













Preview text:
lOMoAR cPSD| 30964149
HANOI UNIVERSITY OF TECHNOLOGY
DEPARTMENT OF GENERAL AND INORGANIC CHEMISTRY * * * * *
LABORATORY EXPERIMENTS FOR GENERAL CHEMISTRY I HANOI 2009 lOMoAR cPSD| 30964149 INTRODUCTION
I. The importance of practice in chemistry
The chemistry is one of the most importance sciences. The logic method for study chemistry
is “practice-theory-practice”. Chemistry consists of both practice and theory, but the theory of
chemistry is developed from experiments.
Studying course of chemistry, the students have to do experiments. It will help the students
understand in more detail the theory and gain patient and careful characters.
II. Organizing and doing experiments 1. Orgainizing
The students in each class are divided into groups, in which each group consist of 15 to 20
students and a leader. When doing experiments, these groups continue being divided into small
groups (2-3 students) for every one have a chance to do experiments by himself or herself.
When getting to the laboratory, the leader of groups have to receive the instruments,
chemicals and check them. If have any things wrong or break, he or she must tell teacher. When
finishing the experiments, students have to arrange chemicals, experimental instruments and take
over by their teacher. During experimental process, the students must follow the rules of the laboratory. 2. Doing Experiments
Each experimental unit, the students have to follow 3 steps.
a. Preparing experiments before entry the laboratory
The preparing consist of
- Understand the theories relating to the experiments
- Guess the results, reactions,etc will receive from experiments.
- Explain and summary all experiments, which are going to do b. Doing experiments
Each student has to understand some following basic points.
- All the glass tools are cleaned before used.
- Chemical bottles are always stood on the shelf. Do not move them to other positions and
change the eyedroppers of each bottle.
- When using the equipments at laboratory, students have to ask supervisor for the first time
use. Do not change the parameters of apparatus, when do not understand.
- Each student group has a specific place for doing experiment, so that do not move around
and make noise in the laboratory. c. Writing report
When finishing experiments, student groups have to clean experiment tube, tools, and
experimental place and then write experimental report.
Students have to fill all the requiments in the report sheet for each experimental unit.
III. Introduction of some experimental tools.
1. Weight measurement – balance.
Balance is an important apparatus in chemistry laboratory. In chemistry laboratory, there are
two kinds of balance based on sensitivity.
a. Technical balance: sensitivity is 0.01gram
b. Analytical balance: sensitivity is 0.0001gram
Based on the operation principles, we could be divided - Mechanical balance - Electrical balance
Nowadays, the digital electrical balances are usually used in almost laboratories.
The balance is setup equilibrium on the table. Page 1 lOMoAR cPSD| 30964149
Plug in 220Velectric source. Turn on the balance and wait 1 to 2 minutes for stability.
Do not put chemicals directly on the balance plate (for instance: the solid chemicals must
cover with water-resisted paper.
Do not balance with too hot or too cold things.
The weight does not over the limit of balance.
Do not try balance by your hands.
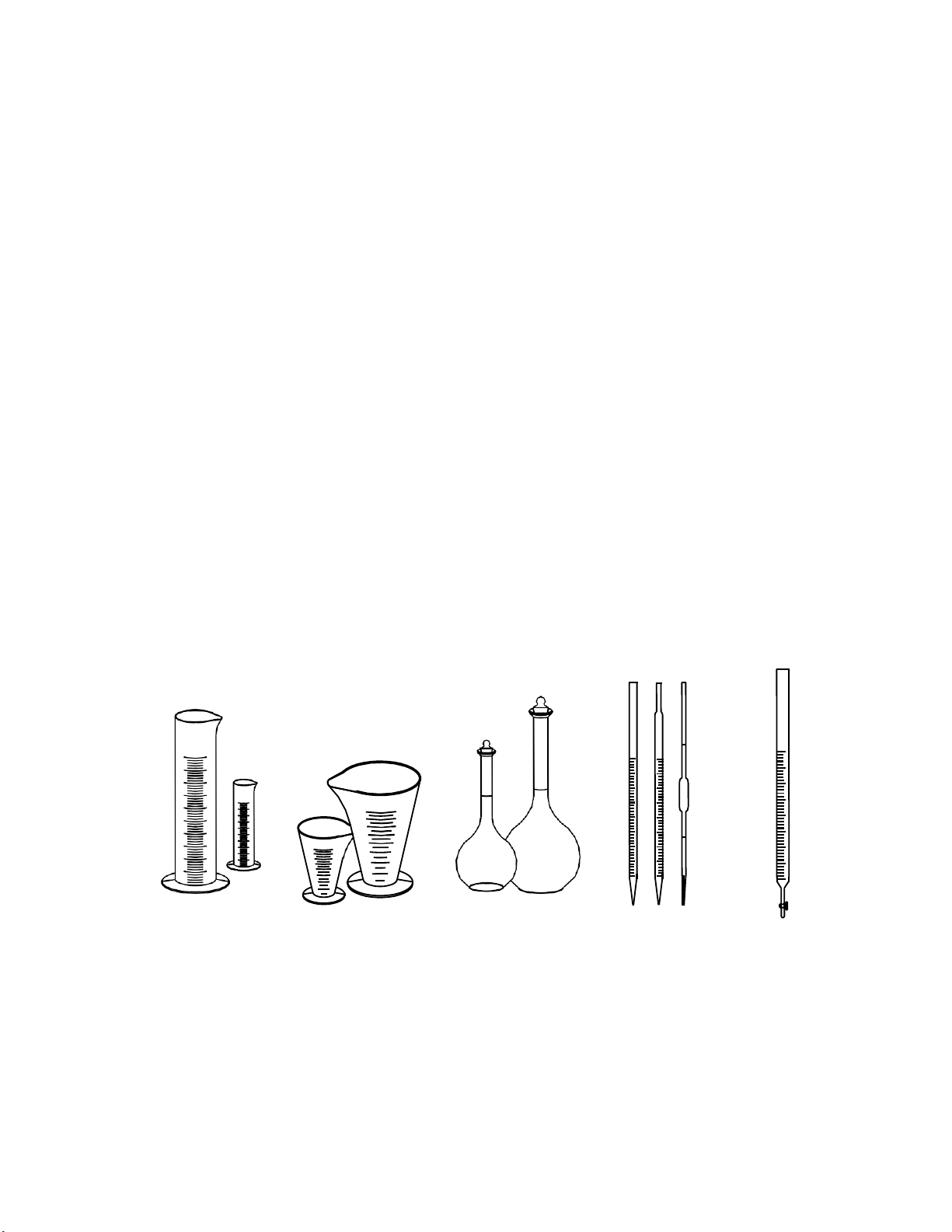
2. Fluid Volume Measurements
a. Graduated Cylinder:
Graduated cylinders are specifically designed to make accurate liquid volume measurements.
