

Preview text:
lOMoAR cPSD| 45254322
1.A chemical compound with which the electrodes of a cell are in contact is called an: A. insulator B. agent C. electrolyte (Correct) D. acid
2.The electrolyte in a lead-acid cell is: A. sulfuric acid and water (Correct)
B. hydrochloric acid and water C. potassium D. sulfuric acid
3.The electrolyte used for copperplating is most often a solution of water and: A. copper sulfate (Correct) B. vinegar C. sulfuric acid D. hydrochloric acid
4.A pure plating metal that is placed within the electrolyte of an electroplating assembly is referred to as the: A. cathode B. plate C. anode (Correct) D. bar
5.In an electroplating assembly, the object to be plated is known as the: A. cathode (Correct) B. target C. anode D. plate
6.The change from ions to atoms or molecules in electrolysis is due to: A. the passage of
electrons through the solution. B. electrolytic dissociation.
C. the ions giving up their charges to the electrodes (Correct)
D. the ions receiving their charges from the electrodes
7.In electrolytic conduction we have the passage of current through a chemical solution: A. by the transfer of
ions through the medium. (Correct)
B. by the transfer of electrons through the medium.
C. by the transfer of molecules through the medium.
D. by the transfer of atoms through the medium.
8.During the action of a cell the anode goes into solution as positively charged ions, and so the plate is left: A. electronegative. (Correct) B. electropositive. C. neutral. D. unchanged.
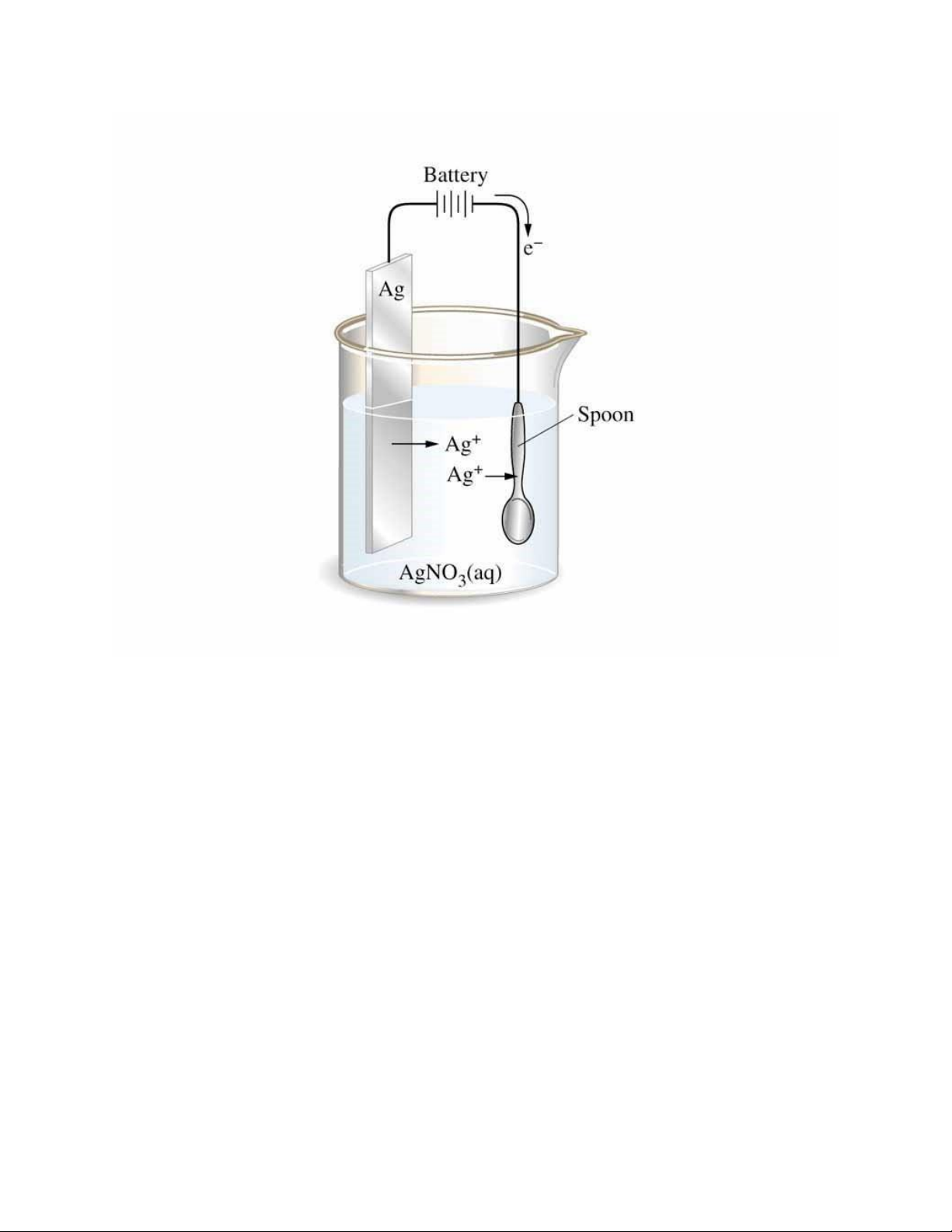
9.This set of apparatus is used to..? lOMoAR cPSD| 45254322 A. Generate electricity B. Discharge metal ion
