SO CTAO DUC
VA DAO
TAO
9tg'
DAKLAK
ON
CTTiNH
TIITIC
(Di
thi
g6m
02 trang)
Ciu 1:
(4,0
di6m)
rt
rnr
cHeN Hec srr\H
GrOr riNn rHCS
NAnn ugc zora-zors
TVT6N: HOA
HOC
(Thdi
gian
ldm
bei 150
phrit,
ttOng
k€
giao
d6)
Ngny
thi: l0l4l20l9
1.
Chgn c6c-ch6t
thich hgrp
thay
vio c6c
chil c6i rOi viiSt c6c
phucrng
trinh ho6 hgc
thgc hiQn
nht'ng
chuytin
e6i noa hoc
sau:
+
H2SO4
fli
KhiA
x<
\
+
H,o
+
HCr
+
NaoH. to
+
HNo"
P
?
Khi B
fo*e
dich B?,
?
Khi B
f'
?l'
Biist
khi A dtng
ct6 nap
cho binh chira ch6y.
Z.Odtch6y
cacbon
trong khdng
khi d nhiQt
d0 cao
thu
dugc
h6n
hqp khi A. Cho A t6c dgng
vdi FeO
nung n6ng
dugc khi B
vd h6n hqp
ch6t r8, C. Cho
B
t6c dpng vdi dung dich nudc
v6i trong
thu dugc tiSt
ma K
vd dung dich D,
dun
n6ng D lai
thu
dugc t<iSt
tria
K.
Cho C tan
trong
dung dich HCl,
thu dugc khi
vd dung dich E. Cho E tdc dUng vdi dung dich NaOH du
dugc hidroxit k6t ma F.
Nung F
trong kh6ng khf tdi khi5i lu-o. ng kh6ng O6i ttru dugc ctr6t ran
G..Bii5t c6c
phin
