





Preview text:
PHẦN KHỞI ĐỘNG •NHÓM NÀO NHANH
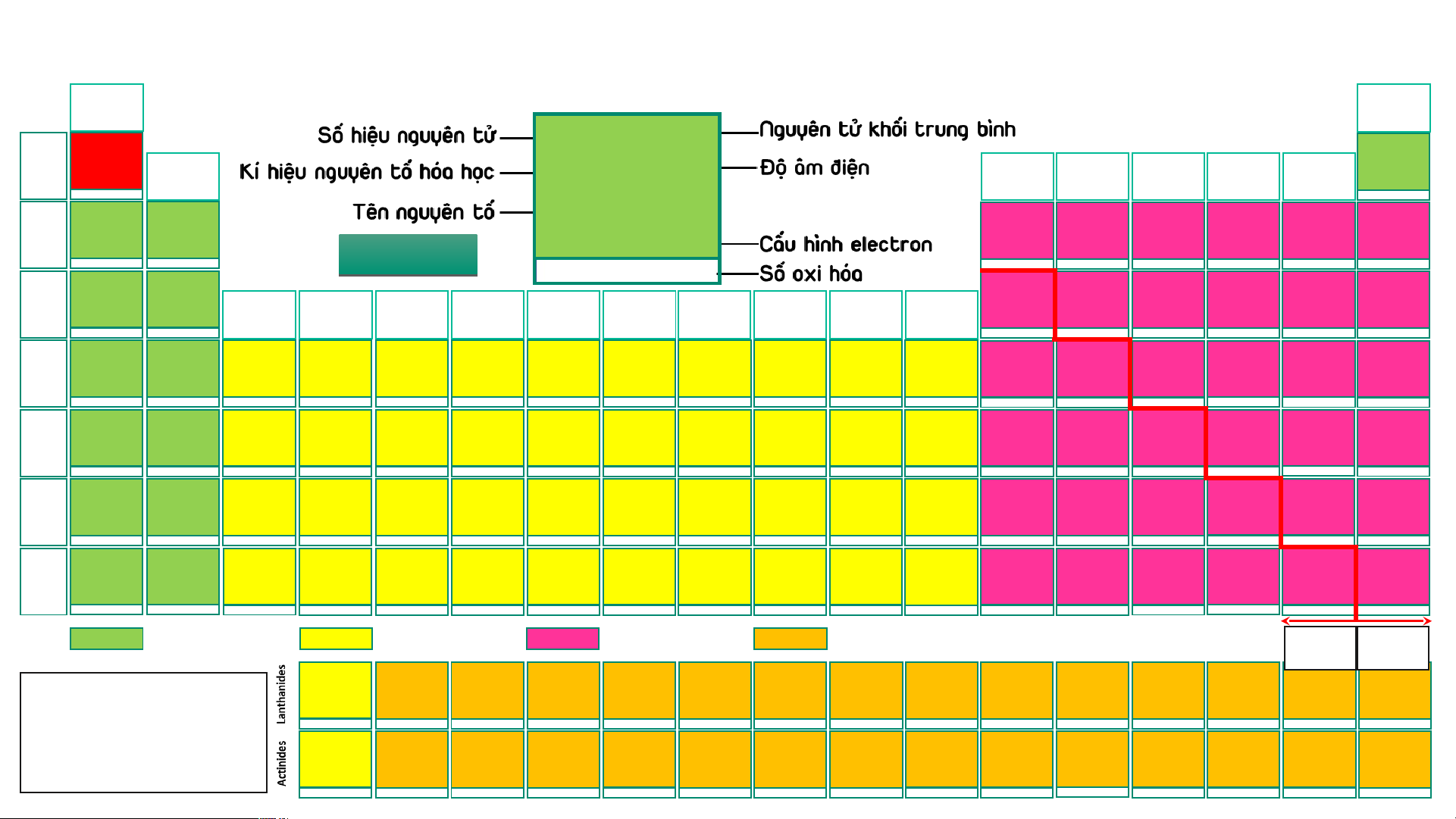
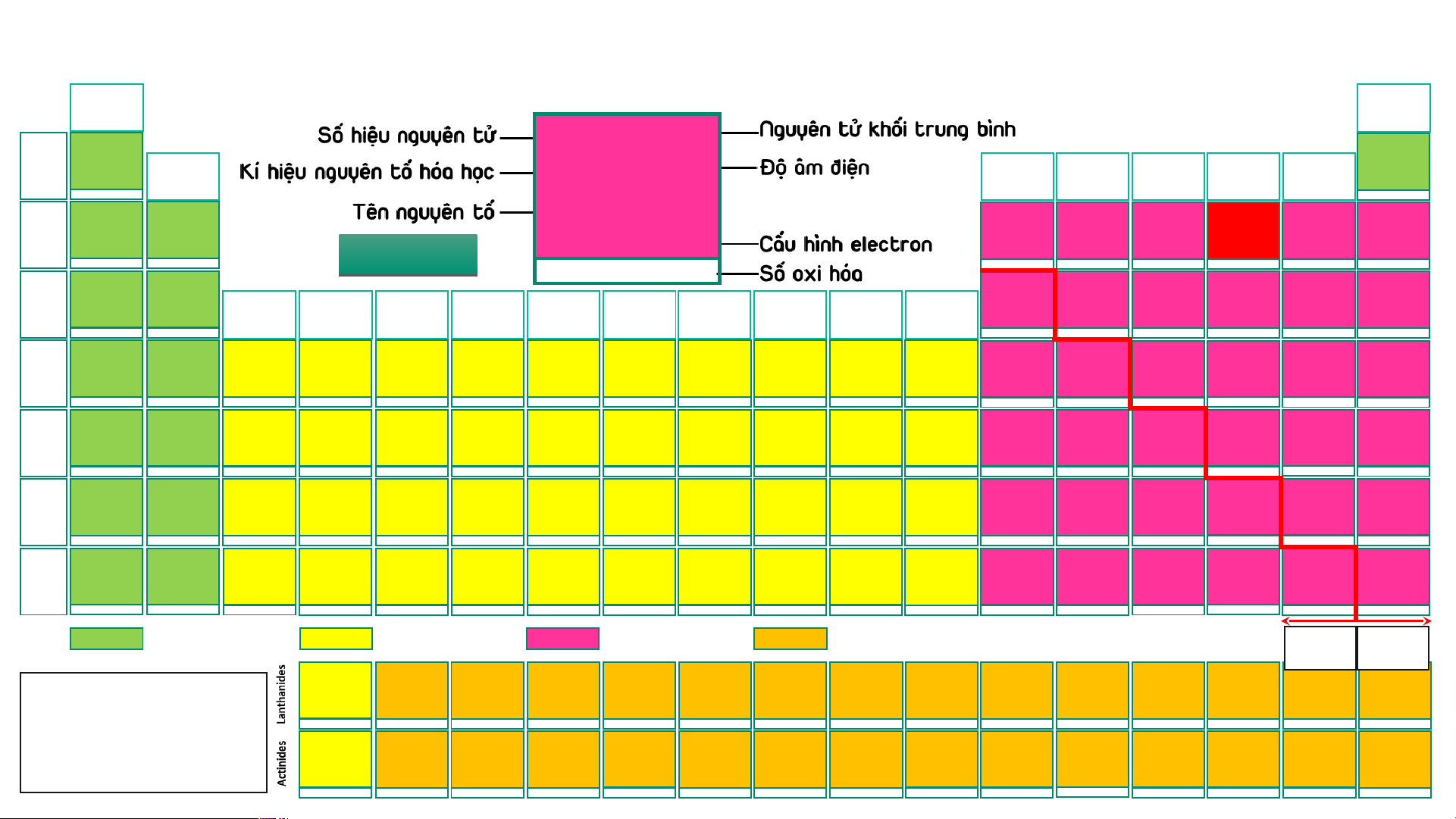
BẢNG TUẦN HOÀN CÁC NGUYÊN TỐ HÓA (IA) HỌC
Design: ThS. Nguyễn (VIIIA) (1) Phú Hoạt (18) 1 1,008 1 1,008 2 4,003 H 2,20 He 1 Hydrogen (IIA) (IIIA) (IVA) (VA) (VIA) (VIIA) Helium 1s1 -1, 1 H 2,20 1s2 (2) (13) (14) (15) (16) (17) 3 6,94 4 9,01 5 10,81 6 12,01 7 14,007 8 15,999 9 18,998 10 20,18 Hydrogen Li 0,98 Be 1,57 B 2,04 C 2,55 N 3,04 O 3,44 F 3,98 Ne 2 Lithium Beryllium Boron Carbon Nitrogen Oxygen Fluorine Neon 1s1 1s22s1 1s22s2 1s22s22p1 1s22s22p2 1s22s22p3 1s22s22p4 1s22s22p5 1s22s22p6 1 2 AU A D U I D O I H O 3 -4,-3,-2,-1,0,1,2,3,4 -3, 1, 2, 3, 4, 5 -2,-1, [-1/2,-1/3,1],2 -1 -1, 1 11 22,989 12 24,31 13 26,98 14 28,09 15 30,97 16 32,06 17 35,45 18 39,95 Na 0,93 Mg 1,31 Al 1,61 Si 1,90 P 2,19 S 2,58 Cl 3,16 Ar 3 Sodium Magnesium (IIIB) (IVB) (VB) (VIB) (VIIB) (VIIIB) (VIIIB) (VIIIB) (IB) (IIB) Aluminium Silicon Phosphorus Sulfur Chlorine Argon [Ne]3s1 [Ne]3s2 [Ne]3s23p1 [Ne]3s23p2 [Ne]3s23p3 [Ne]3s23p4 [Ne]3s23p5 [Ne]3s23p6 (3) (4) (5) (6) (7) (8) (9) (10) (11) (12) 1 2 3 4 -3, [1], 3, [4], 5 -2, -1, [1, 2] 4, 6 -1, 1, 3, [4], 5, 7 19 39,098 20 40,08 21 44,96 22 47,90 23 50,94 24 51,996 25 54,94 26 55,85 27 58,93 28 58,71 29 63,54 30 65,38 31 69,72 32 72,64 33 74,92 34 78,96 35 79,91 36 83,80 K 0,82 Ca 1,00 Sc 1,36 Ti 1,54 V 1,63 Cr 1,66 Mn 1,55 Fe 1,83 Co 1,88 Ni 1,91 Cu 1,90 Zn 1,65 Ga 1,81 Ge 2,01 As 2,18 Se 2,55 Br 2,96 Kr 3,00 4 Potassium Calcium Scandium Titanium Vanadium Chromium Manganese Iron Cobalt Nickel Copper Zinc Gallium Germanium Arsenic Selenium Bromine Krypton [Ar]4s1 [Ar]4s2 [Ar]3d14s2 [Ar]3d24s2 [Ar]3d34s2 [Ar]3d54s1 [Ar]3d54s2 [Ar]3d64s2 [Ar]3d74s2 [Ar]3d84s2 [Ar]3d104s1 [Ar]3d104s2 [Ar]3d104s24p1 [Ar]3d104s24p2 [Ar]3d104s24p3 [Ar]3d104s24p4 [Ar]3d104s24p5 [Ar]3d104s24p6 1 2 3 2, 3, 4 2, [3], 4, 5 2, 3, 4, 6 2, 3, 4, [5], 6, 7 2, 3, [4, 5, 6] 2, [3], [4] 2, [3], [4] 1, 2 2 3 2, 4 -3, 3, 5 -2, 4, 6 -1, 1, [3], [4], 5, 7 2, 4 37 85,47 38 87,62 39 88,91 40 91,22 41 92,91 42 95,94 43 (99) 44 101,07 45 102,91 46 106,40 47 107,87 48 112,41 49 114,82 50 118,69 51 121,75 52 127,60 53 126,90 54 131,30 Rb 0,82 Sr 0,95 Y 1,22 Zr 1,33 Nb 1,60 Mo 2,16 Tc 1,90 Ru 2,20 Rh 2,28 Pd 2,20 Ag 1,93 Cd 1,69 In 1,78 Sn 1,96 Sb 2,05 Te 2,10 I 2,66 Xe 2, 60 5 Rubidium Strontium Yttrium Zirconium Niobium Molybdenum Technetium Ruthenium Rhodium Paladium Silver Cadmium Indium Tin Antimony Tellurium Iodine Xenon [Kr]5s1 [Kr]5s2 [Kr]4d15s2 [Kr]4d25s2 [Kr]4d45s1 [Kr]4d55s1 [Kr]4d55s2 [Kr]4d75s1 [Kr]4d85s1 [Kr]4d105s0 [Kr]4d105s1 [Kr]4d105s2 [Kr]4d105s25p1 [Kr]4d105s25p2 [Kr]4d105s25p3 [Kr]4d105s25p4 [Kr]4d105s25p5 [Kr]4d105s25p6 1 2 3 [2], [3], 4 [2], [3], [4], 5 2, 3, 4, [5], 6 3, 4, [5], [6], 7 2, 3, 4, [5, 6], 8 2, 3, 4 2, [3], 4 1, [2] 2 1, 3 2, 4 -3, 3, [4], 5 -2, [2], 4, 6 -1, 1, 3, 5, 7 2, 4, 6 55 132,91 56 137,31 72 178,49 73 180,95 74 183,85 75 186,20 76 190,20 77 192,20 78 195,09 79 196,97 80 200,59 81 204,37 82 207,20 83 208,98 84 (209) 85 (210) 86 (222) 57 – 71 Cs 0,79 Ba 0,89 Hf 1,30 Ta 1,50 W 2,36 Re 1,90 Os 2,20 Ir 2,20 Pt 2,28 Au 2,54 Hg 2,00 Tl 1,80 Pb 1,80 Bi 1,90 Po 2,00 At 2,20 Rn 6 Caesium Barium Lanthanides Hafnium Tantalum Tungsten