











Preview text:
lOMoARcPSD|47231818 lOMoARcPSD|47231818
• Section 15-1: What is the nature of the atmosphere?
• Section 15-2: What are the major air pollution problems?
• Section 15-3: How should we deal with air pollution?
• Section 15-4: How might the earth’s climate change in the future?
• Section 15-5: What are some possible effects of a warmer atmosphere?
• Section 15-6: What can we do to slow projected climate change?
• Section 15-7: How have we depleted ozone in the
stratosphere and what can we do about it? lOMoARcPSD|47231818 Components of the Earth’s System 4 Fig. 3-2, p. 42 lOMoARcPSD|47231818 t E S
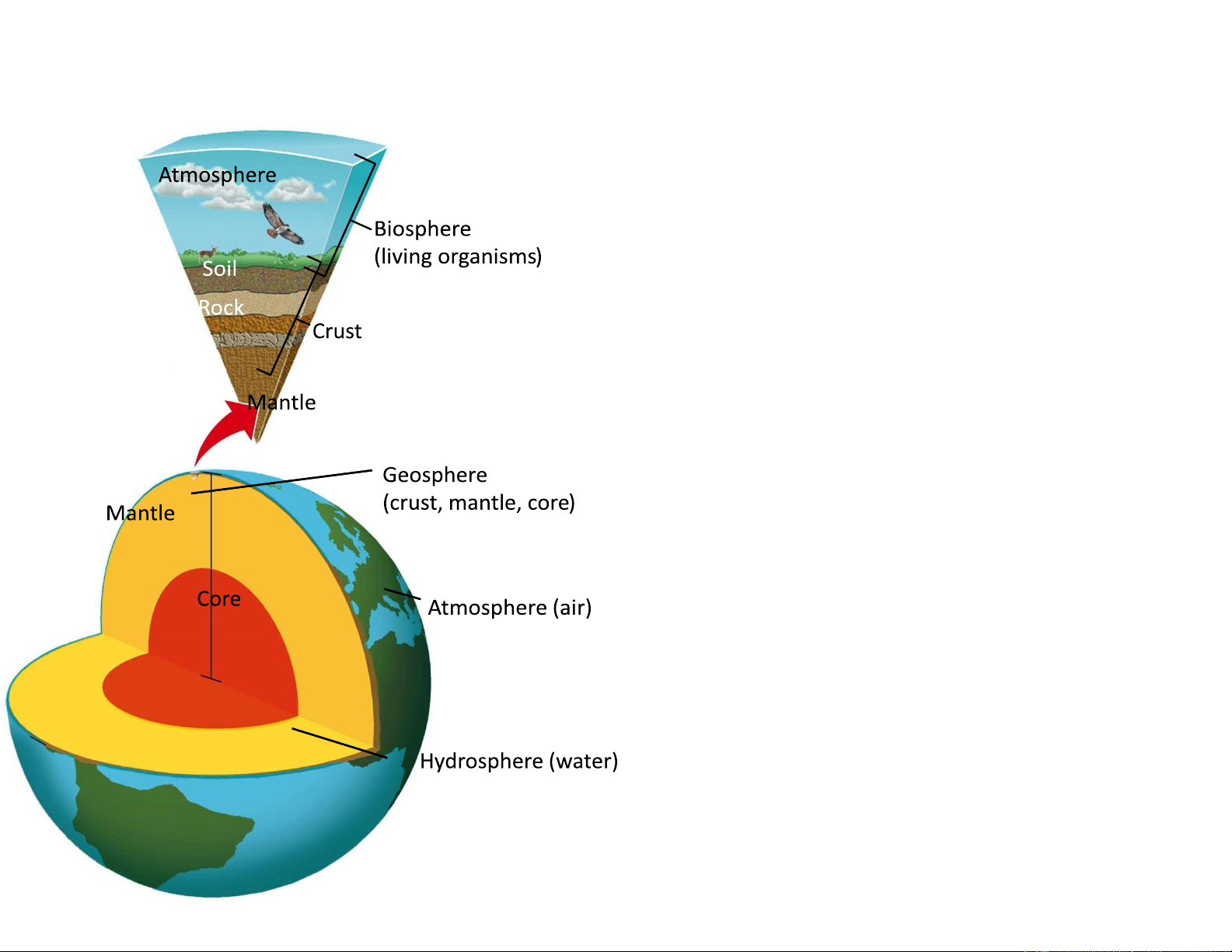
p n n t s f h a r t h ’ s y s t
Includes parts of other physical components of the Earth’s system Represents life on Earth
Includes both nonrenewable fossil fuels
and renewable soil chemicals (nutrients)
Includes the troposphere which is the air layer
about 7–17km above sea level. It contains
greenhouse gases that absorb and release
energy which warms the inner layer of the atmosphere.