img xiy ra hoin
tonn. X6C dinh thanh
phan
Criu A, B, C, D, K, E, F, G vd
vi6t
cr4c
phuong
trinh hori hgc.
CAu 2:
(4,0
tli6m)
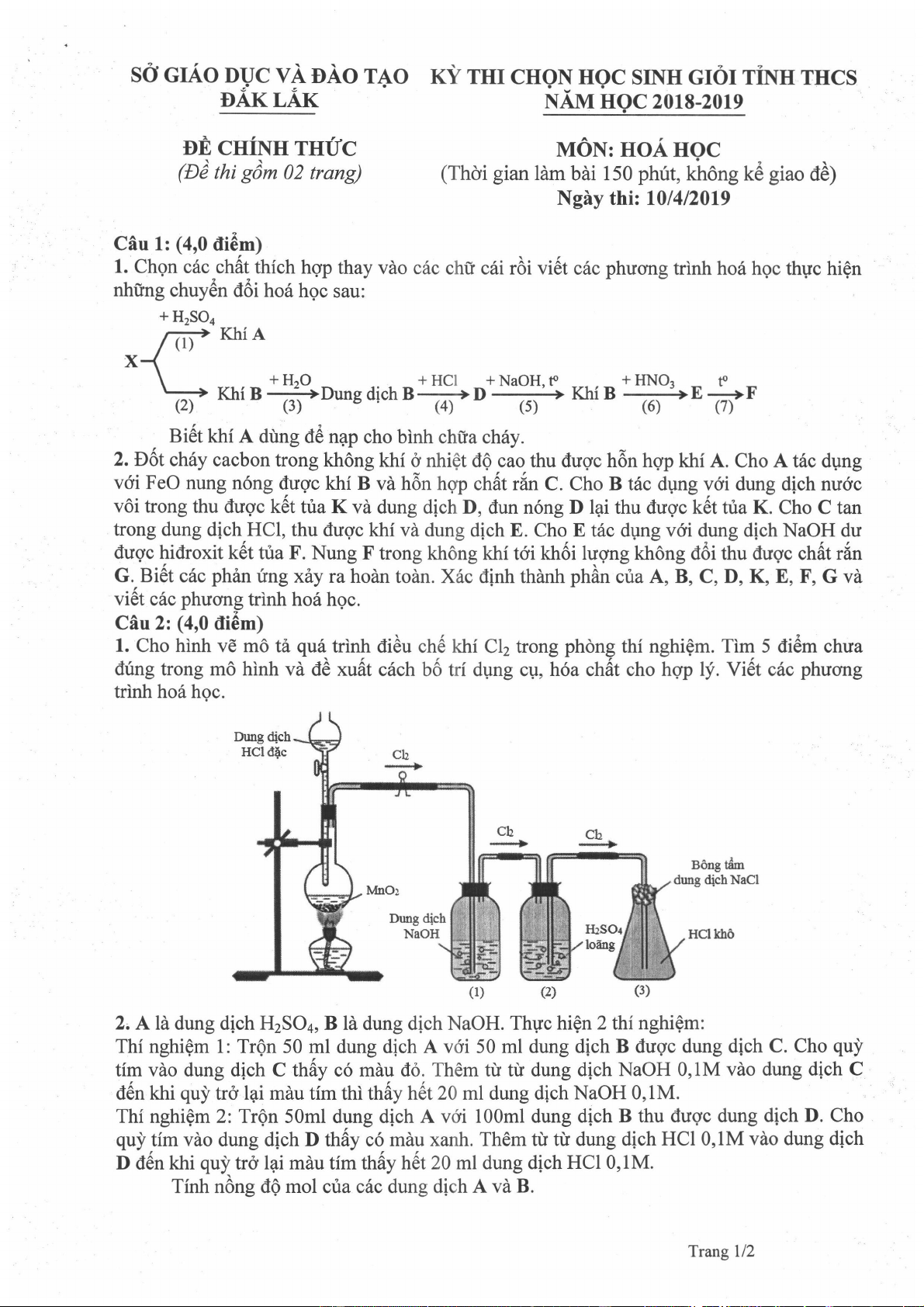
1.
Cho
hinh
vE m6 t6
qu6
trinh iti6u
chiS
khi
Cl2 trong
phdng
thi nghiQm. Tim 5 di6m chua
dtng trong mO hinh vd
iI6 xu6t c6ch
bO tri dung cp,
h6a
chft
cho hqp ly. YiAt cdc
phucrng
trinh ho6 hgc.
B6ng dm
dmgdchNaCl
HCllifi6
2, Ald dung dich HzSOa, B ld dung
dlch NaOH.
Thgc hiQn
2
thi
nghiQm:
Thi nghiQm l: TrQn
50 ml dung dich A vdi 50 ml
dung dich B dugc dung
dich C. Cho
qu!
tim vdo dung dich C th6y c6 mdu
d6.
Th0m tir tt dung dich
NaOH O,lM vdo
dung dich C
<l6n
khi
qu)
tr&
lpi mdu tim
thi thdy hOt
20 ml dung dich
NaOH 0,lM.
Thi nghiQm 2: TrQn 50m1 dung dich A v6i 100m1 dung
dich
B
thu dugc
dung dich
D. Cho
qu)tim
vdo dung dich D th6y
cQ
mdu xanh. ThCm
tir tir dung dich
HCI O,lM
vdo dung dich
D d6n khi
qui
trd lpi mdu tim thdy h6t 20 ml dung
dich HCI
0,lM.
Tinh n6ng
dQ mol
oia cic dung dich
A vd B.