Rhenium Osmium Iridium Platinum Gold Mercury Thallium Lead Bismuth Polonium Astatine Radon [Xe]6s1 [Xe]6s2 [Xe]4f145d26s2 [Xe]4f145d36s2 [Xe]4f145d46s2 [Xe]4f145d56s2 [Xe]4f145d66s2 [Xe]4f145d76s2 [Xe]4f145d96s1 [Xe]4f145d106s1 [Xe]4f145d106s2 [Xe]4f145d106s26p1 [Xe]4f145d106s26p2 [Xe]4f145d106s26p3 [Xe]4f145d106s26p4 [Xe]4f145d106s26p5 [Xe]4f145d106s26p6 1 2 [2], [3], 4 [2], [3], [4], 5 2, [3], [4], [5], 6 [2], 3, 4, [5], [6], 7 2, 3, 4, [6], 8 2, 3, 4, [6] 2, [3], 4, [6] 1, 3 1, 3 1, 3 2, 4 3, 5 -2, 2, 4, 6 -1, 1, 3, 5, 7 [4] 87 (223) 88 (226) 104 (267)* 105 (268)* 106 (269)* 107 (270)* 108 (277)* 109 (278)* 110 (281)* 111 (282)* 112 (285)* 113 (286)* 114 (289)* 115 (290)* 116 (293)* 117 (294)* 118 (294)* 89 – 103 Fr 0,70 Ra 0,90 Rf Db Sg Bh Hs Mt Ds Rg Cn Nh Fl Mc Lv Ts Og 7 Francium Radium Actinides Rutherfordium Dubnium Seaborgium Bohrium Hassium Meitnerium Darmstadtium Roentgenium Copernicium Nihonium Flerovium Moscovium Livermorium Tennessium Oganesson [Rn]7s1 [Rn]7s2 [Rn]5f146d27s2 [Rn]5f146d37s2 [Rn]5f146d47s2 [Rn]5f146d57s2 [Rn]5f146d67s2 [Rn]5f146d77s2 [Rn]5f146d87s2 [Rn]5f146d97s2 [Rn]5f146d107s2 [Rn]5f146d107s27p1 [Rn]5f146d107s27p2 [Rn]5f146d107s27p3 [Rn]5f146d107s27p4 [Rn]5f146d107s27p5 [Rn]5f146d107s27p6 1 2 Các nguyên tố s Các nguyên tố d Các nguyên tố p Các nguyên tố f Kim Phi loại kim 57 138,91 58 140,12 59 140,91 60 144,24 61 (147) 62 150,35 63 151,96 64 157,25 65 158,93 66 162,50 67 164,93 68 167,26 69 168,93 70 173,04 71 174,97 La 1,10 Ce 1,12 Pr 1,13 Nd 1,14 Pm 1,13 Sm 1,17 Eu 1,20 Gd 1,20 Tb 1,10 Dy 1,22 Ho 1,23 Er 1,24 Tm 1,25 Yb 1,10 Lu 1,27
- Lưu ý: Số oxi hóa Lanthanum Cerium Praseodymium Neodymium Promethium Samarium Europium Gadolinium Terbium Dysprosium Homium Erbium Thulium Ytterbium Lutetium [Xe]5d16s2 [Xe]4f25d06s2 [Xe]4f35d06s2 [Xe]4f45d06s2 [Xe]4f55d06s2 [Xe]4f65d06s2 [Xe]4f75d06s2 [Xe]4f75d16s2 [Xe]4f95d06s2 [Xe]4f105d06s2 [Xe]4f115d06s2 [Xe]4f125d06s2 [Xe]4f135d06s2 [Xe]4f145d06s2 [Xe]4f145d16s2 dương trong bảng 3 3, 4 3, 4 [2], 3, 4 3 2, 3 2, 3 3 3, 4 3, 4 3 3 (2), 3 2, 3 3
tuần hoàn không kèm 89 (227) 90 232,04 91 231,04 92 238,03 93 (237) 94 (244) 95 (243) 96 (247) 97 (247) 98 (251) 99 (252) 100 (257) 101 (258) 102 (259) 103 (266) thêm dấu Ac 1,10 Th 1,30 Pa 1,50 U 1,38 Np 1,36 Pu 1,28 Am 1,13 Cm 1,28 Bk 2,20 Cf 1,30 Es 1,30 Fm 1,30 Md 1,30 No 1,30 Lr Actinium Thorium Protactinium Uranium Neptunium Plutonium Americium Curium Berkelium Califormium Einsteinium Fermium Mendelevium Nobelium Lawrencium
- (*): Đồng vị bền [Rn]6d17s2 [Rn]5f06d27s2 [Rn]5f26d17s2 [Rn]5f36d17s2 [Rn]5f46d17s2 [Rn]5f66d07s2 [Rn]5f76d07s2 [Rn]5f76d17s2 [Rn]5f96d07s2 [Rn]5f106d07s2 [Rn]5f116d07s2 [Rn]5f126d07s2 [Rn]5f136d07s2 [Rn]5f146d07s2 [Rn]5f146d17s2 3 4 4, 5 [3], 4, [5], 6 [3], 4, 5, 6 [3], 4, 5, 6 [3], 4, 5, 6 3 3, 4 3 3 3 2, 3 2, 3 3
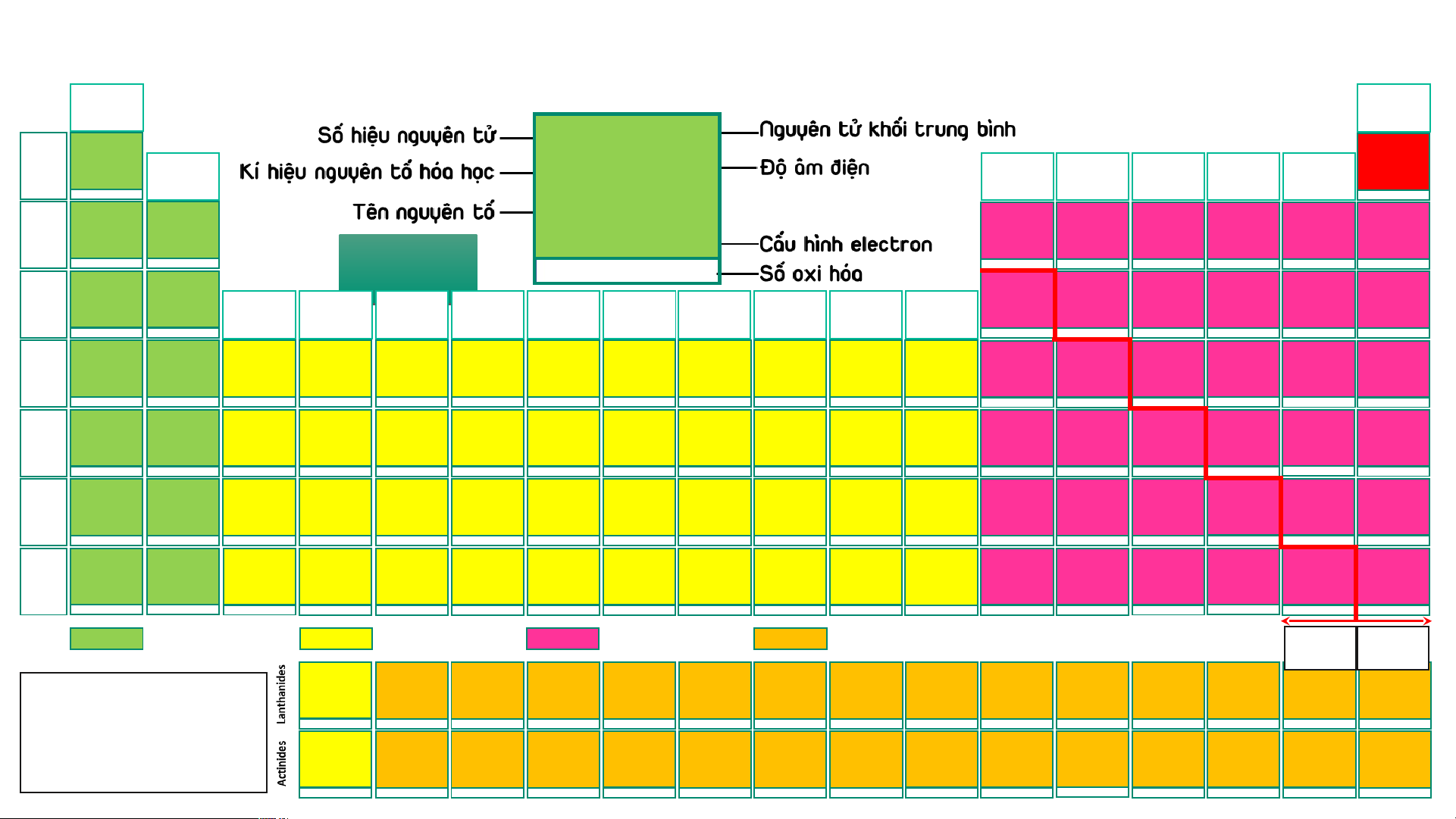
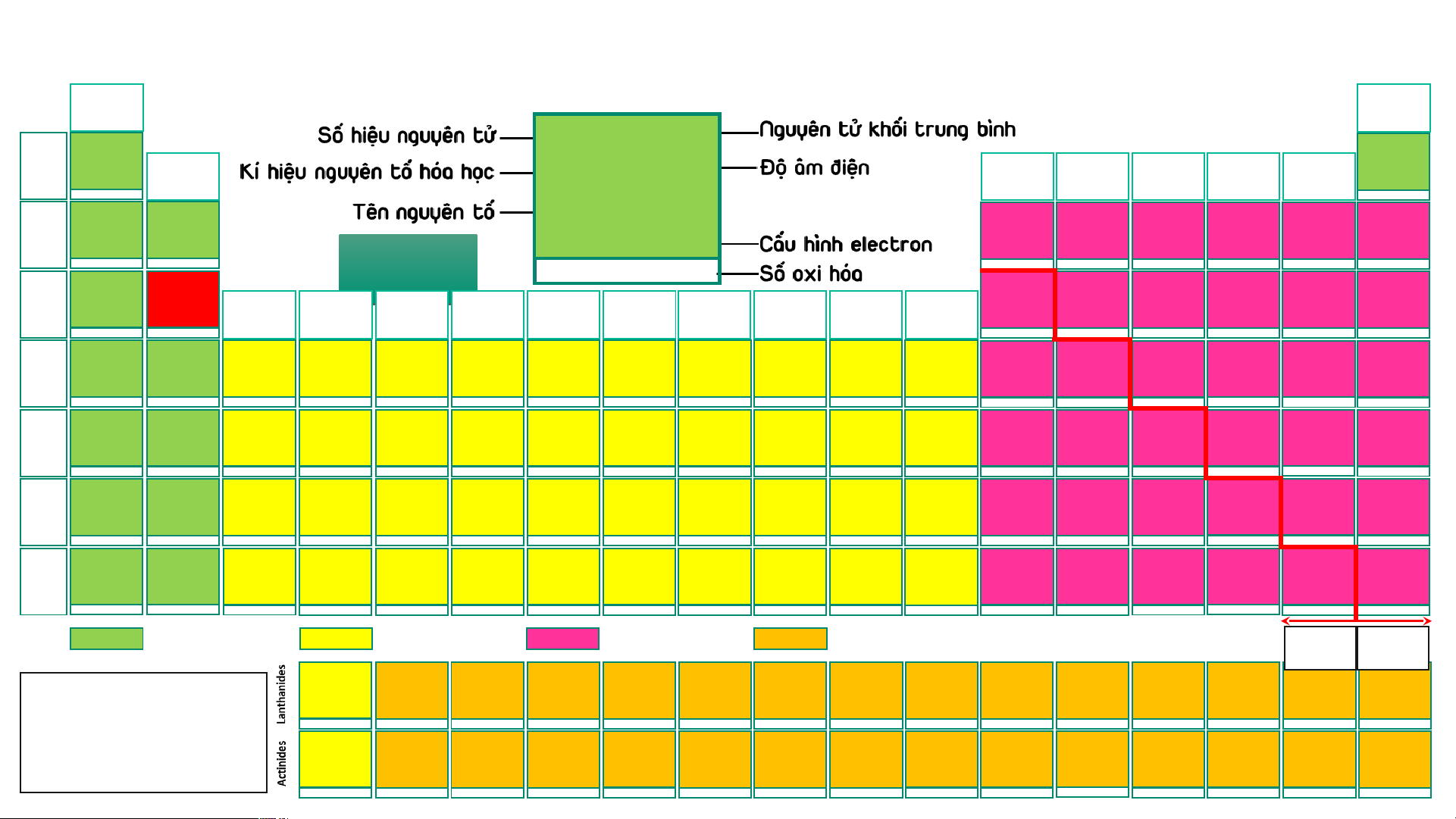
BẢNG TUẦN HOÀN CÁC NGUYÊN TỐ HÓA (IA) HỌC