Contains all water on or near the Earth’s surface,
In all forms (liquid, solid ice or permafrost and vapor) 5 lOMoARcPSD|47231818 p n nts f th t sph r
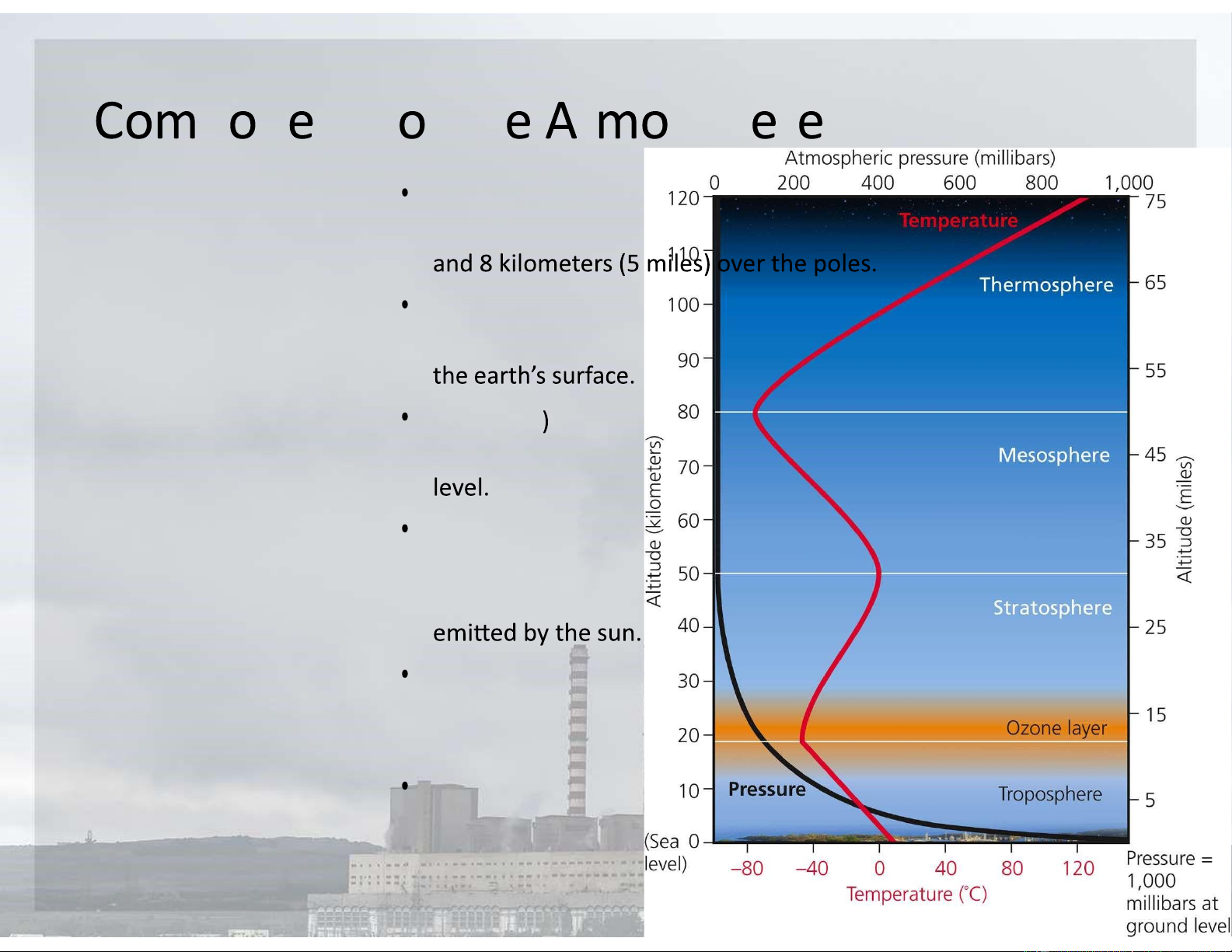
The troposphere: about 17 kilometers
(11 miles) above sea level at the equator
The stratosphere: about 17 to about 48