Trangll2
C0u 3:
(5,0
tli6m)
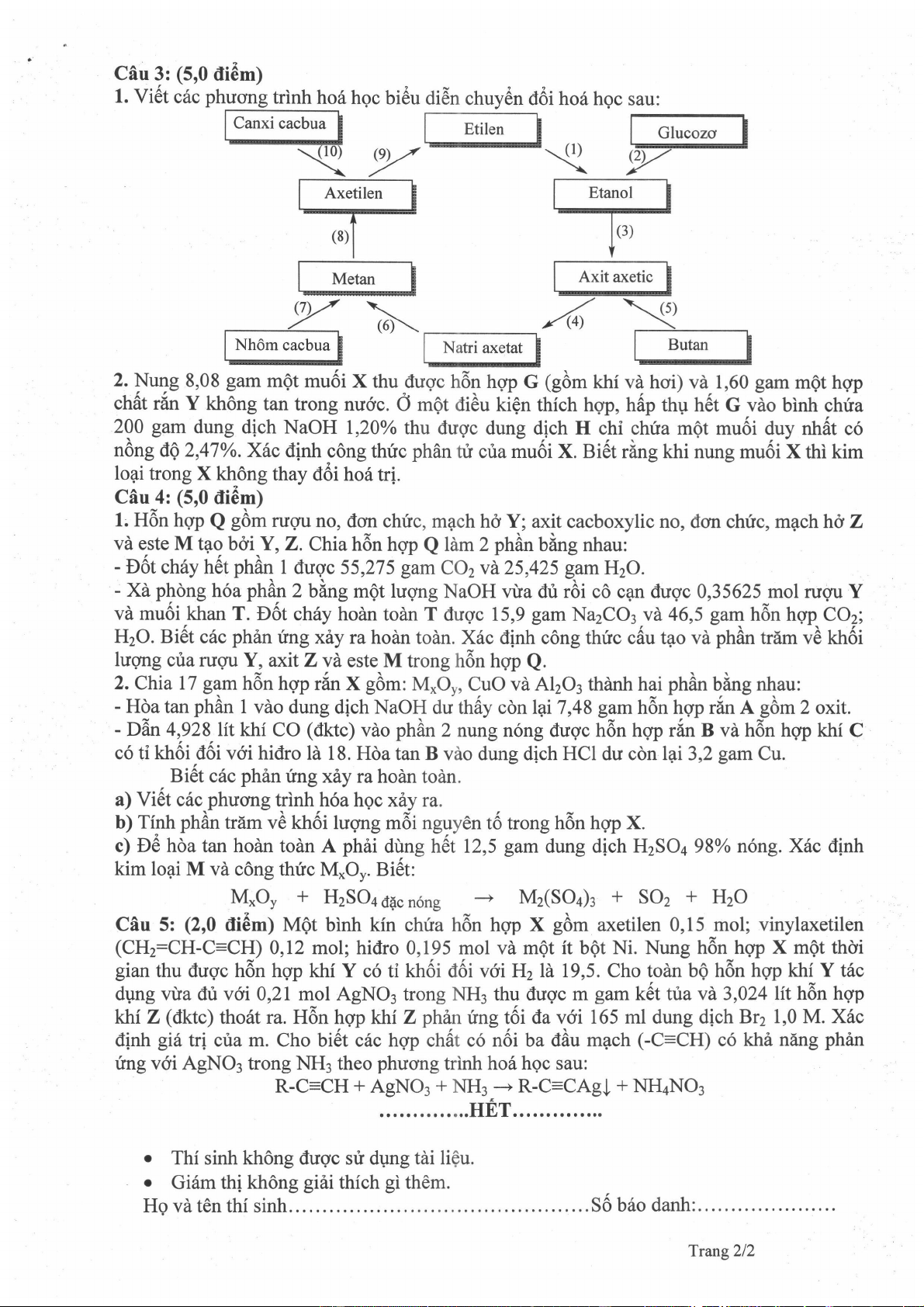
1.
Vi6t c6c
phuong
trinh
ho6 hgc
bi6u
di6n
chuyOn eOi noa hgc
sau:
Etilen
Metan
Natri axetat
l-c"r-i *.b* l
L--I
xv
Y
,>--
,6
Y
-.*b""1
-lNut,i;.t"rl
f-"f
I Ntrt
'-I
!,...E
2. Nung
8,08
gam
mQt
mu5i X
thu.d*q" h6n hqp
G
(g6m
khi vi hoi)
vd.1,60
gam
mQt hqrp
ch6t ran
Y kh6ng
tan trong
nu6c. O mQt
ili6u kiQn thich
hqrp,
h6p
thu hiSt G vdo binh
chrli
200
gam
dung dich NaOH
l,20yo thu
dugc dung d-ich H
chi chria mQt mui5i duy nh6t
c6
n6ng
d0 2,47%.