Design: ThS. Nguyễn (VIIIA) (1) Phú Hoạt (18) 1 1,008 2 4,003 2 4,003 H 2,20 He 1 Hydrogen (IIA) (IIIA) (IVA) (VA) (VIA) (VIIA) He Helium 1s1 (2) (13) (14) (15) (16) (17) 1s2 -1, 1 3 6,94 4 9,01 5 10,81 6 12,01 7 14,007 8 15,999 9 18,998 10 20,18 Helium Li 0,98 Be 1,57 B 2,04 C 2,55 N 3,04 O 3,44 F 3,98 Ne 2 Lithium Beryllium Boron Carbon Nitrogen Oxygen Fluorine Neon 1s2 1s22s1 1s22s2 1s22s22p1 1s22s22p2 1s22s22p3 AUD A I UD O I 1s22s22p4 1s22s22p5 1s22s22p6 1 2 3 -4,-3,-2,-1,0,1,2,3,4 -3, 1, 2, 3, 4, 5 -2,-1, [-1/2,-1/3,1],2 -1 11 22,989 12 24,31 13 26,98 14 28,09 15 30,97 16 32,06 He H 17 35,45 18 39,95 Na 0,93 Mg 1,31 Al 1,61 Si 1,90 P 2,19 S 2,58 Cl 3,16 Ar 3 Sodium Magnesium (IIIB) (IVB) (VB) (VIB) (VIIB) (VIIIB) (VIIIB) (VIIIB) (IB) (IIB) Aluminium Silicon Phosphorus Sulfur Chlorine Argon [Ne]3s1 [Ne]3s2 [Ne]3s23p1 [Ne]3s23p2 [Ne]3s23p3 [Ne]3s23p4 [Ne]3s23p5 [Ne]3s23p6 (3) (4) (5) (6) (7) (8) (9) (10) (11) (12) 1 2 3 4 -3, [1], 3, [4], 5 -2, -1, [1, 2] 4, 6 -1, 1, 3, [4], 5, 7 19 39,098 20 40,08 21 44,96 22 47,90 23 50,94 24 51,996 25 54,94 26 55,85 27 58,93 28 58,71 29 63,54 30 65,38 31 69,72 32 72,64 33 74,92 34 78,96 35 79,91 36 83,80 K 0,82 Ca 1,00 Sc 1,36 Ti 1,54 V 1,63 Cr 1,66 Mn 1,55 Fe 1,83 Co 1,88 Ni 1,91 Cu 1,90 Zn 1,65 Ga 1,81 Ge 2,01 As 2,18 Se 2,55 Br 2,96 Kr 3,00 4 Potassium Calcium Scandium Titanium Vanadium Chromium Manganese Iron Cobalt Nickel Copper Zinc Gallium Germanium Arsenic Selenium Bromine Krypton [Ar]4s1 [Ar]4s2 [Ar]3d14s2 [Ar]3d24s2 [Ar]3d34s2 [Ar]3d54s1 [Ar]3d54s2 [Ar]3d64s2 [Ar]3d74s2 [Ar]3d84s2 [Ar]3d104s1 [Ar]3d104s2 [Ar]3d104s24p1 [Ar]3d104s24p2 [Ar]3d104s24p3 [Ar]3d104s24p4 [Ar]3d104s24p5 [Ar]3d104s24p6 1 2 3 2, 3, 4 2, [3], 4, 5 2, 3, 4, 6 2, 3, 4, [5], 6, 7 2, 3, [4, 5, 6] 2, [3], [4] 2, [3], [4] 1, 2 2 3 2, 4 -3, 3, 5 -2, 4, 6 -1, 1, [3], [4], 5, 7 2, 4 37 85,47 38 87,62 39 88,91 40 91,22 41 92,91 42 95,94 43 (99) 44 101,07 45 102,91 46 106,40 47 107,87 48 112,41 49 114,82 50 118,69 51 121,75 52 127,60 53 126,90 54 131,30 Rb 0,82 Sr 0,95 Y 1,22 Zr 1,33 Nb 1,60 Mo 2,16 Tc 1,90 Ru 2,20 Rh 2,28 Pd 2,20 Ag 1,93 Cd 1,69 In 1,78 Sn 1,96 Sb 2,05 Te 2,10 I 2,66 Xe 2, 60 5 Rubidium Strontium Yttrium Zirconium Niobium Molybdenum Technetium Ruthenium Rhodium Paladium Silver Cadmium Indium Tin Antimony Tellurium Iodine Xenon [Kr]5s1 [Kr]5s2 [Kr]4d15s2 [Kr]4d25s2 [Kr]4d45s1 [Kr]4d55s1 [Kr]4d55s2 [Kr]4d75s1 [Kr]4d85s1 [Kr]4d105s0 [Kr]4d105s1 [Kr]4d105s2 [Kr]4d105s25p1 [Kr]4d105s25p2 [Kr]4d105s25p3 [Kr]4d105s25p4 [Kr]4d105s25p5 [Kr]4d105s25p6 1 2 3 [2], [3], 4 [2], [3], [4], 5 2, 3, 4, [5], 6 3, 4, [5], [6], 7 2, 3, 4, [5, 6], 8 2, 3, 4 2, [3], 4 1, [2] 2 1, 3 2, 4 -3, 3, [4], 5 -2, [2], 4, 6 -1, 1, 3, 5, 7 2, 4, 6 55 132,91 56 137,31 72 178,49 73 180,95 74 183,85 75 186,20 76 190,20 77 192,20 78 195,09 79 196,97 80 200,59 81 204,37 82 207,20 83 208,98 84 (209) 85 (210) 86 (222) Cs 57 – 71 0,79 Ba 0,89 Hf 1,30 Ta 1,50 W 2,36 Re 1,90 Os 2,20 Ir 2,20 Pt 2,28 Au 2,54 Hg 2,00 Tl 1,80 Pb 1,80 Bi 1,90 Po 2,00 At 2,20 Rn 6 Caesium Barium Lanthanides Hafnium Tantalum Tungsten Rhenium Osmium Iridium Platinum Gold Mercury Thallium Lead Bismuth Polonium Astatine Radon [Xe]6s1 [Xe]6s2 [Xe]4f145d26s2 [Xe]4f145d36s2 [Xe]4f145d46s2 [Xe]4f145d56s2 [Xe]4f145d66s2 [Xe]4f145d76s2 [Xe]4f145d96s1 [Xe]4f145d106s1 [Xe]4f145d106s2 [Xe]4f145d106s26p1 [Xe]4f145d106s26p2 [Xe]4f145d106s26p3 [Xe]4f145d106s26p4 [Xe]4f145d106s26p5 [Xe]4f145d106s26p6 1 2 [2], [3], 4 [2], [3], [4], 5 2, [3], [4], [5], 6 [2], 3, 4, [5], [6], 7 2, 3, 4, [6], 8 2, 3, 4, [6] 2, [3], 4, [6] 1, 3 1, 3 1, 3 2, 4 