kilometers (from 11 to 30 miles) above
Ozone (O 3 (the ozone layer): roughly 17–
30 kilometers (11–19 miles) above sea
Stratospheric ozone: produced when
some of the oxygen molecules there
interact with ultraviolet (UV) radiation
Mesosphere: 50 km (31 miles) above
Earth's surface and goes up to 85 km (53
miles) high. (windows2universe.org)
Thermosphere: about 80-90 km (56
miles) to between 500 and 1,000 km (311 to 621 miles) above sea level What ar th aj r air p lluti n pr bl s Section 15-2 lOMoARcPSD|47231818 Wh r D ir P llutants fr
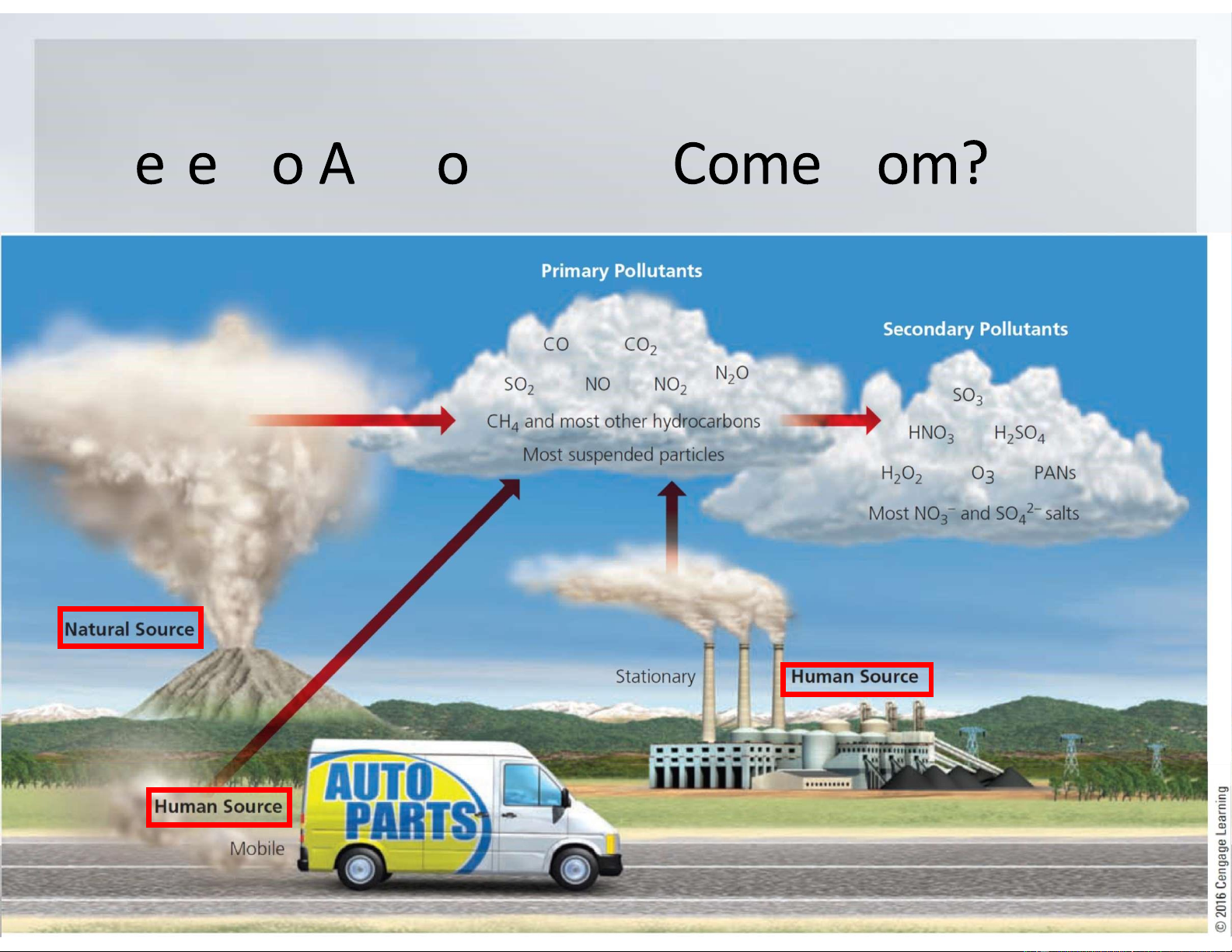
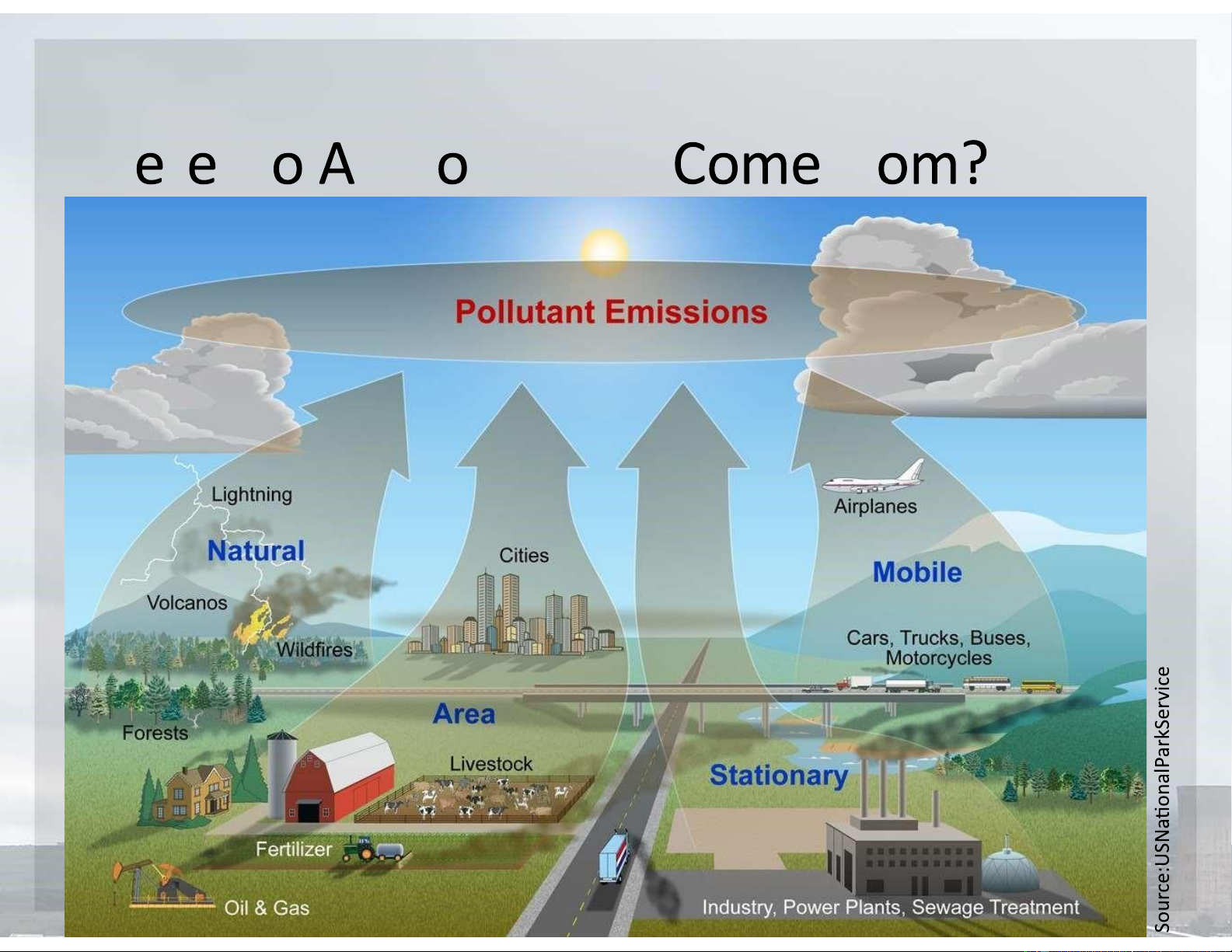
Where Do Air Pollutants Come from?