The volume is read from the lowest portion of the meniscus of the liquid; that is, the lowest
portion of the convex dip of the liquid as it sits in the graduated cylinder. Graduated cylinders are
available in a number of sizes: 5ml, 10ml, …1000ml, 2000ml. b. Graduated Cone:
Graduated Cone shape is designed to make suspension or emulsion solutions. Graduated
Cone shape is available in sizes: 50ml, 100ml, …1000ml, 2000ml.
c. Volumetric Flask:
A volumetric flask is used to make up a solution of fixed volume very accurately. This piece
of glassware is characterized by a long neck with a line for measuring a specified volume.
Volumetric flasks usually are made of borosilicate glass. They may have flat or round bottoms
(usually flat). Typical sizes are 25, 50, 100, 250, 500, 1000 ml. d. Pipet:
A pipet is used to measure small amounts of solution very accurately. A pipet bulb is used to
draw solution into the pipet. Typical sizes are 1, 2, 5, 10, 25, 50, 100, and 250.
e. Buret: a burette (also buret) is a vertical cylindrical piece of laboratory glassware with a
volumetric graduation on its full length and a precision tap, or stopcock, on the bottom. It is used
to dispense known amounts of a liquid reagent in experiments for which such precision is
necessary, such as a titration experiment. Typical sizes are 5, 10, 25, 50, 100, 250ml.
a: Graduated cylinders b. Graduated cone c. Volumetric flask d. Pipets e. Buret
IV. Basically manipulations in laboratory
1. Clean laboratory glasswares a. Mechanical clean
Laboratory glassware is washed by clout, and brush. Do not use strong force of the end brush on the bottom of test tube.
b. Chemical clean
Soak the glassware in sulfochromic solution (milled 5g K2Cr2O7 dissolved in 100ml
concentrated H2SO4 at 60-70oC). Page 2 lOMoAR cPSD| 30964149
If instruments, whom is adhensived oils or organics soak in KMnO4 solution for 10 mintutes
and then cleaned by HCl or H2C2O4 solution.
2. Filter and clean precipitate
To separate precipitate from solution we must filter and clean by funnel. - Clean funnel
- Fold and set filter paper in funnel. Use distillated water to make wet whole filter paper.
- Pour the solution flow throught filter paper on funnel. The fluid go throught the filter pores,
precipitate is detained on filter paper.
- To pour distillated water on the precipitate for cleaning. V. Experiments 1. Purposes
To help students practice with instruments in chemistry laboratory and understand basically
manipulations when use instruments.
To practice with balance and fabricate a solution with concentration CM in laboratory.
2. Practices: fabricate 50ml solution Na2SO4 1M from salt crystal
- Calculate the amout of Na2SO4 crystal for making 50ml solution of Na2SO4 1M.
- To put this amout of Na2SO4 into graduated flask 50ml.
- Pour aproximately 40-45ml distilled water in this graduated flask.
- Mix this solution by using glass stick.
- Add more the distilled water in graduated flask until 50ml volume, and
mix solution, we got the solution Na2SO4 1M (if solution is not transparent,
or clear you must filter it by filter paper). Unit 1 CHEMICAL EQUILIBRIUM I. SUMMARY 1.Chemical equilibrium.
Equilibrium is a state in which there are no observable changes as time goes by. When a
chemical reaction has reached the equilibrium state, the concentrations of reactants and products
remain constant over time, and there are no visible changes in the system.
In thermochemistry, chemical equilibrium is a stable state, in which Gsystem = 0.
The equilibrium constant is a dimensionless quantity; a certain ratio of reactant and product
concentrations at equilibrium and a constant temperature. For a reversible reaction, in the solution, we often use Kc.
Ex: Equilibrium constant of follow equation: Fe3+ + CNS - Fe(CNS)2+ (1) Red color [Fe(CNS)2 ] Kc = [Fe3 ][ CNS ]
Kc depends on temperature and the nature of substances, does not depend on concentrations.
2. Factors that influence on chemical equilibrium.
* Le Chatelier’s principle:
If a change of conditions is applied to a system at equilibrium, the system shifts in the
direction that reduces the stress to more toward a new state of equilibrium.
a. Changes in concentration. Page 3 lOMoAR cPSD| 30964149
If we increase concentration of reactants, the system adjusts in such a way so that its
concentration decreases and the forward reaction favors.
Ex for (1): If we add FeCl3 or NH4CNS to the solution (1), the equilibrium shifts from left to
right (the red color of the solution (1) deepens). The forward reaction favors, that means more Fe(SCN)3 is formed.
b. Changes in temperature.
If we increase temperature, equilibrium shifts to endothermic reaction and if we decrease the
temperature, equilibrium will shift to exothermic reaction. Ex: N2O4 2 NO2 (2)
The forward reaction is endothermic, the reverse reaction is exothermic so: if we increase the
temperature, equilibrium will convert to the forward reaction and the dark brown color deepens
and if we decrease the temperature equilibrium will shift to the reverse reaction and the dark brown color lightens.
3. Equilibrium in weak acids and bases.
a. Equilibrium in weak acids.
For example, in CH3COOH solution, there is a balance: CH3COOH + H2O CH3COO- + H3O+ (3)
Because the concentration of H3O+ is more than 10-7M so Methyl orange changes to red – orange color.
If we add CH3COONa to this solution: CH3COONa CH3COO- + Na+
Equilibrium (3) will shifts to the reverse direction and the concentration of H3O+ will
decrease so the red – orange color will change to light orange.
b. Equilibrium in weak bases.
For example, there is a balance: NH + 3 + H2O NH4 (4)
This is a base solution so phenolphthalein will change to pink color.
If we add NH4Cl to this solution NH4Cl NH 4 + + Cl-
The equilibrium (4) will shift to the reverse direction and pink color lightens.