3, 5 -2, 2, 4, 6 -1, 1, 3, 5, 7 [4] 87 (223) 88 (226) 104 (267)* 105 (268)* 106 (269)* 107 (270)* 108 (277)* 109 (278)* 110 (281)* 111 (282)* 112 (285)* 113 (286)* 114 (289)* 115 (290)* 116 (293)* 117 (294)* 118 (294)* Fr 89 – 103 0,70 Ra 0,90 Rf Db Sg Bh Hs Mt Ds Rg Cn Nh Fl Mc Lv Ts Og 7 Francium Radium Actinides Rutherfordium Dubnium Seaborgium Bohrium Hassium Meitnerium Darmstadtium Roentgenium Copernicium Nihonium Flerovium Moscovium Livermorium Tennessium Oganesson [Rn]7s1 [Rn]7s2 [Rn]5f146d27s2 [Rn]5f146d37s2 [Rn]5f146d47s2 [Rn]5f146d57s2 [Rn]5f146d67s2 [Rn]5f146d77s2 [Rn]5f146d87s2 [Rn]5f146d97s2 [Rn]5f146d107s2 [Rn]5f146d107s27p1 [Rn]5f146d107s27p2 [Rn]5f146d107s27p3 [Rn]5f146d107s27p4 [Rn]5f146d107s27p5 [Rn]5f146d107s27p6 1 2 Các nguyên tố s Các nguyên tố d Các nguyên tố p Các nguyên tố f Kim Phi loại kim 57 138,91 58 140,12 59 140,91 60 144,24 61 (147) 62 150,35 63 151,96 64 157,25 65 158,93 66 162,50 67 164,93 68 167,26 69 168,93 70 173,04 71 174,97 La 1,10 Ce 1,12 Pr 1,13 Nd 1,14 Pm 1,13 Sm 1,17 Eu 1,20 Gd 1,20 Tb 1,10 Dy 1,22 Ho 1,23 Er 1,24 Tm 1,25 Yb 1,10 Lu 1,27
- Lưu ý: Số oxi hóa Lanthanum Cerium Praseodymium Neodymium Promethium Samarium Europium Gadolinium Terbium Dysprosium Homium Erbium Thulium Ytterbium Lutetium [Xe]5d16s2 [Xe]4f25d06s2 [Xe]4f35d06s2 [Xe]4f45d06s2 [Xe]4f55d06s2 [Xe]4f65d06s2 [Xe]4f75d06s2 [Xe]4f75d16s2 [Xe]4f95d06s2 [Xe]4f105d06s2 [Xe]4f115d06s2 [Xe]4f125d06s2 [Xe]4f135d06s2 [Xe]4f145d06s2 [Xe]4f145d16s2 dương trong bảng 3 3, 4 3, 4 [2], 3, 4 3 2, 3 2, 3 3 3, 4 3, 4 3 3 (2), 3 2, 3 3
tuần hoàn không kèm 89 (227) 90 232,04 91 231,04 92 238,03 93 (237) 94 (244) 95 (243) 96 (247) 97 (247) 98 (251) 99 (252) 100 (257) 101 (258) 102 (259) 103 (266) thêm dấu Ac 1,10 Th 1,30 Pa 1,50 U 1,38 Np 1,36 Pu 1,28 Am 1,13 Cm 1,28 Bk 2,20 Cf 1,30 Es 1,30 Fm 1,30 Md 1,30 No 1,30 Lr Actinium Thorium Protactinium Uranium Neptunium Plutonium Americium Curium Berkelium Califormium Einsteinium Fermium Mendelevium Nobelium Lawrencium
- (*): Đồng vị bền [Rn]6d17s2 [Rn]5f06d27s2 [Rn]5f26d17s2 [Rn]5f36d17s2 [Rn]5f46d17s2 [Rn]5f66d07s2 [Rn]5f76d07s2 [Rn]5f76d17s2 [Rn]5f96d07s2 [Rn]5f106d07s2 [Rn]5f116d07s2 [Rn]5f126d07s2 [Rn]5f136d07s2 [Rn]5f146d07s2 [Rn]5f146d17s2 3 4 4, 5 [3], 4, [5], 6 [3], 4, 5, 6 [3], 4, 5, 6 [3], 4, 5, 6 3 3, 4 3 3 3 2, 3 2, 3 3
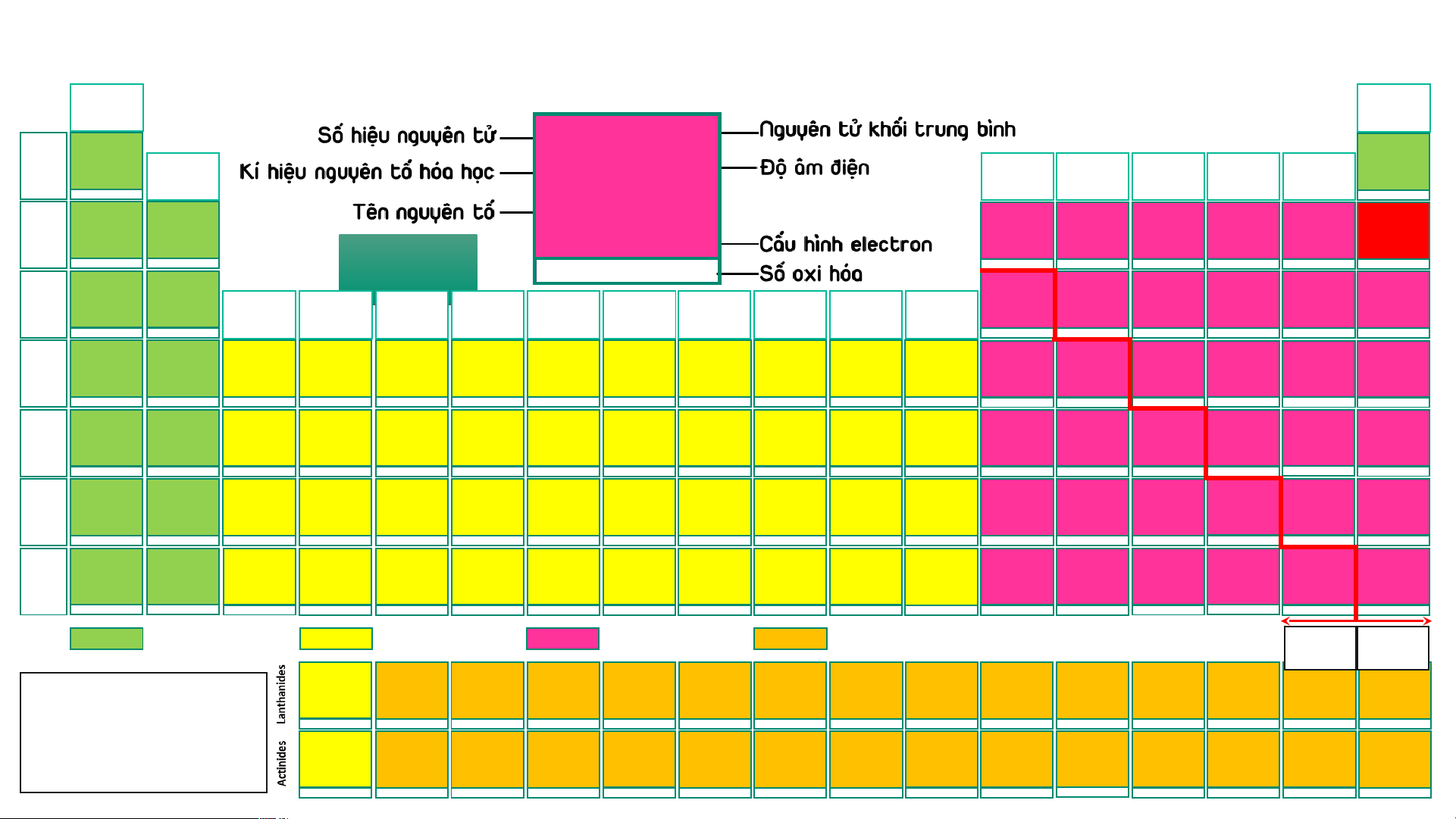
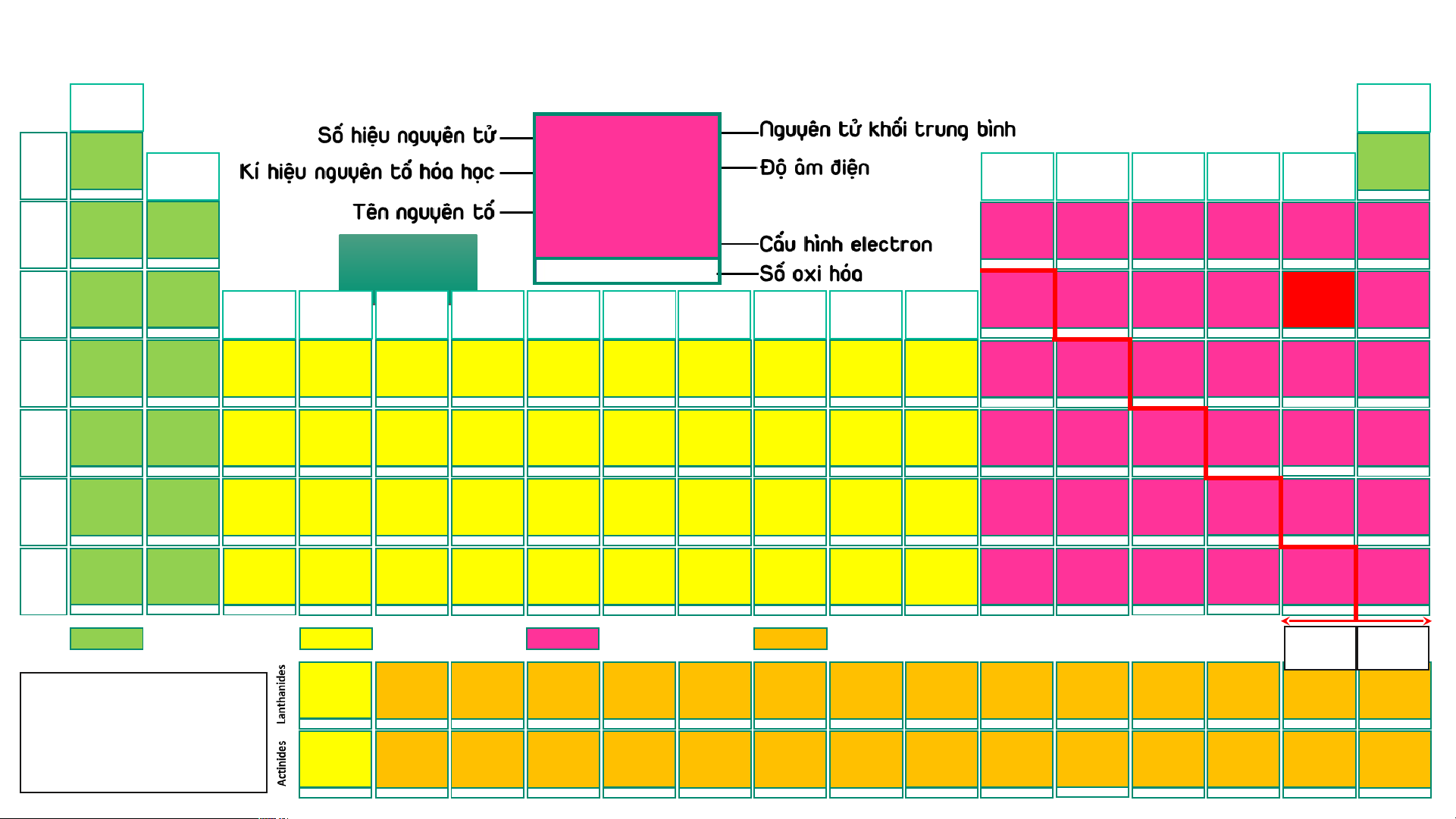
BẢNG TUẦN HOÀN CÁC NGUYÊN TỐ HÓA (IA) HỌC
Design: ThS. Nguyễn (VIIIA) (1) Phú Hoạt (18) 1 1,008 10 20,18 2 4,003 H 2,20 He 1 Hydrogen (IIA) (IIIA) (IVA) (VA) (VIA) (VIIA) Helium Ne 1s1 1s2 (2) (13) (14) (15) (16) (17) -1, 1 3 6,94 4 9,01 5 10,81 6 12,01 7 14,007 8 15,999 9 18,998 Neon 10 20,18 Li 0,98 Be 1,57 B 2,04 C 2,55 N 3,04 O 3,44 F 3,98 Ne 2 Lithium Beryllium Boron Carbon Nitrogen Oxygen Fluorine 1s22s22p6 Neon 1s22s1 1s22s2 1s22s22p1 1s22s22p2 1s22s22p3 AUD A I UD O I 1s22s22p4 1s22s22p5 1s22s22p6 1 2 3 -4,-3,-2,-1,0,1,2,3,4 -3, 1, 2, 3, 4, 5 -2,-1, [-1/2,-1/3,1],2 -1 11 22,989 12 24,31 13 26,98 14 28,09 15 30,97 16 32,06 Ne N 17 35,45 18 39,95 Na 0,93 Mg 1,31 Al 1,61 Si 1,90 P 2,19 S 2,58 Cl 3,16 Ar 3 Sodium Magnesium (IIIB) (IVB) (VB) (VIB) (VIIB) (VIIIB) (VIIIB) (VIIIB) (IB) (IIB) Aluminium Silicon Phosphorus Sulfur Chlorine Argon [Ne]3s1 [Ne]3s2 [Ne]3s23p1 [Ne]3s23p2 [Ne]3s23p3 [Ne]3s23p4 [Ne]3s23p5 [Ne]3s23p6 (3) (4) (5) (6) (7) (8) (9) (10) (11) (12) 1 2 3 4 -3, [1], 3, [4], 5 -2, -1, [1, 2] 4, 6 -1, 1, 3, [4], 5, 7 19 39,098 20 40,08 21 44,96 22 47,90 23 50,94 24 51,996 25 54,94 26 55,85 27 58,93 28 58,71 29 63,54 30 65,38 31 69,72 32 72,64 33 74,92 34 78,96 35 79,91 36 83,80 K 0,82 Ca 1,00 Sc 1,36 Ti 1,54 V 1,63 Cr 1,66 Mn 1,55 Fe 1,83 Co 1,88 Ni 1,91 Cu 1,90 Zn 1,65 Ga 1,81 Ge 2,01 As 2,18 Se 2,55 Br 2,96 Kr 3,00 4 Potassium Calcium Scandium Titanium Vanadium Chromium Manganese Iron Cobalt Nickel Copper Zinc Gallium Germanium Arsenic Selenium Bromine Krypton [Ar]4s1 [Ar]4s2 [Ar]3d14s2 [Ar]3d24s2 [Ar]3d34s2 [Ar]3d54s1 [Ar]3d54s2 [Ar]3d64s2 [Ar]3d74s2 [Ar]3d84s2 [Ar]3d104s1 [Ar]3d104s2 [Ar]3d104s24p1 [Ar]3d104s24p2 [Ar]3d104s24p3 [Ar]3d104s24p4 [Ar]3d104s24p5 [Ar]3d104s24p6 1 2 3 2, 3, 4 2, [3], 4, 5 2, 3, 4, 6 2, 3, 4, [5], 6, 7 2, 3, [4, 5, 6] 2, [3], [4] 2, [3], [4] 1, 2 2 3 2, 4 -3, 3, 5 -2, 4, 6 -1, 1, [3], [4], 5, 7 2, 4 37 85,47 38 87,62 39 88,91 40 91,22 41 92,91 42 95,94 43 (99) 44 101,07 45 102,91 46 106,40 47 107,87 48 112,41 49 114,82 50 118,69 51 121,75 52 127,60 53 126,90 54 131,30 Rb 0,82 Sr 0,95 Y 1,22 Zr 1,33 Nb 1,60 Mo 2,16 Tc 1,90 Ru 2,20 Rh 2,28 Pd 2,20 Ag 1,93 Cd 1,69 In 1,78 Sn 1,96 Sb 2,05 Te 2,10 I 2,66 Xe 2, 60 5 Rubidium Strontium Yttrium Zirconium Niobium Molybdenum Technetium Ruthenium Rhodium Paladium Silver Cadmium Indium Tin Antimony Tellurium Iodine Xenon [Kr]5s1 [Kr]5s2 [Kr]4d15s2 [Kr]4d25s2 [Kr]4d45s1 [Kr]4d55s1 [Kr]4d55s2 [Kr]4d75s1 [Kr]4d85s1 [Kr]4d105s0 [Kr]4d105s1 [Kr]4d105s2 [Kr]4d105s25p1 [Kr]4d105s25p2 [Kr]4d105s25p3 [Kr]4d105s25p4 [Kr]4d105s25p5 [Kr]4d105s25p6 1 2 3 [2], [3], 4 [2], [3], [4], 5 2, 3, 4, [5], 6 3, 4, [5], [6], 7 2, 3, 4, [5, 6], 8 2, 3, 4 2, [3], 4 1, [2] 2 1, 3 2, 4 -3, 3, [4], 5 -2, [2], 4, 6 -1, 1, 3, 5, 7 2, 4, 6 55 132,91 56 137,31 72 178,49 73 180,95 74 183,85 75 186,20 76 190,20 77 192,20 78 195,09 79 196,97 80 200,59 81 204,37 82 207,20 83 208,98 84 (209) 85 (210) 86 (222) Cs 57 – 71 0,79 Ba 0,89 Hf 1,30 Ta 1,50 W 2,36 Re 1,90 Os 2,20 Ir 2,20 Pt 2,28 Au 2,54 Hg 2,00 Tl 1,80 Pb 1,80 Bi 1,90 Po 2,00 At 2,20 Rn 6 Caesium Barium Lanthanides Hafnium Tantalum Tungsten Rhenium Osmium Iridium Platinum Gold Mercury Thallium Lead Bismuth Polonium Astatine Radon [Xe]6s1 [Xe]6s2 [Xe]4f145d26s2 [Xe]4f145d36s2 [Xe]4f145d46s2 [Xe]4f145d56s2 [Xe]4f145d66s2 [Xe]4f145d76s2 [Xe]4f145d96s1 [Xe]4f145d106s1 [Xe]4f145d106s2 [Xe]4f145d106s26p1 [Xe]4f145d106s26p2 [Xe]4f145d106s26p3 [Xe]4f145d106s26p4 [Xe]4f145d106s26p5 [Xe]4f145d106s26p6 1 2 [2], [3], 4 [2], [3], [4], 5 2, [3], [4], [5], 6 [2], 3, 4, [5], [6], 7 2, 3, 4, [6], 8 2, 3, 4, [6] 2, [3], 4, [6] 1, 3 1, 3 1, 3 2, 4 3, 5 -2, 2, 4, 6 -1, 1, 3, 5, 7 [4] 87 (223) 88 (226) 104 (267)* 105 (268)* 106 (269)* 107 (270)* 108 (277)* 109 (278)* 110 (281)* 111 (282)* 112 (285)* 113 (286)* 114 (289)* 115 (290)* 116 (293)* 117 (294)* 118 (294)* Fr 89 – 103 0,70 Ra 0,90 Rf Db Sg Bh Hs Mt Ds Rg Cn Nh Fl Mc Lv Ts Og 7 Francium Radium Actinides Rutherfordium Dubnium Seaborgium Bohrium Hassium Meitnerium Darmstadtium Roentgenium Copernicium Nihonium Flerovium Moscovium Livermorium Tennessium Oganesson [Rn]7s1 [Rn]7s2 [Rn]5f146d27s2 [Rn]5f146d37s2 [Rn]5f146d47s2 [Rn]5f146d57s2 [Rn]5f146d67s2 [Rn]5f146d77s2 [Rn]5f146d87s2 [Rn]5f146d97s2 [Rn]5f146d107s2 [Rn]5f146d107s27p1 [Rn]5f146d107s27p2 [Rn]5f146d107s27p3 [Rn]5f146d107s27p4 [Rn]5f146d107s27p5 [Rn]5f146d107s27p6 1 2 Các nguyên tố s Các nguyên tố d Các nguyên tố p Các nguyên tố f Kim Phi loại kim 57 138,91 58 140,12 59 140,91 60 144,24 61 (147) 62 150,35 63 151,96 64 157,25 65 158,93 66 162,50 67 164,93 68 167,26 69 168,93 70 173,04 71 174,97 La 1,10 Ce 1,12 Pr 1,13 Nd 1,14 Pm 1,13 Sm 1,17 Eu 1,20 Gd 1,20 Tb 1,10 Dy 1,22 Ho 1,23 Er 1,24 Tm 1,25 Yb 1,10 Lu 1,27
- Lưu ý: Số oxi hóa Lanthanum Cerium Praseodymium Neodymium Promethium Samarium Europium Gadolinium Terbium Dysprosium Homium Erbium Thulium Ytterbium Lutetium [Xe]5d16s2 [Xe]4f25d06s2 [Xe]4f35d06s2 [Xe]4f45d06s2 [Xe]4f55d06s2 [Xe]4f65d06s2 [Xe]4f75d06s2 [Xe]4f75d16s2 [Xe]4f95d06s2 [Xe]4f105d06s2 [Xe]4f115d06s2 [Xe]4f125d06s2 [Xe]4f135d06s2 [Xe]4f145d06s2 [Xe]4f145d16s2 dương trong bảng 3 3, 4 3, 4 [2], 3, 4 3 2, 3 2, 3 3 3, 4 3, 4 3 3 (2), 3 2, 3 3
tuần hoàn không kèm 89 (227) 90 232,04 91 231,04 92 238,03 93 (237) 94 (244) 95 (243) 96 (247) 97 (247) 98 (251) 99 (252) 100 (257) 101 (258) 102 (259) 103 (266) thêm dấu Ac 1,10 Th 1,30 Pa 1,50 U 1,38 Np 1,36 Pu 1,28 Am 1,13 Cm 1,28 Bk 2,20 Cf 1,30 Es 1,30 Fm 1,30 Md 1,30 No 1,30 Lr Actinium Thorium Protactinium Uranium Neptunium Plutonium Americium Curium Berkelium Califormium Einsteinium Fermium Mendelevium Nobelium Lawrencium
- (*): Đồng vị bền [Rn]6d17s2 [Rn]5f06d27s2 [Rn]5f26d17s2 [Rn]5f36d17s2 [Rn]5f46d17s2 [Rn]5f66d07s2 [Rn]5f76d07s2 [Rn]5f76d17s2 [Rn]5f96d07s2 [Rn]5f106d07s2 [Rn]5f116d07s2 [Rn]5f126d07s2 [Rn]5f136d07s2 [Rn]5f146d07s2 [Rn]5f146d17s2 3 4 4, 5 [3], 4, [5], 6 [3], 4, 5, 6 [3], 4, 5, 6 [3], 4, 5, 6 3 3, 4 3 3 3 2, 3 2, 3 3
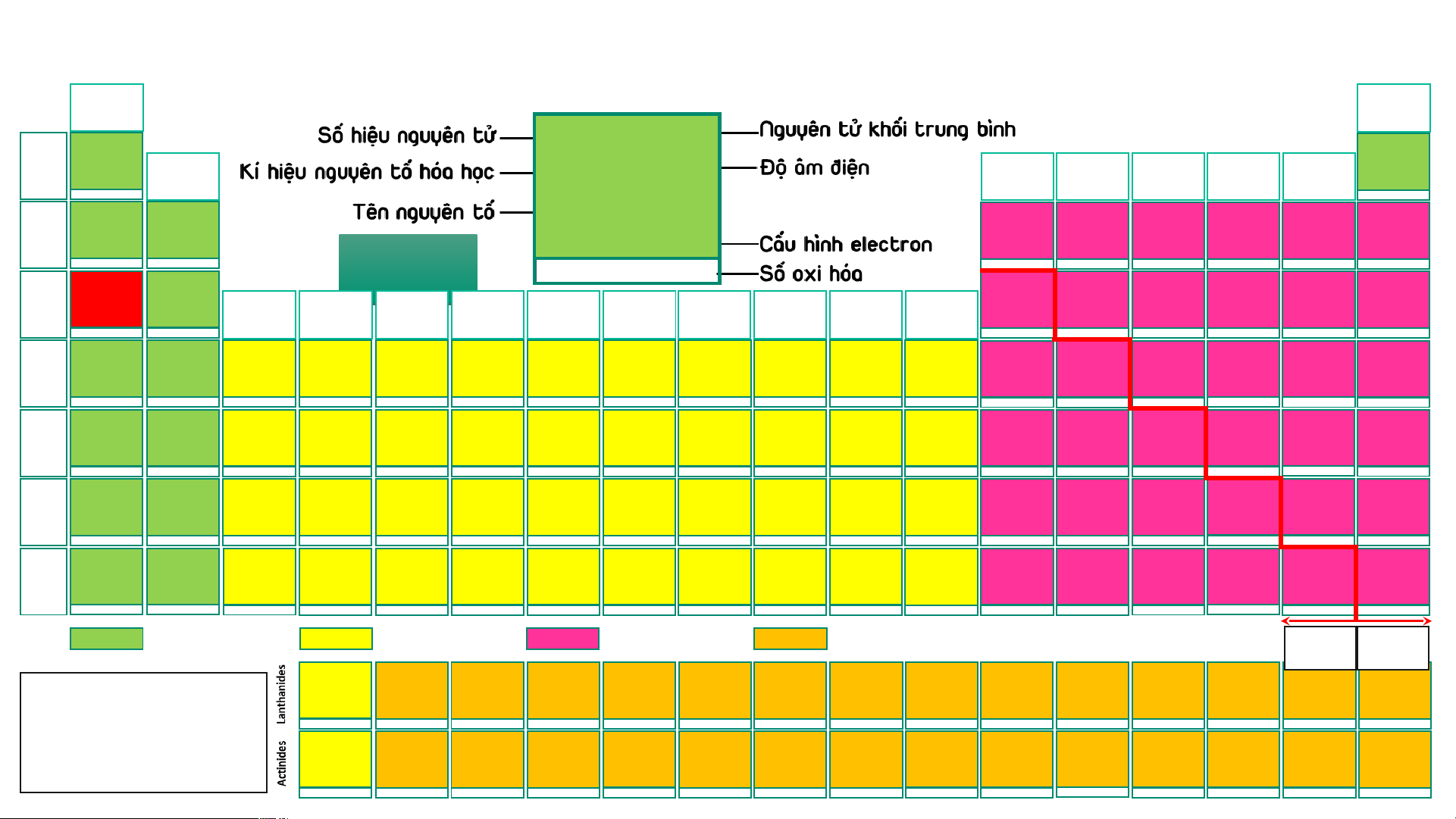
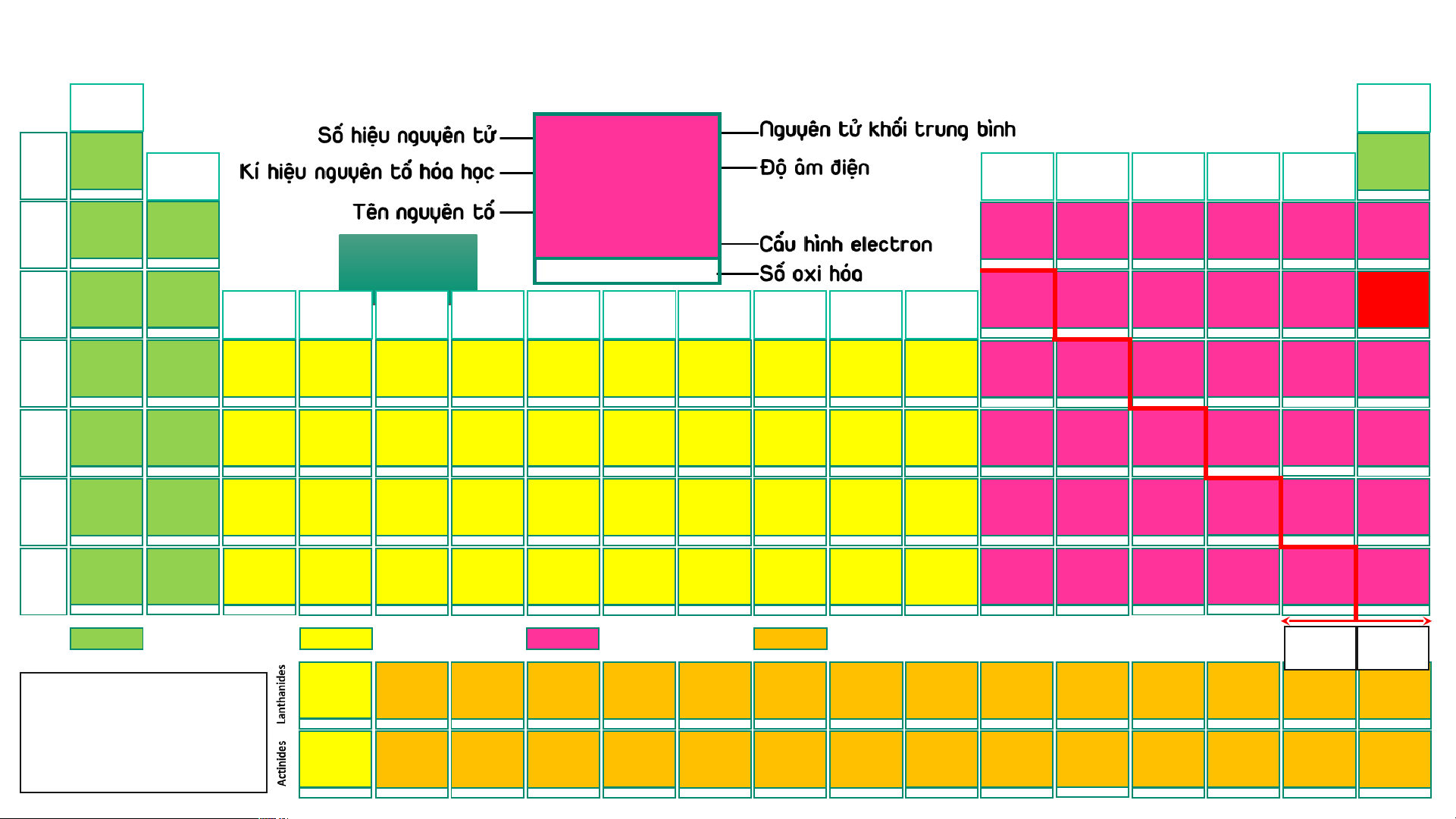
BẢNG TUẦN HOÀN CÁC NGUYÊN TỐ HÓA (IA) HỌC
Design: ThS. Nguyễn (VIIIA) (1) Phú Hoạt (18) 1 1,008 11 22,989 2 4,003 H 2,20 He 1 Hydrogen (IIA) (IIIA) (IVA) (VA) (VIA) (VIIA) Helium Na 0,93 1s1 1s2 (2) (13) (14) (15) (16) (17) -1, 1 3 6,94 4 9,01 5 10,81 6 12,01 7 14,007 8 15,999 9 18,998 10 20,18 Sodium Li 0,98 Be 1,57 B 2,04 C 2,55 N 3,04 O 3,44 F 3,98 Ne 2 Lithium Beryllium Boron Carbon Nitrogen Oxygen Fluorine Neon [Ne]3s1 1s22s1 1s22s2 1s22s22p1 1s22s22p2 1s22s22p3 1s22s22p4 1s22s22p5 1s22s22p6 2 AUD A I UD O I 1 3 -4,-3,-2,-1,0,1,2,3,4 -3, 1, 2, 3, 4, 5 -2,-1, [-1/2,-1/3,1],2 -1 1 11 22,989 12 24,31 13 26,98 14 28,09 15 30,97 16 32,06 17 35,45 18 39,95 Na 0,93 Mg 1,31 Na N Al 1,61 Si 1,90 P 2,19 S 2,58 Cl 3,16 Ar 3 Sodium Magnesium (IIIB) (IVB) (VB) (VIB) (VIIB) (VIIIB) (VIIIB) (VIIIB) (IB) (IIB) Aluminium Silicon Phosphorus Sulfur Chlorine Argon [Ne]3s1 [Ne]3s2 [Ne]3s23p1 [Ne]3s23p2 [Ne]3s23p3 [Ne]3s23p4 [Ne]3s23p5 [Ne]3s23p6 (3) (4) (5) (6) (7) (8) (9) (10) (11) (12) 1 2 3 4 -3, [1], 3, [4], 5 -2, -1, [1, 2] 4, 6 -1, 1, 3, [4], 5, 7 19 39,098 20 40,08 21 44,96 22 47,90 23 50,94 24 51,996 25 54,94 26 55,85 27 58,93 28 58,71 29 63,54 30 65,38 31 69,72 32 72,64 33 74,92 34 78,96 35 79,91 36 83,80 K 0,82 Ca 1,00 Sc 1,36 Ti 1,54 V 1,63 Cr 1,66 Mn 1,55 Fe 1,83 Co 1,88 Ni 1,91 Cu 1,90 Zn 1,65 Ga 1,81 Ge 2,01 As 2,18 Se 2,55 Br 