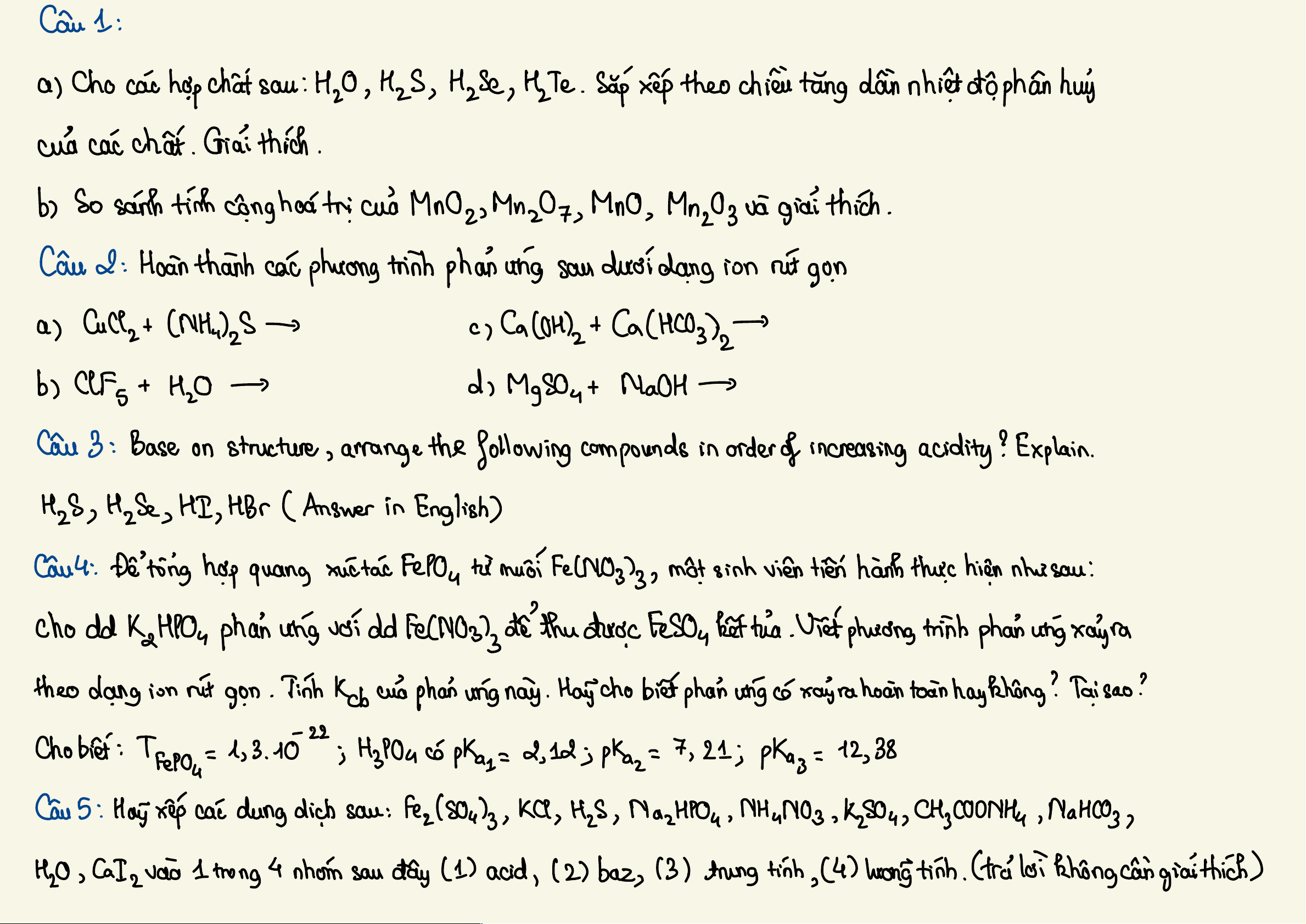
F
↑
-
j
·
e
e
G
E
-
·
-
-
T
g
·
E
s
s
·
·
I
·
I
=
"
S
M
O
E
E
T
u
T
&
·
-
-
Yo
E
.