•Natural sources include dust blown by wind,
pollutants from wildfires and volcanic eruptions,
and volatile organic chemicals released by some plants.
•Most human inputs of outdoor air pollutants come
from the burning of fossil fuels in power plants and
industrial facilities (stationary sources) and in motor vehicles (mobile sources). What Are Air Pollutants? lOMoARcPSD|47231818
•Air pollutants are solid particles, gases, and liquid
droplets in the air that can adversely affect ecosystems
and the health of humans. (Kicinski & Nawrot 2015)
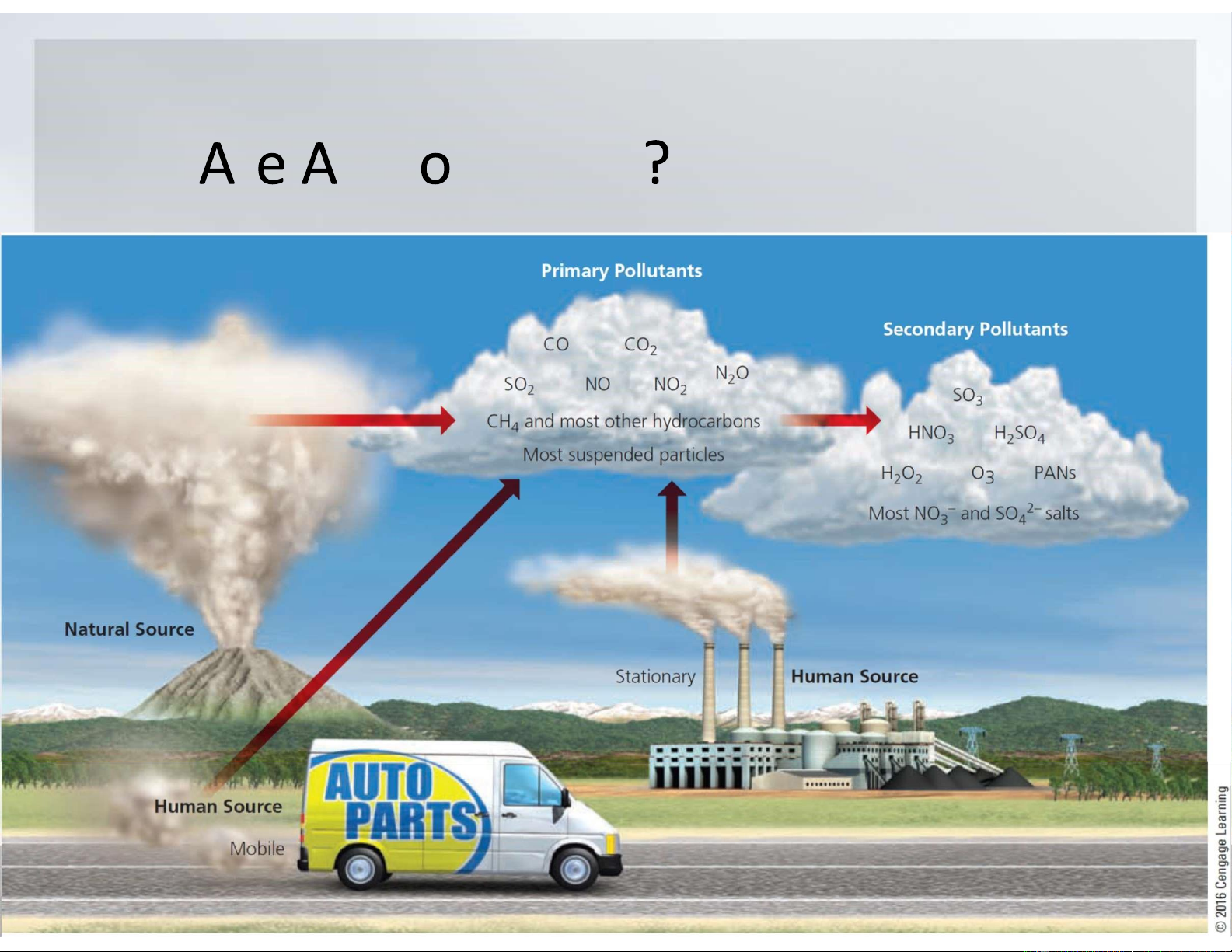
•Primary pollutants are harmful chemicals emitted
directly into the air from natural processes and human activities.