2,96 Kr 3,00 4 Potassium Calcium Scandium Titanium Vanadium Chromium Manganese Iron Cobalt Nickel Copper Zinc Gallium Germanium Arsenic Selenium Bromine Krypton [Ar]4s1 [Ar]4s2 [Ar]3d14s2 [Ar]3d24s2 [Ar]3d34s2 [Ar]3d54s1 [Ar]3d54s2 [Ar]3d64s2 [Ar]3d74s2 [Ar]3d84s2 [Ar]3d104s1 [Ar]3d104s2 [Ar]3d104s24p1 [Ar]3d104s24p2 [Ar]3d104s24p3 [Ar]3d104s24p4 [Ar]3d104s24p5 [Ar]3d104s24p6 1 2 3 2, 3, 4 2, [3], 4, 5 2, 3, 4, 6 2, 3, 4, [5], 6, 7 2, 3, [4, 5, 6] 2, [3], [4] 2, [3], [4] 1, 2 2 3 2, 4 -3, 3, 5 -2, 4, 6 -1, 1, [3], [4], 5, 7 2, 4 37 85,47 38 87,62 39 88,91 40 91,22 41 92,91 42 95,94 43 (99) 44 101,07 45 102,91 46 106,40 47 107,87 48 112,41 49 114,82 50 118,69 51 121,75 52 127,60 53 126,90 54 131,30 Rb 0,82 Sr 0,95 Y 1,22 Zr 1,33 Nb 1,60 Mo 2,16 Tc 1,90 Ru 2,20 Rh 2,28 Pd 2,20 Ag 1,93 Cd 1,69 In 1,78 Sn 1,96 Sb 2,05 Te 2,10 I 2,66 Xe 2, 60 5 Rubidium Strontium Yttrium Zirconium Niobium Molybdenum Technetium Ruthenium Rhodium Paladium Silver Cadmium Indium Tin Antimony Tellurium Iodine Xenon [Kr]5s1 [Kr]5s2 [Kr]4d15s2 [Kr]4d25s2 [Kr]4d45s1 [Kr]4d55s1 [Kr]4d55s2 [Kr]4d75s1 [Kr]4d85s1 [Kr]4d105s0 [Kr]4d105s1 [Kr]4d105s2 [Kr]4d105s25p1 [Kr]4d105s25p2 [Kr]4d105s25p3 [Kr]4d105s25p4 [Kr]4d105s25p5 [Kr]4d105s25p6 1 2 3 [2], [3], 4 [2], [3], [4], 5 2, 3, 4, [5], 6 3, 4, [5], [6], 7 2, 3, 4, [5, 6], 8 2, 3, 4 2, [3], 4 1, [2] 2 1, 3 2, 4 -3, 3, [4], 5 -2, [2], 4, 6 -1, 1, 3, 5, 7 2, 4, 6 55 132,91 56 137,31 72 178,49 73 180,95 74 183,85 75 186,20 76 190,20 77 192,20 78 195,09 79 196,97 80 200,59 81 204,37 82 207,20 83 208,98 84 (209) 85 (210) 86 (222) 57 – 71 Cs 0,79 Ba 0,89 Hf 1,30 Ta 1,50 W 2,36 Re 1,90 Os 2,20 Ir 2,20 Pt 2,28 Au 2,54 Hg 2,00 Tl 1,80 Pb 1,80 Bi 1,90 Po 2,00 At 2,20 Rn 6 Caesium Barium Lanthanides Hafnium Tantalum Tungsten Rhenium Osmium Iridium Platinum Gold Mercury Thallium Lead Bismuth Polonium Astatine Radon [Xe]6s1 [Xe]6s2 [Xe]4f145d26s2 [Xe]4f145d36s2 [Xe]4f145d46s2 [Xe]4f145d56s2 [Xe]4f145d66s2 [Xe]4f145d76s2 [Xe]4f145d96s1 [Xe]4f145d106s1 [Xe]4f145d106s2 [Xe]4f145d106s26p1 [Xe]4f145d106s26p2 [Xe]4f145d106s26p3 [Xe]4f145d106s26p4 [Xe]4f145d106s26p5 [Xe]4f145d106s26p6 1 2 [2], [3], 4 [2], [3], [4], 5 2, [3], [4], [5], 6 [2], 3, 4, [5], [6], 7 2, 3, 4, [6], 8 2, 3, 4, [6] 2, [3], 4, [6] 1, 3 1, 3 1, 3 2, 4 3, 5 -2, 2, 4, 6 -1, 1, 3, 5, 7 [4] 87 (223) 88 (226) 104 (267)* 105 (268)* 106 (269)* 107 (270)* 108 (277)* 109 (278)* 110 (281)* 111 (282)* 112 (285)* 113 (286)* 114 (289)* 115 (290)* 116 (293)* 117 (294)* 118 (294)* 89 – 103 Fr 0,70 Ra 0,90 Rf Db Sg Bh Hs Mt Ds Rg Cn Nh Fl Mc Lv Ts Og 7 Francium Radium Actinides Rutherfordium Dubnium Seaborgium Bohrium Hassium Meitnerium Darmstadtium Roentgenium Copernicium Nihonium Flerovium Moscovium Livermorium Tennessium Oganesson [Rn]7s1 [Rn]7s2 [Rn]5f146d27s2 [Rn]5f146d37s2 [Rn]5f146d47s2 [Rn]5f146d57s2 [Rn]5f146d67s2 [Rn]5f146d77s2 [Rn]5f146d87s2 [Rn]5f146d97s2 [Rn]5f146d107s2 [Rn]5f146d107s27p1 [Rn]5f146d107s27p2 [Rn]5f146d107s27p3 [Rn]5f146d107s27p4 [Rn]5f146d107s27p5 [Rn]5f146d107s27p6 1 2 Các nguyên tố s Các nguyên tố d Các nguyên tố p Các nguyên tố f Kim Phi loại kim 57 138,91 58 140,12 59 140,91 60 144,24 61 (147) 62 150,35 63 151,96 64 157,25 65 158,93 66 162,50 67 164,93 68 167,26 69 168,93 70 173,04 71 174,97 La 1,10 Ce 1,12 Pr 1,13 Nd 1,14 Pm 1,13 Sm 1,17 Eu 1,20 Gd 1,20 Tb 1,10 Dy 1,22 Ho 1,23 Er 1,24 Tm 1,25 Yb 1,10 Lu 1,27