C. electroplate silver spoon (Correct)
D. eliminate impurities from silver spoon.
10.In which direction does electricity 昀氀 ow in a voltaic cell (battery)?
A. Electrons 昀氀 ow from the cathode to the anode
B. Electrons 昀氀 ow from left to right
C. Electrons 昀氀 ow from anode to cathode (Correct)
D. Electrons 昀氀 ow from right to left
11.In electrolytic cell, anode will attract... A. Cations B. Anions (Correct) C. Electrons D. Atoms
12.In electrolytic cell, anode electrode is connected to...
A. Positive Terminal (Correct) B. Negative Terminal C. Both terminals D. Neither of the terminals
13.The process in which electrical energy is used to bring about a chemical change lOMoAR cPSD| 45254322 A. fuel cell B. battery C. electrolysis (Correct) D. voltaic cell
14.An electrochemical cell that is used to convert chemical energy into electrical energy A. electrolysis B. electrode C. voltaic cell (Correct) D. anode
15.Which of the following is a di 昀昀 erence between a voltaic and electrolytic cell?
A. electrons 昀氀 ow from anode to cathode
B. Charges on anode and cathode are opposites (Correct)
C. both are used as batteries in di 昀昀 erent ways
D. both can be used for metal plating
16.What does the cell / battery do in a circuit with a light bulb?
A. Changes electrical energy into chemical energy
B. Changes chemical energy into light energy (Correct) C. Uses up electrical energy
D. Changes chemical energy into motion
17.A combination of two or more electrochemical cells A. electrode B. electrolyte C. battery (Correct) D. cathodes and anodes
18.an electrochemical cell in which the electrolyte is a paste (gel) A. wet cell B. dry cell (Correct) C. paste cell D. half dry cell
19.A primary cell is a cell that... A. can only be used once (Correct)
B. can be recharged and used again C. is reusable D. is used again and again
20.Which of the following is an advantage of lithium ion cells?
A. High energy density and high discharge rates
B. Charge quickly and have a high number of cycles
C. High energy density and low discharge rate (Correct) D. Cheap to make
21.What is a disadvantage of a lead acid battery? A. They are expensive to manufacture.
B. They have a high self discharge rate.
C. They pose an explosion risk when overcharged. (Correct)
D. Lead is a plastic that is harmful for the environment.
22.What is the function of a battery charger? A. produce low-
voltage direct current (Correct) lOMoAR cPSD| 45254322
B. produce high-voltage direct current
C. produce low-voltage alternating current
D. produce high-voltage alternating current
23.What is a disadvantage of using a nickel cadmium battery? A. Expensive to produce.
B. Known to cause electrical 昀椀 res.
C. Harmful to the environment after it is disposed. (Correct)
D. Overcharging leads to production of hydrogen gas, which can explode.
24.What is the meaning of "Electrochemical equivalent" in Vietnamese?
A. tương đương điện hóa
B. đương lượng điện hóa (Correct)
C. điện hóa tương đương
D. lượng điện hóa tương đương
25.What does the word "electrolysis" mean in Vietnamese? A. điện phân (Correct) B. điện hóa C. phân tích điện D. điện tử
26.What does the word "electrolyte" mean in Vietnamese?
A. chất điện phân (Correct) B. dung dịch cách điện C. chất cách điện D. dung dịch dẫn điện
27.What does the word "rechargeable batteries" mean in Vietnamese? A. Pin sạc (Correct) B. Pin nạp lại C. Pin sạc được D. Pin tái tạo
28.What does the word "alkaline cell" mean in Vietnamese? A. Pin kiềm (Correct) B. Pin ka li C. Pin tiểu D. Pin liti
29.What does the term "memory e 昀昀 ect" mean in Vietnamese?
A. hiệu ứng nhớ (Correct) B. hiệu ứng kỷ niệm C. tác động nhớ D. tác dụng nhớ
30.What does the term "internal resistance" mean in Vietnamese? A. Nội trở (Correct) B. Trở kháng nội vụ
C. Sự cản trở từ bên trong D. Chống lại bên trong