•Primary pollutants react with one another and with
other normal components of air to form new harmful
chemicals, called secondary pollutants. What r ir P llutants lOMoARcPSD|47231818 Wh r D ir P llutants fr lOMoARcPSD|47231818 What r Maj r ir P llutants Carbon oxides.
Carbon monoxide (CO): from motor vehicle exhaust, burning of forests and
grasslands, tobacco smoke, and open fires and inefficient stoves used for cooking.
CO reacts with hemoglobin in red blood cells and reduces the ability of blood to
transport oxygen to body cells and tissues.
Long-term exposure can trigger heart attacks and aggravate lung diseases such as asthma and emphysema.
At high levels, CO can cause headache, nausea, drowsiness, mental impairment, collapse, coma, and death.
Carbon dioxide (CO 2 from natural carbon cycle, human activities, 2 absorbing forests and grasslands Contribute to global warming lOMoARcPSD|47231818
What Are Major Air Pollutants? • Particulates
• Suspended particulate matter (SPM): A variety of solid particles and liquid
droplets small and light enough to remain suspended in the air for long periods.
• PM-10 (with diameters less than 10 micrometers): Fine particles • PM-2.5: ultrafine
• 38% comes from human sources such as coal-burning power and industrial
plants, motor vehicles, road construction, and tobacco smoke
• Irritate the nose and throat, damage the lungs, aggravate asthma and bronchitis, and shorten life • Ozone lOMoARcPSD|47231818
• Causes coughing and breathing problems, aggravates lung and heart
diseases, reduces resistance to colds and pneumonia, and irritates the eyes, nose, and throat.
• Damages plants, rubber in tires, fabrics, and paints lOMoARcPSD|47231818 What r Maj r ir P llutants
Volatile organic compounds (VOCs).
Organic compounds as gases in the atmosphere
Methane: from natural sources, rice paddies, landfills, oil and natural gas wells, and cows.
Benzene and other liquids: from industrial solvents, dry-cleaning fluids,
and components of gasoline, plastics, and other products.
Sulfur dioxide and sulfuric acid.
2 from natural sources, combustion of sulfur-containing coal in
electric power and industrial plants and oil refining and smelting of
2 can be converted to sulfuric acid (H 2 4 lOMoARcPSD|47231818 What r Maj r ir P llutants
Nitrogen oxides and nitric acid.
Irritate the eyes, nose, and throat; aggravate lung ailments such as asthma and
bronchitis; and suppress plant growth and reduce visibility
Nitric oxide (NO): when nitrogen and oxygen gas in air react at the high-
combustion temperatures in automobile engines and coal-burning power and
NO reacts with oxygen to form nitrogen dioxide (NO 2 , a reddish-brown gas
NO 2 reacts with water vapor in the air to form nitric acid (HNO 3 Form photochemical smog
Nitrous oxide (N 2O) is a greenhouse gas