4. Equilibrium of poorly soluble electrolyte.
a. Solubility product constant (T or Ksp)
An electrolyte is even called poorly soluble or insoluble, however, it can dissolve partially to
form the corresponding ions and therefore to reach the equilibrium state between precipitate and ions in the solution. Ex: CaCO 2- 3 Ca2+ + CO3 (5) BaSO 2- 4 Ba2+ + SO4 (6) CaSO 2- 4 Ca2+ + SO4 (7)
In the saturated solution, the dissociation reaction reaches the equilibrium state, the product of
poorly soluble electrolytic ions concentration in the solution is a constant, it is called solubility product and abbreviated to Tt [Ca2+][SO4 2- ] = 4 TCaSO = 6,1.10-5 [Ba2+][SO4 2- ] = 4 TBaSO = 1,1.10-10 [Ca2+][CO 2- 3 ] = TCaCO3 = 4,8.10-9
The less solubility of the electrolyte, the less value of Tt
Tt depends on the nature of poorly soluble electrolyte and temperature, it does not depend on the concentration. Page 4 lOMoAR cPSD| 30964149
b. Condition to form precipitate is the product of poorly soluble electrolyte ions
concentration in the solution must higher than solubility product. Ex: [Ca2+][SO 2- 4 ] > 6,1.10-5 [Ba2+][SO 2- 4 ] > 1,1.10-10 [Ca2+][CO 2- 3 ] > 4,8.10-9
c. Condition to dissolve poorly soluble electrolyte is the product of poorly soluble electrolyte
ions concentration must less than solubility product. Ex: [Ca2+][CO 2- 3 ] < 4,8.10-9 5. Hydrolysis of salt.
a. Definition: Hydrolysis of salt is a reaction of weak acid anion with water or weak base
cation with water which changes the pH of solution.
b. Characteristic of salt hydrolytic reaction.
- Just weak acid anion and base cation in salt is hydrolyzed. Strong acid anion and strong base
cation in salt is not be hydrolyzed.
- Hydrolysis is a reversible reaction, it obeys chemistry equilibrium rules. c. Types of hydrolysis.
- Salt which is made from weak acid anion and strong base cation, weak acid anion will be hydrolyzed to form OH – Ex: CH3COONa CH3COO- + Na+ CH3COO- + H2O CH3COOH + OH- Na 2- 2CO3 2Na+ + CO3 CO 2- - 3 + H2O HCO3 + OH-
- Salt which is made from weak base cation and strong acid anion, weak base cation will be hydrolyzed to form H3O+. Ex: NH + 4Cl NH4 + Cl- NH + 4 + H2O NH3 + H3O+
- Salt which is made from weak base cation and weak acid anion, both anion and cation will be hydrolyzed. II. EXPERIMENTAL SECTION.
1. Factors that affect chemical equilibrium.
1.1. Changes in concentration.
Look at this reaction: Fe3+ + CNS - Fe(CNS)2+
Pour into a 100 ml beaker about 20 ml distilled water, add 1 drop of FeCl3 (saturated
solution) and 1 drop of NH4CNS (saturated solution). Share the solution to four test–tubes, each
tube have about 1 ml (about 10 drops or 1 cm of the height of solution in the test–tube).
The first test-tube is remaining to comparison.
The second test-tube is added 1-2 drops of saturated FeCl3.
The third test-tube is added 1-2 drops of saturated Nh4CNS.
The forth test-tube is added some crystal of NH4Cl.
Observation and compare the color of solution in four test-tubes. Explain it.
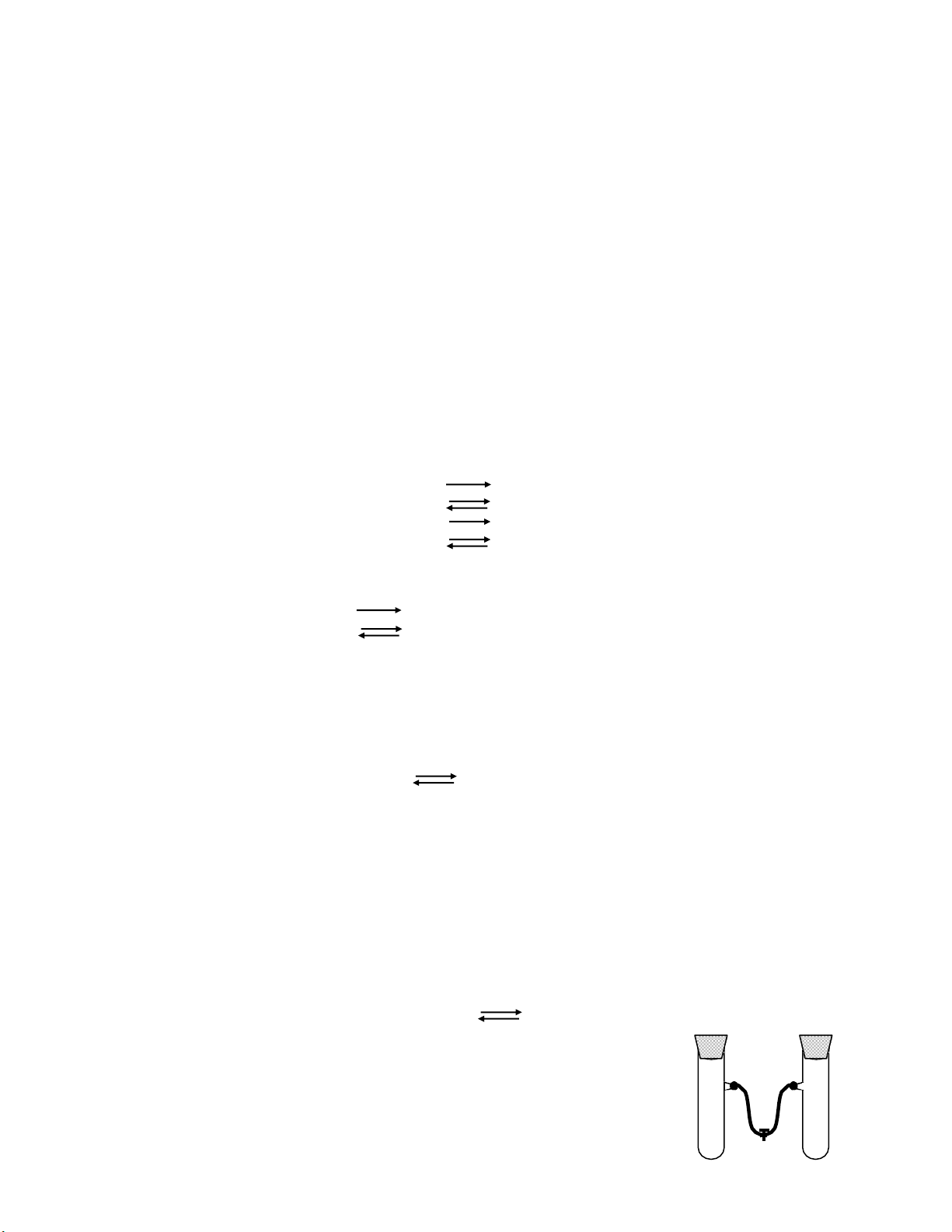
1.2. Changes in temperature.
Let’s see the influence of temperature on this reaction: 2 NO2 N2O4
Using two connected test-tube which contain NO2 (the color is
brown), open the lock (K) to have the same color in two test-tubes then
close the lock. Put test-tube number 1 into ice water with white salt.
Test-tube number 2 is remained to observe. Look at the changes in color
of test-tube number 1 in the cold water, after that put it into hot water Page 5 lOMoAR cPSD| 30964149
and observe the color changes. Use Le Chatelier’s principle to consider that reaction is endothermic or exothermic.
