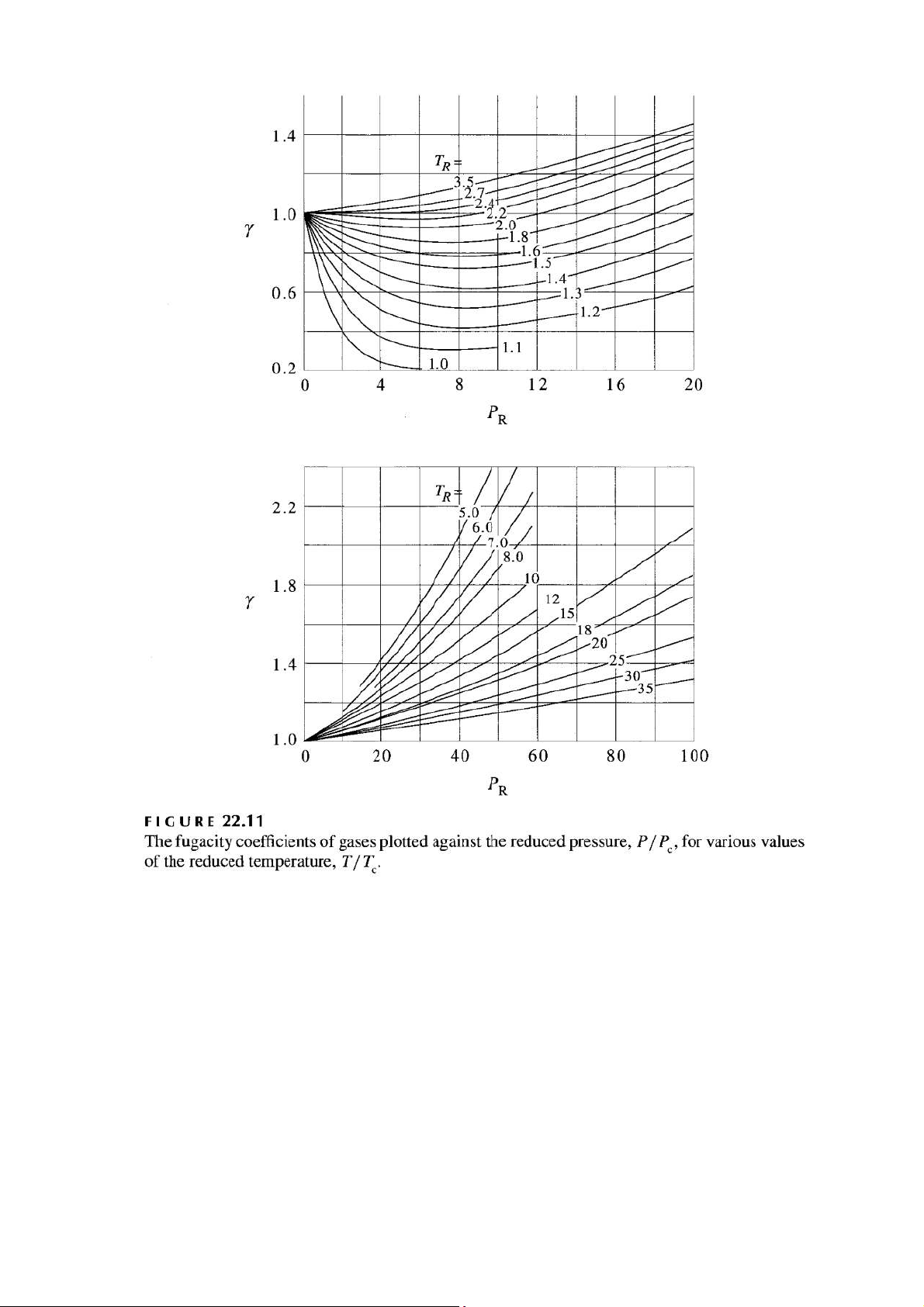
Use Figure 22.11 and the data in Table 16.5 to estimate the fugacity of ethane at 360 K and 1000 atm. (McQ&S, 22-30)