X6c
ctinh
c6ng thric
phdn
trl cria mu6i X. Bitit r6ng khi nung mu6i
X thi kim
lo4i
trong X
kh6ng thay
d6i ho6
tri.
CAu
4:
(5,0
tli6m)
1; H6n
hqp
Q
g6m
rugu
no, don chrlc,
mpch h0 Y; a><it
cacboxylic no, don chfc, m4ch hO Z
vi e_ste
M t4g
bOi
Y, Z.
Chiah6n hqrp
Q
ldm
2
phan
blng nhau:
-
OOt
ch6y hiSt
phAn
I
dugc
55,275
gam
CO2
vit25,425
gam
H2O.
-
Xa
phdng
h6a
phry
2 bang mQt luqne
NaOH vira dri iOi
.O c4n duqc 0,35625 mol rugu Y
vd mu6i khan
T. O6t chay
hoan toan T
ttugc 15,9
gam
Na2CO3 vd 46,5
gam
h5n
hqp CO2;
HzO. Bi6t
c6c
phan
ring xiy ra hodn
todn. X6c <Ifnh c6ng thr?c c6u tao vd
phdn
tram v€ kh5i
luqng
cria ruqu Y,
axit Z vd
este
M
trong h6n hqrp
Q.
2. Chia 17
g1m
h5n hqp
ran X
g6m:
M*Oy,
CuO vd Al2O3 thanh hai
phdn
bdng nhau:
-
Hgu
tan
phAn
1
vdo dung
dich
NaOH
du th6y cdn 14i
7,48
gamh6n
hqp ran I
FOm
2
oxit.
-
Dan 4P28.lit khi
CO
(ttktc)
vdo
phAn
2 nung n6ng dugc h6n hqrp riin
g
vd h5n hqp khi C
c6 ti kh6i
<l6i vdri hidro ln 18.
Hda tan B
vdo
dung dich HCI
du
cdn lqi3,2
gam
Cu.
.
gi6t
c6c
phan
img xiy
ra hodn todn.
a)
Vi6t c6c
phuong
trinh
h6a hgc xiry
ru.
b)
TtS
phdn
tram ve
nrOi luqng m6i nguy6n ti5
trong
h6n hgp X.
c) EC hoa
tan
hoan
todn A
phii
dung h}t 12,5
gam
dung dich
HzSO
4
98yo
n6ng. Xdc ilinh
kim lo4i M
vd c6ng thrlc M*Or. Bitit:
M"Oy
+
HzSO+
d{c n6ng
-+
M2(SO4L
+
SOz
+
HzO
Cffu 5:
(2,0
tli6m) MOt
binh kin chria h6n hqp X
g6m
axetilen
0,15 mo[; vinylaxetilen
(CH2:CH-C=CH)
0,12 mol;
hidro 0,195 mol vi
mQt it bQt Ni. Nung h6n hqrp
X
mQt
thoi
gian
thu
dugc
h6n
hqp khf Y c6 ti kh5i
eOi
vOi H2 la 19,5. Cho toan b0
h5n hqp khi Y t6c
dung vta dri v6i 0,21 mol AgNO3
trong
NH3
thu
dugc m
gam
ktit tfra vd3,024lft
h6n hqp
khi Z
(dktc)
tho6t
ra.
Hdn hqrp kti Z
phin
fng ti5i da vdi
165 ml dung dich
Brz 1,0 M. X6c
tlinh
gi6
tr! cta m. Cho
biiit c6c
hqp
ch6t c6
ni5i ba dAu m4ch
(-C=CH)
c6 khi nEng
phan
img vdi AgNO3 trong NH3 theo
phuong
trinh
ho5 hgc sau:
R-c=cH
.
i*: .1. Yfni. 1:::.:.1l'
+
NHaNo3
o
Thi sinh kh6ng dugc stl dpng tdi liOu.
.
Gi6m thi kh6ng
giii
thich
gi
th€m.
Trang2l2
Bấm Tải xuống để xem toàn bộ.