2. Equilibrium in electrolytic solution.
2.1. Color of indicators in various environments. Take 3 test-tubes N1: 10 drops of 2N H2SO4
N2: 10 drops of distilled water N3: 10 drops of 2N NaOH
Put a piece of litmus paper into each test-tube. Observe the color in each test-tube
Do the same but replace litmus paper by phenolphthalein and methyl orange. Write down the result into the table: Color indicator
Color of indicator in various environments Acid Neutral Base Litmus paper Methyl orange Phenolphthalein
Note: use only one drop of indicators.
2.2. Equilibrium in week acids and weak bases.
a. Weak acids: Put 2 ml (20 drops) of Acetic acid 2N (CH3COOH) into test-tube, add 1 drop
of methyl orange then share the solution into 2 test-tubes. N1: Remain to observe
N2: Add some CH3COONa crystals; shake the test-tube to dissolve them. Observe and
compare the color in 2 test-tubes. Explain it.
b. Weak bases: Put 2 ml (20 drops) of 2N NH3 to test-tube, add 1 drop of phenolphthalein.
Observe the color then share the solution into 2 test-tubes. N1: Remain to observe
N2: Add some NH4Cl crystals; shake the test-tube to dissolve them.
Observe the change in color of the solution and explain it.
2.3. Poorly soluble electrolyte
a. Condition to form precipitate.
Put into 2 test-tubes as follow:
N1: 5 drops of 0.1M CaCl2 and 5 drops of saturated BaSO4 solution.
N2: 5 drops of 0.1M BaCl2 and 5 drops of saturated CaSO4 solution
In which test-tube wil have precipitate? What is it? Explain it by calculating each case in
detail. (TBaSO4 = 1.1x10-10; TCaSO4 = 6.1x10-5)
b. Condition to dissolve precipitate
Prepare CaCO3 by adding into a test-tube 10 drops of 0.1M Na2CO3 and 10 drops of 0.1M
CaCl2. Add slowly drop by drop of HCl 2N into precipitate which we’ve just had. Observe and
explain what happen did. Write down the chemical equation. 2.4. Hydrolysis of salt
Take 2 test-tubes; put some NH4Cl crystals into N1 and some CH3COONa crystal into N2.
Add 2 ml (20drops) of distilled water into each test-tube.
Use pH paper to measure the pH values of these solutions, which environment these solutions
belong to (acid or base). Explain and write down the chemical equation of hydrolysis reaction. Page 6 lOMoAR cPSD| 30964149 Unit 2 Page 7 lOMoAR cPSD| 30964149 Page 8 lOMoAR cPSD| 30964149 Page 9 lOMoAR cPSD| 30964149 Page 10 lOMoAR cPSD| 30964149 Unit 3
FACTORS THAT AFFECT REACTION RATE I. SUMMARY.
1. Reaction rate (v) is a quantity specific to how fast the reaction takes place. It is measured
by the change in concentration of a reactant or a product over a period of time. Average rate: C C C v = ± 2 1 = ± t t t 2 1
C is the change in concentration (mol/l) of a studied substance from C1 to C2 over a period of time from t1 to t2. Instant rate: C dC v = lim = ± t dt
2. Factors that affect reaction rate.
2.1. Influence of concentration.
Reaction rate is directly proportional to the product of reactants with suitable exponent. (Mass interacting law) Ex: aa + bB cC + dd v = k [A]m [B]n Page 11 lOMoAR cPSD| 30964149
[A], [B] are concentration (mol/l) of A, B
m, n are numbers that must be determined experimentally. They are called the reaction order with respect to A and B.
m + n : The overall reaction order.
K: The rate constant. It depends on the nature of reactants and temperature.
2.2. Influence of temperature. * The Van’t Hoff’s rule v2 = v1. (T2 – T1)/10
- Temperature Coefficient ( = 2 – 4)
This is an experimental rule; it is only applied for the low range of temperatures. The Arrhenius equation E lnk = - a ln C lnv RT 1
C: a constant characterizes for each reaction T E
a: The activation energy of the reaction (in kJ/mol), It can be
determined experimentally by graphical method. (lnk – 1/T or lnv – E 1/T) tg = - a
When the temperature increases, the reaction rate increases R because:
-The speed of atom is increased make the collision frequency increase.
- In high temperature molecules are less stable so they react easily.
2.3. Influence of catalyst
- Catalyst is a substance that increases the rate of a chemical reaction without itself being consumed.
- In homogeneous catalysis, the reactants and the catalyst are in the same phases.
- In heterogeneous catalysis, the reactants and the catalyst are in different phases. II. EXPERIMENTAL SECTION.
1. Influence of concentration.
To study the influence of concentration on the rate of chemical reaction we investigate the following reaction:
H2SO4 + Na2S2O3 = H2SO3 + Na2SO4 + S
S is precipitated give the turbid milk color. The average reaction rate can be determined by
the change in concentration of one of products (such as sulfur) over a period of time as follows: C v t
C is the change in concentration of sulfur from the beginning of reaction (C1 = 0) to the time
when the initial precipitate can be observed. Here we stipulate C = 1 (stipulated unit) so the
reaction rate can count by stipulated unit as:
Using stop-watch to determine t (from the beginning of reaction to the time when we see the precipitate) Method:
- Take five test-tubes and drop into each one 2.5 ml 1M H2SO4 by pipette.
- Using pipette take into another five test-tubes mixture of H2O and 0.1M Na2S2O3
follow the ratio in the table. Page 12 lOMoAR cPSD| 30964149 -
Pour test-tubes (contain H2SO4) into test-tubes (contain mixture of H2O and 0.1M Na2S2O3) one by one. -
Using stop-watch to determine and record t (t is the time from the reaction
begin to the time when precipitate appear)
Make a plot to express the dependence of the reaction rate on the concentration of Na2S2O3.
Use the plot to define the reaction order with respect to Na2S2O3. Make a conclusion about the
influence of reactant’s concentration on the reaction rate.
2. Influence of temperature. Look at this reaction:
5H2C2O4 + 2 KMnO4 + H2SO4 = 10 CO2 + 2 MnSO4+ K2SO4+ 8 H2O
KMnO4 solution is violet when reaction end, it becomes colorless. So that with this reaction,
t is counted from the beginning to the time when solution being colorless. Stipulated rate of the
reaction is counted follow this equation: 1 v = t Method:
- Take 5 test-tubes and pour 2 ml 0.05N KMnO4 into each one. Take 5 other test-tubes, pour 2
ml 0.1M H2C2O4 which is acidified by H2SO4. Measure the reaction rate at 5 different temperatures (50C each step).