i
*
↳
=
I
E
Ju
·
-
~
-
-
a
&
o
-
·
E
g
=
·
-
↳
·
-
·
↳
i
E
al
·
-

Cu
?:
a
H20
,
HzS
,
HzSe
,
Hate
dulhop
chat
CHT
.
b)
MnO
,
MnzOz
,
MnOz
delhopchfion
;
MnzOz
banchtlfhCHT
Nhiet
to
phanhuy
cera
hop
chat
CHT
Chi
cobente
CHIT
=>
Mn
,
Of
c
tinh
CHT
walk
loinh
at
Dober
CHTY
hi
Mucting
nange
M
E
Tick
CHT
chopchation
-
Chi STDPC
macation
y
The
tich
ven
phu
X
MDBPC
cu
anion
↑
1
Matctixenphu'T
Cynt
quantrognhat)
MnO
,
MnzOz
,
MnOz
c cing
anion
=>
XeF
TDP(cuacation
Hate
He
HS
Ho
#
Parcation
thi
at
/
(y euto
quantro
.
ngnhat)
Mucci
drig
nang
S
r
+
1
the rich
ven
phu"
7
Che
:
d'od
...
do
Mat
co
xemphu
12/
=>
Hoben
tinhe
CHT
:
Hate
<HiSe
<
H2S
<
H20
Mut
"
Mu
+
3
Mu
+
2
=>
tophanhuy
tang
dantheothitu
:
HTe
,
HaSe
,
Hz
S
,
H20
+
4
+
3
+
2
=>
TDPC
au
Mnt"
>
Mn
+
3 cMatz
=>
Tirh
CHT
ca
MnOz
>
MnLOs
>
MnO
Kethan
:
Tick
CHT
gian
dan
theo
the
Hi Mn 207
,
MnOz
,
Mnz
Oz
,
MnO
Cu2
:
as
C2
+
ga-
-
Cust
c)
Caf
+
+
OH
+
HCO3
-
CaCOz
+
+
H0
b)
CeF z
+
H20
-
>
5HF
+
H
+
+
ClO3-
d
>
Mg
+
20H-
-
Mg(OH)2
Can3
:
HzS
,
HiSe
,
HI
,
HBr
are
all
hydracids
.
The
acidity
of
hydracids
increases
when
:
the
polarity
of
H-X
bonding
increases
(X
=
S
,
Se
,
I
,
Br)
E
the
bonding
strength
of
H-X
decreases
#
Central
atoms
are
in
same
group
:
the
bonding
strength
of
H-X
is
more
important
than
the
polarity
of
H-X
bonding
+
S
and
Se
are
in
group
VIA7
From
the
top
to
bottom
,
the
radius
of
central
atom
increases
+
Brand
I
are
in
group
VIA
Overlappies
ar ea
incr eases
Electr on
density
decrease
eases
=>
[Ac idity
of
Mese
is
stronger
than h
S
Acidity
of
HI
is
stronger
than
HBr
↓
Central
atoms
are
in
same
period
:
the
polarity
of
H-X
bond
is
more
important
than
the
bonding
Strength
of
H-X
++
Se
and
Br
are
in
period
4
.
From
the
left
to
right
,
the
electronegativity
of
central
atom
increases
=>
Polarity
of
H-X
bond
increases
=>
Acidity
of
M-X
increases
=>
Acidity
of
HBr
is
stronger
than
HSe
CONCL USION
:
The
order
of
increasing
acidity
:
H2S
,
HSe
,
MBr
,
HI
Cu4
:
Cu5
:
Fest
+
CHPO-
>
FePOut
+
HzPOn
Kob
A
cid
:
Fez
(SOn's
,
MzS
,
My NOz
Kob
=
[H2POn]
Baz
:
Khongc
[Fe3
+
(HPOR2
=
O
[H2POn]
-
-
-
Trungtinh
:
KC
,
K.SOy
,
Calz
1
=
*
E
]
3
Long
tinh
:
NazHPOu
,
CHzCOONHy
,
MaMCOs
,
MO
[H
2
-
2
=
(Trepoy)
=
Kazkaz
*
(Kaz)
=
(1
,
3
.
1822)
-
*
x
(10 7
,
21)
- 7
x
1812
,
38
~
1016
,
72
K
*=
10
=
10
Kock
*
Phan vng
hantin
a
Bấm Tải xuống để xem toàn bộ.