- Each experiment; soak two test-tubes (one contains KMnO4 and other contains H2C2O4) in
thermo regulator to reach the required temperature and remain in 5 minutes. Note the
temperature, mix two solutions and shake well. Determined t (from the time we start mixing to
the time solution being colorless)
Note: (after mixing, the solution has to soak in thermo regulator)
- Save the result, determine Ea and make a conclusion about the influence of temperature to the reaction rate. 3. Influence of catalyst Look at this reaction: 2H2O2 = 2H2O + O2
At room temperature, H2O2 disintegrates slowly. If catalyst (Ex: K2CrO4) is present, it will
disintegrate very fast. Mechanism of catalysis is making unstable, dark brown intermediate compound. H2O2 K
2CrO4 + H2O + H2O2 K2 CrO4 H2O H2O2 K 2 CrO4 K2CrO4 + 1/2 O2 + 2H2O H2O Method:
Pour 1 ml H2O2 10% into test-tube. Observe whether there is any bubble of O2 or not. Add some
drops of saturated solution K2CrO4. Observe the change in color and speed of gas released from the solution.
Make a conclusion about the influence of catalyst on the reaction rate. Page 13 lOMoAR cPSD| 30964149 Unit 4
Determine a reaction order of oxidation HCOOH by KMnO4 By using UV-VIS Method I. Summary 1. Regulation
a. Chemical reaction:
1A1 + 2A2 + 3A3 + ... = ’1A’1 + ’2A’2 + ’3A’3 + ... (1)
According to mass interating law:
v = kA n1 A n2 A n3 ... (2) 1 2 3 Where:
n1, n2, n3... is a reaction order with respect to A1, A2, A3.... k: The rate constant
Total of n = n1 + n2 + n3 ..... is overall reaction order
To determine the overall reaction order, firstly, we have to determine a reaction order with respect to each reactant.
To determine the reaction order (eg: n1 with respect to A1), have to find conditions so as to
change the concentration of [A1], while the concentrations of other reactants keep unchanged
(that means, their concentrations are many times higher than that of [A1]. Equation (2) is rewritten:
v = kA n1 A n 2 A n3 ... k A n1 (3) 1 2 3 1 1 (where k1 = kA 2 n2 A 3n3 ... const ) dA
Follows the concept of rate v = 1 so (3) can write like that: dt dA k A 1 1
n1 dA k dt (4) dt 1 1 1 A n1 1
Therefore, to determine the reaction order in A1, we have to investigate the variation of rate
vs. concentration (V- C; equation (3)) or concentration vs. time (C – t; equation (4)). Compare the
measured results and the obtained results for equation (4), we can define the reaction order in A1. Reaction order V- C C – t 0 v = k C – C0 = -kt C 1 V = kC ln kt C0 1 1 kt 2 V = kC2 C C 0
By the same experiments, we will obtain n2, n3… and then the overall reaction order.
b. Determine the reaction order of oxidation of HCOOH by KMnO4 Chemical reaction: 2MnO -
4 + 3HCOOH + 2H3O+ = 2MnO2 + 3CO2 + 6H2O (5) Page 14 lOMoAR cPSD| 30964149 Mass interacting law: v = k[MnO - 4 ]n1[HCOOH]n2[H3O+]n3 (6)
In this work, we will determine the reaction order with respect to KMnO4 (n1). In general, we
have to keep the concentrations of [HCOOH] and [H3O+] constant, whereas change the concentration of [MnO - 4 ].
Equation (6) can be rewritten: d MnO d MnO 4 n1 4 v = k MnO k dt (6’) dt 1 4 hay 1 MnO4 n1
To determine the reaction order n1, we have to investigate the relationship between the concentration of [MnO - 4 ] vs time t.
2. Use UV – VIS method to determine the reaction order
UV – Vis method is similar to other method, we will not investigate directly the relation
between C vs time t. We investigate the relation between absorbance A and time t. Based on Lambert - Beer law: D = ().l.C (7) a.Regulation
Radiating a monochromatic light which has a wavelength, with I0 initial intensity pass
through a solution, contained in cuvet which has a l length, one part of light will be absorbed,
another part will be transmitted It and another part will be reflected Ir. We have: I0 = Ia + It + Ir (8) I l t
T (%) is called transmittance. The coefficient lg
A D is an absorbance (A) or Io T (D).
According to Lambert – Beer law: A = ().l.C (9)
Where: A: absorbance; l: a length of cuvet: C: a concentration of solution:
(): Molar absorbed factor. This factor change by changing and it characterizes for each substance.
Thus, if we measure absorbance of a substance at a known wavelength, moreover l = constant
and A = k.C so absorbance only depends on C (concentration).
That means, to investigate the relation between A – t instead of the relation between C – t.
b. The condition to do the experiment
Use spectrophotometer 20D to measure the absorbance A. Because the instrument operates at
visible region and ultra-violet region (UV – Vis) so it only applies to solution which contains
color reagents. For example: KMnO4, MnO2, K2Cr2O7… In this work, KMnO4 has a violet color and MnO2 has a brown color.
Lambert – Beer law is only applied for dilute solution dA
The sensitivity of instrument is S =
.l S. That means the bigger S, the bigger dC
() (l = constant). Because depends on so we have to build a plot of = f() before doing the
experiments to determine the value of maximum corresponding to the maximum absorbance Amax II. Experiments
1. Steps to do the experiments Step 1: Preparation Page 15 lOMoAR cPSD| 30964149 Reagent and glasswares: - 0.03M KMnO4 - Magnetic stirrer - 0.1M HCOOH - 50ml Graduated cylinder - 0.04M K2HPO4 - 100ml beakers - Stopwatch - 5ml, 1ml Pipettes
Switch on Spectrophotometer within 15minutes before doing the experiments.
Step 2: Investigation of absorbance spectrum of KMnO4
Calibrate the machine (set off 0.00A using the distilled water)
Take 50ml distilled water into 100ml beaker, after that using the pipette to take 0.5ml KMnO4 into the beaker.
Measure absorbance of this solution at various wavelengths = 510; 515; 520; 525; 530;
535nm. Build a plot of wavelength vs. absorbance
Determine the to which corresponds Amax.
Caution: We have to recalibrate the machine, when changing wavelength of light. Step 3:
Measure absorbance of mixture reaction.
Use 5ml pipette to take 5ml 0.1 M HCOOH into 100ml beaker.
• Use graduated cylinder to take 50ml K2HPO4 and put it into the solution above.
• Put the stirring bar into this beaker. Put it on the magnetic stirrer and switch on the machine.
• Use 1ml pipette to take quickly 0.5ml KMnO4 into the solution above.
• Use the stopwatch and turn on the stopwatch at the time putting 0.5ml KMnO4 into the beaker.
• After 510seconds, pour amount of the reacting solution into the cuvet and put it into the spectrophotometer.
• Record result (absorbance A) after 10 seconds. During measure the A will decrease. The
measurement will be taken place until A = const (within 10 minutes). 2. Results
Use buffer K2HPO4, pH of this solution is constant and calculated as formula: [HPO 2 4 ] pH = pK a + lg pK 6 (11) a [H2 PO ] 4
[HCOOH] = 5.10-4 mol.l-1 >> [MnO -4] = 0,15.10-4 mol.l-1
and constant during the process.
To determine the reaction order in KMnO4 (n1), we have to investigate the dependance of [MnO -
4 ] vs. time (t). If n1 is equal to 1, the plot of ln(A- A ) vs. t is a straight-line. (If it is
not, the reaction order in KMnO4 is not equal to 1) and maybe it is 2, 3 …
At (t = 0), the reaction does not take place, in the solution has only KMnO4; the
Absorbance A0 only depends on concentration of [MnO -] 4 0 A0 = 1[MnO -] .l (12) 4 o where -
1: Molar Absorb factor of MnO4 .
At t, because the reaction takes place a part so KMnO4 converts to MnO2: the solution includes both MnO -
4 and MnO2, the measured absorbance: A =( -
1. [MnO4 ] + (2. [MnO2 .aq]).l (13)
A = 2[MnO2.aq].l = 2[MnO -] .l (14) 4 o Where: [MnO - -
4 ], [MnO2.aq]: concentration of MnO4 , MnO2.aq at a time of t: Page 16 lOMoAR cPSD| 30964149
2 : Molar Absorb factor of MnO2.aq.
Rely on Mass conservation law: [ 4 MnO - o ] = 4 [MnO - ] + [MnO2 ].aq (15)
From (12),(13), (14) and (15) we have: MnO A A ln 4 ln A (16) MnO 4 A 0 0
So, we can check the straight-line of ln[MnO -
4 ] - t by the straight –line instead of ln(A-A) - t
(because A0, A are constant).
If the plot of ln(A-A) - t is straight-line so the reaction order in MnO - 4 is 1. Question
1. How to determine the reaction order?
2. What is the purpose of this lesson?
3.Why is the concentration of HCOOH many times much more than that of KMnO4?
4.How to determine the A in this lesson? Check the formula (16). UNIT 5
EQUILIBRIA IN ACID- BASE SOLUTIONS PURPOSE
- Determine ionization constant of weak-acid.
- Determine concentration of acid by titration method of acid-base. I. Principle of pH- meter 1. General principle:
When one metal is brought in contact with another, a voltage difference occurs due to their
differences in electron mobility. When a metal is brought in contact with a solution of salts or
acids, a similar electric potential is caused, which has led to the invention of batteries. Similarly,
an electric potential develops when one liquid is brought in contact with another one, but a
membrane is needed to keep such liquids apart.
A pH meter measures essentially the electro-chemical potential between a known liquid
inside the glass electrode (membrane) and an unknown liquid outside. Because the thin glass bulb
allows mainly the agile and small hydrogen ions to interact with the glass, the glass electrode
measures the electro-chemical potential of hydrogen ions or the potential of hydrogen. To
complete the electrical circuit, also a reference electrode is needed. Note that the instrument does
not measure a current but only an electrical voltage, yet a small leakage of ions from the
reference electrode is needed, forming a conducting bridge to the glass electrode. A pH meter
must thus not be used in moving liquids of low conductivity (thus measuring inside small containers is preferable). 2. Introduction pH-meter
Using pH-meter and measured steps are guided at laboratory.
II. DEFINE IONIZATION CONSTANT OF WEAK ACID 1. Principle
Equilibrium in week-acid solution: HA + H2O H3O+ + A- (1)
Equilibrium constant of reaction (1) is called ionization constant of weak-acid HA (Ka): Page 17 lOMoAR cPSD| 30964149 [H O ].[A ] K a = 3 (2) [HA] pH [H3O+] = [HA] .K (3) a [A ] -lg[H O+] = lg [ A ] 3 - lgKa [ HA] pH = lg [ A ] + pKa (4) K p a [ HA] [ A ] Figure 1 lg X lg [HA]
pH index (4) is measured by pH-meter. To calculate value of constant pKa, we must estimate [ A ] [ A ] the ratio of . The ration of
is estimated by approximate method. Because of ionized [ HA] [ HA]
percent of HA decreased when increased concentration of common ions in solution of weak-acid,
so when add salt of NaA (mean A-) into solution of HA the equilibrium concentrations of HA
and A- are approximately equal to initial concentrations of Ca and Cm, respectively.
The equation (4) is expressed: C C pH = lg m + pK = lgX + pK (5) Where m a a X Ca Ca
The equation (5) is expressed in Figure 1, the pKa is estimated
2. Experiments and Results
Prepare 5 samples with various ratios of volumes from two solutions CH3COOH and CH3COONa.
Measure pH of each sample and note in Table 1.
Calculate value of X and note in Table 1, draw the plot (pH vs lgX) and estimate value of pKa from the plot.
III. Titration of strong acid (HCl) by strong base (NaOH)
Reaction of titration: HCl + NaOH = NaCl + H2O Ionized equation: H3O+ + OH- = 2H2O
1.Principle of titration Method
Titration is a general class of experiment where a known property of one solution is used to
infer an unknown property of another solution.
From balanced chemical equation find out equivalence concentration : CNA .VA = CNB.VB Where
NA, NB: equivalence concentrations of acid and base, respectively.
VA, VB: volume of acid and base at equivalence point. V Figure out: C C B (6) N . A NB VA Page 18 lOMoAR cPSD| 30964149
We use this instrumentation to calculate the amount of unknown acid in the receiving flask by
measuring the amount of base, or titrate; it takes to neutralize the acid. There is a way to know
when the solution has been neutralized. Uses a pH meter in
the receiving flask adding base slowly until the pH reads exactly 7. Titration Curves
A titration curve is drawn by plotting data attained
during a titration, titrate volume on the x-axis and pH on the y-axis.
The titration curve serves to profile the unknown
solution. In the shape of the curve lies much chemistry and
an interesting summary of what we have learned so far about
Figure 2. Apparatus for titration acids and bases.
The titration of a strong acid with a strong base produces the following titration curve:
Figure 3. Titration plot of a strong base titrating a strong acid
Note the sharp transition region near the equivalence point on the above Figure. Also
remember that the equivalence point for a strong acid-strong base titration curve is exactly 7
because the salt produced does not undergo any hydrolysis reactions.
2. Experiments and Results Uses pipette to estimate Solution NaOH 0,1N
exactly 20ml solution of acid HCl (unknown concentration) and
pour in flask 100ml (contained stirring bar). Add more 20ml distilled
water into flask (to increase the volume of solution) Glass electrode of pH-meter Stand flask on the
magnetic motor, manual stirring
Solution HCl unknown concentration speed for mixing solution as Magnetic motor Stirring shown in Figure. bar Pour 0.1 N NaOH into Figure 4 Page 19 lOMoAR cPSD| 30964149 buret 25ml.
Drop various volumes of NaOH from burette to flask of HCl solution and
measrure pH index of solution in flask for each dropped volume of NaOH.
Note the values of pH index and make table 2 in report form.
Draw graph of pH = f(VNaOH), and estimate VNaOH = Ve at equivalence point.
Calculate concentration of acid HCl according to (6) Unit 6
DETERMINING THE SOLUBILITY PRODUCT CONSTANT OF POOR ELECTROLYTES
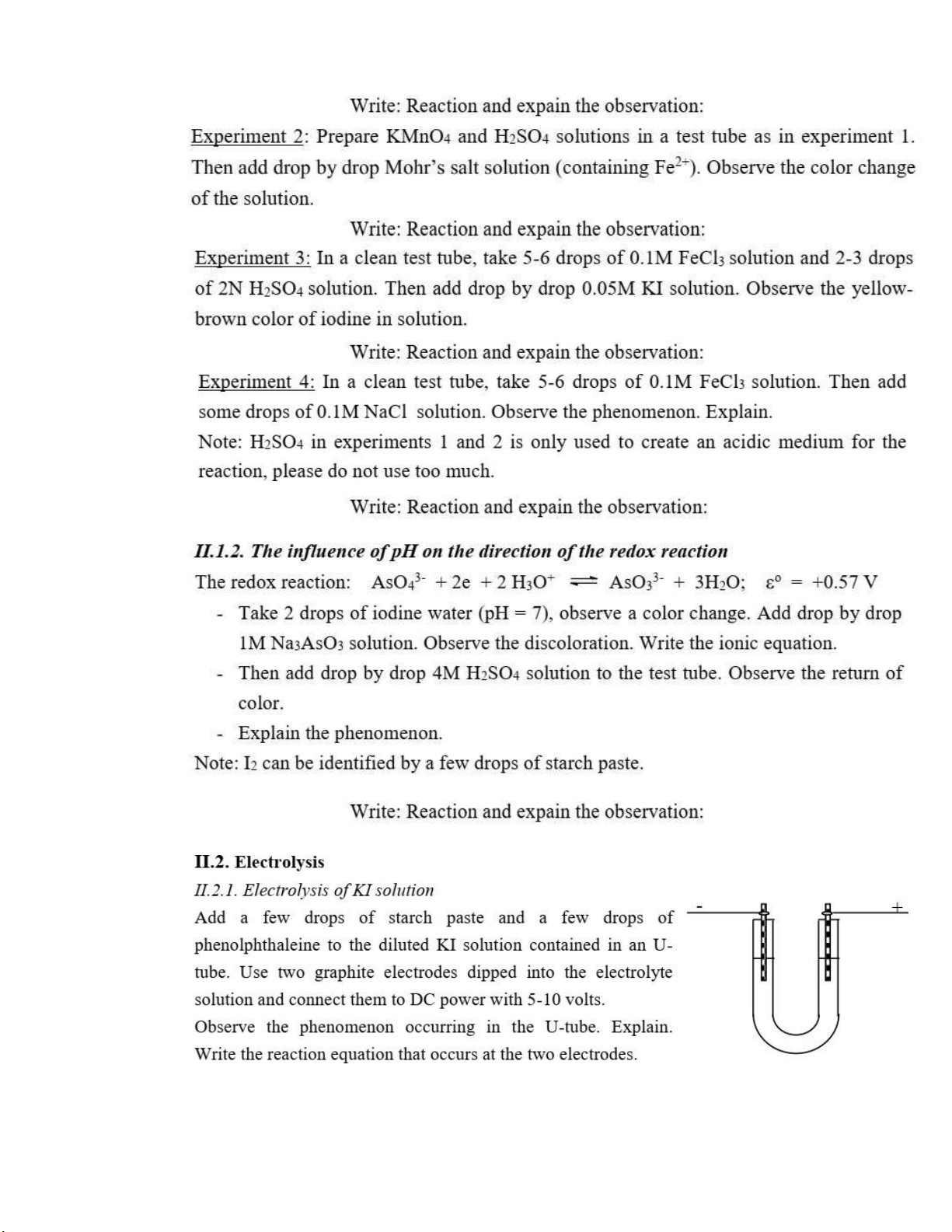
(By using the conductivity Meter)
Purpose: - How to use the conductivity Meter
-Determine the solubility product constant of CaSO4 I. Summary: 1. Regulation:
In a saturated aqueous solution, it always has the equilibrium between AnBm and the ions present in it: A nBm nAm+ + mBn-
The solubility product constant of AnBm is calculated by the following formula: T n m = a xa
const (T const) n A m B Am Bn
Because AnBm is very slightly soluble in water so the concentration of ions is very small, we
can replace activity by concentration of the ions involved in the equilibrium, each raised to a
powder equal to the stoichiometric coefficient of the ioes. Like other equilibrium constant, Tt depends on temperature
So, we can calculate a solubility product constant T if we know its concentration. The
concentrations of Am+ & Bn+ are determined by quantity method or by analytical chemistry
method ( titration by volume …) or by physico – chemical method (conductivity meter; milivon meter …).
In this lesson, students will determine the solubility product constant of CaSO4 by conductivity meter. 2. General concepts:
a. Conductivy G: A wire has a l(m) length and A(m2) square and specific resistance (m)
so its resistance is calculated by formula :
Conductivity G of solution is a factor that characterizes for electro conductivity ability of the
solution; it is a inversely proportional ratio of R R = x l G = 1/R A
Where: R- resistance (); G – conductivity (S = -1).
b. Specific conductivity 1 A G 1 A R l l K
Where, a stoichiometry 1/ is a specific conductivity & designed as . The specific
conductivity is a conductivity of one tube which has a 1m length; 1 m2 square. Unit of is [S/m]. Page 20 lOMoAR cPSD| 30964149
K = l/A (m-1) is a electrode factor which characterizes to geometry of solution that has a
electric current passes through. K b Gb
c.Molar conductivity : is a conductivity of solution that contain 1 mole of electrolyte within
1 m distance of 2 electrodes. Unit of is Sm2/mol.
Theory of Kohrousch: When diluting a solution, increases to maximum 0 and then
constant. 0 is a maximum molarity conductivity, depends on temperature. n o i (T) C i i1 Where: Ci: concentration of i ion
0i(T): maximum molarity conductivity of i ion at temperature T
Note: This formula only use for dilution solutions
3. Determine the product constant by conductivity meter
RC includes 2 platinum sheets. Each sheet has a A m2 and the distance between two sheets of
l m. RE is calibrating resistance. When immerse an electrode into a solution, electric source 250
mV and frequency of 50 – 4kHz, depends on conductivity of the measured solution. Between 2
electrodes, there is a electric current passes through RC and has the potential difference UC (vol). Because IC = IE so U U R C , since R C C << RE > U U C R R R C E R C E
that means UC is modified and performance on the machine.
Because each electrode has a difference K; it depends on l and A values. To have the same
result, we have to calibrate by 0.01M KCl solution. It has a known conductivity value follow the
temperature. After calibrating, measure conductivity G or specific conductivity of solution;
depends on each machine. Measured value depends on temperature of solution, frequency of
electric source; ratio of blending, bubble; electrode factor…
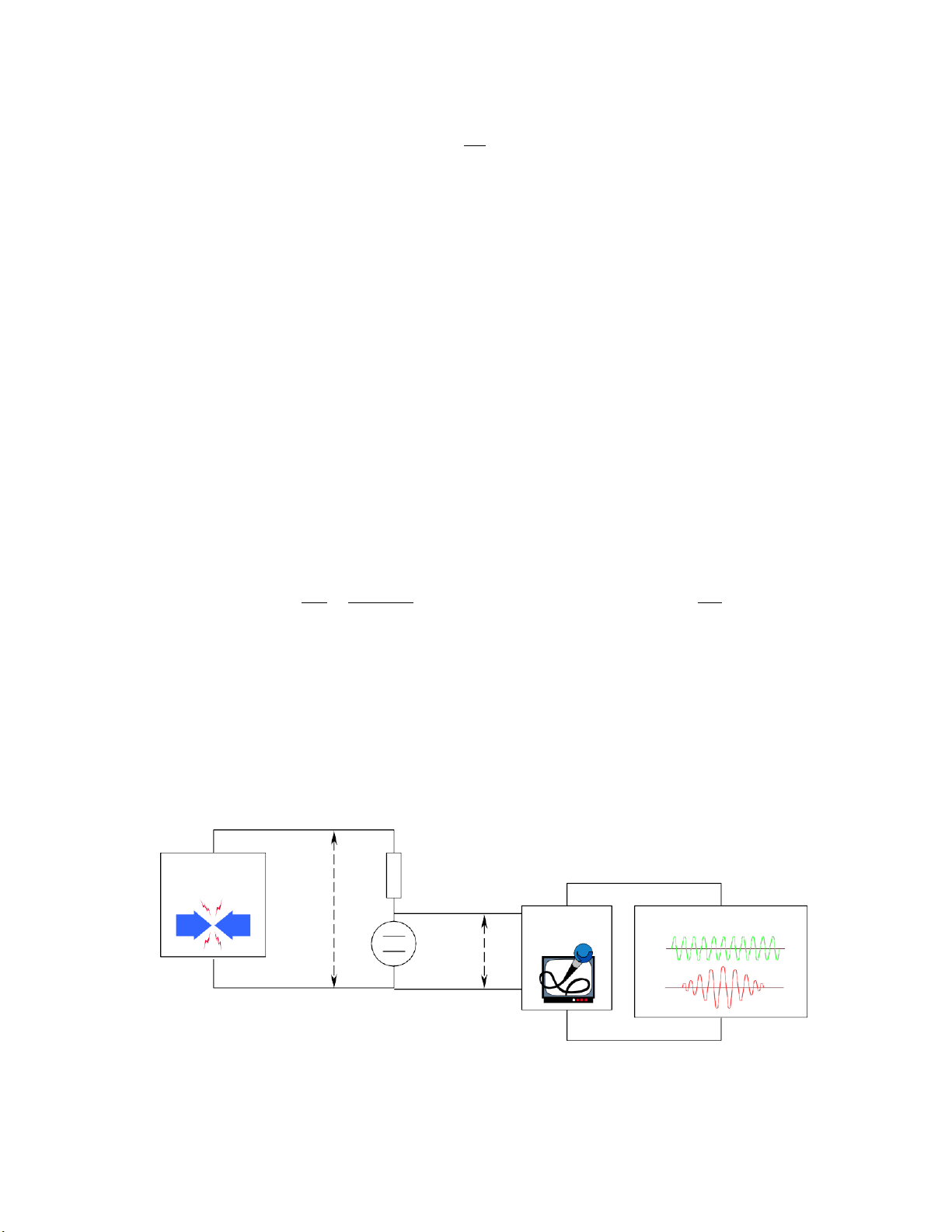
Based on the relation between 0 and , we can calculate the product constant of poorly electrolyte. ®IÖn ¸ p R xo a y c hiÒu E U khuyÕc h
Vo nmet c hia ®é [, S] R C ®¹ i C U
Eg: There are ions Ca2+ and SO 2- 4
in the CaSO4 solution. Because it’s a dilute solution so a of CaSO -2
4 saturated solution is calculated by following formula: a = [oT(Ca+2) + oT(SO4 )] . C
C [mol/m3] is concentration of ions. The similar with 0.01 M KCl, we have b which is calculated Page 21 lOMoAR cPSD| 30964149 by formula:
b = [o(K+) + o(Cl-)] . 0.01/1000
The electrode factor is calculated by formula:
The maximum molarity conductivity depends on temperature and calculated by formula:
o(T) = o(25oC) . [1 + x . (t - 25)] where:
t – temperature of solution [oC].
o and x of ions are taken in this table: 1 do x o dt Ions Ca+2 SO -2 4 K+ Cl- o (25oC) 119. 10-4 l59.6. 10-4 73.52. 10-4 76.34. 10-4 X 211. 10-4 196. 10-4 189. 10-4 188. 10-4
Concentration C [mol/m3] is calculated by formula: C a
o(Ca2) o(SO 2) 4 II. Experiments 1. Reagent and apparatus
-Conductivity Meter Denver-Model 30 machine or Jenway 4310; Magnetic; 25 ml beaker and
250 ml beaker; three-50 ml beakers; a glass funnel, filterating paper.
-Pure crystal CaSO4.2H2O; The standard solution of 0.01 M KCl. 2. Experiments 2.1. Titration:
Adding 0.01 M KCl solution into 25 ml beaker. Immerse a electrode into this solution. Stable
the electrode and release all bubbles. Read a temperature on the machine.
2.2. Measure a conductivity of saturated solution CaSO4
-Devide a saturated solution into 3 beakers (25ml) (2/3 of beaker).
-Immerse a electrode into solution.
-Read a result after stability.
- Calculate an average solubility product constant Question 1.
What is a solubility product constant. What factor does it depend? Why? 2.
Condition to form a precipitate and dissolve a precipitate. 3.
A relationship between a solubility product constant and solubility? Page 22