- Lưu ý: Số oxi hóa Lanthanum Cerium Praseodymium Neodymium Promethium Samarium Europium Gadolinium Terbium Dysprosium Homium Erbium Thulium Ytterbium Lutetium [Xe]5d16s2 [Xe]4f25d06s2 [Xe]4f35d06s2 [Xe]4f45d06s2 [Xe]4f55d06s2 [Xe]4f65d06s2 [Xe]4f75d06s2 [Xe]4f75d16s2 [Xe]4f95d06s2 [Xe]4f105d06s2 [Xe]4f115d06s2 [Xe]4f125d06s2 [Xe]4f135d06s2 [Xe]4f145d06s2 [Xe]4f145d16s2 dương trong bảng 3 3, 4 3, 4 [2], 3, 4 3 2, 3 2, 3 3 3, 4 3, 4 3 3 (2), 3 2, 3 3
tuần hoàn không kèm 89 (227) 90 232,04 91 231,04 92 238,03 93 (237) 94 (244) 95 (243) 96 (247) 97 (247) 98 (251) 99 (252) 100 (257) 101 (258) 102 (259) 103 (266) thêm dấu Ac 1,10 Th 1,30 Pa 1,50 U 1,38 Np 1,36 Pu 1,28 Am 1,13 Cm 1,28 Bk 2,20 Cf 1,30 Es 1,30 Fm 1,30 Md 1,30 No 1,30 Lr Actinium Thorium Protactinium Uranium Neptunium Plutonium Americium Curium Berkelium Califormium Einsteinium Fermium Mendelevium Nobelium Lawrencium
- (*): Đồng vị bền [Rn]6d17s2 [Rn]5f06d27s2 [Rn]5f26d17s2 [Rn]5f36d17s2 [Rn]5f46d17s2 [Rn]5f66d07s2 [Rn]5f76d07s2 [Rn]5f76d17s2 [Rn]5f96d07s2 [Rn]5f106d07s2 [Rn]5f116d07s2 [Rn]5f126d07s2 [Rn]5f136d07s2 [Rn]5f146d07s2 [Rn]5f146d17s2 3 4 4, 5 [3], 4, [5], 6 [3], 4, 5, 6 [3], 4, 5, 6 [3], 4, 5, 6 3 3, 4 3 3 3 2, 3 2, 3 3
BẢNG TUẦN HOÀN CÁC NGUYÊN TỐ HÓA (IA) HỌC
Design: ThS. Nguyễn (VIIIA) (1) Phú Hoạt (18) 1 1,008 8 15,999 2 4,003 H 2,20 He 1 Hydrogen (IIA) (IIIA) (IVA) (VA) (VIA) (VIIA) Helium O 3,44 1s1 1s2 (2) (13) (14) (15) (16) (17) -1, 1 3 6,94 4 9,01 5 10,81 6 12,01 7 14,007 8 15,999 9 18,998 10 20,18 Oxygen Li 0,98 Be 1,57 B 2,04 C 2,55 N 3,04 O 3,44 F 3,98 Ne 2 Lithium Beryllium Boron Carbon Nitrogen Oxygen Fluorine Neon 1s22s22p4 1s22s1 1s22s2 1s22s22p1 1s22s22p2 1s22s22p3 AUD A I UD O I O O 1s22s22p4 1s22s22p5 1s22s22p6 1 2 3 -4,-3,-2,-1,0,1,2,3,4 -3, 1, 2, 3, 4, 5 -2,-1, [-1/2,-1/3,1],2 -1 -2,-1, [-1/2,-1/3,1],2 11 22,989 12 24,31 13 26,98 14 28,09 15 30,97 16 32,06 17 35,45 18 39,95 Na 0,93 Mg 1,31 Al 1,61 Si 1,90 P 2,19 S 2,58 Cl 3,16 Ar 3 Sodium Magnesium (IIIB) (IVB) (VB) (VIB) (VIIB) (VIIIB) (VIIIB) (VIIIB) (IB) (IIB) Aluminium Silicon Phosphorus Sulfur Chlorine Argon [Ne]3s1 [Ne]3s2 [Ne]3s23p1 [Ne]3s23p2 [Ne]3s23p3 [Ne]3s23p4 [Ne]3s23p5 [Ne]3s23p6 (3) (4) (5) (6) (7) (8) (9) (10) (11) (12) 1 2 3 4 -3, [1], 3, [4], 5 -2, -1, [1, 2] 4, 6 -1, 1, 3, [4], 5, 7 19 39,098 20 40,08 21 44,96 22 47,90 23 50,94 24 51,996 25 54,94 26 55,85 27 58,93 28 58,71 29 63,54 30 65,38 31 69,72 32 72,64 33 74,92 34 78,96 35 79,91 36 83,80 K 0,82 Ca 1,00 Sc 1,36 Ti 1,54 V 1,63 Cr 1,66 Mn 1,55 Fe 1,83 Co 1,88 Ni 1,91 Cu 1,90 Zn 1,65 Ga 1,81 Ge 2,01 As 2,18 Se 2,55 Br 2,96 Kr 3,00 4 Potassium Calcium Scandium Titanium Vanadium Chromium Manganese Iron Cobalt Nickel Copper Zinc Gallium Germanium Arsenic Selenium Bromine Krypton [Ar]4s1 [Ar]4s2 [Ar]3d14s2 [Ar]3d24s2 [Ar]3d34s2 [Ar]3d54s1 [Ar]3d54s2 [Ar]3d64s2 [Ar]3d74s2 [Ar]3d84s2 [Ar]3d104s1 [Ar]3d104s2 [Ar]3d104s24p1 [Ar]3d104s24p2 [Ar]3d104s24p3 [Ar]3d104s24p4 [Ar]3d104s24p5 [Ar]3d104s24p6 1 2 3 2, 3, 4 2, [3], 4, 5 2, 3, 4, 6 2, 3, 4, [5], 6, 7 2, 3, [4, 5, 6] 2, [3], [4] 2, [3], [4] 1, 2 2 3 2, 4 -3, 3, 5 -2, 4, 6 -1, 1, [3], [4], 5, 7 2, 4 37 85,47 38 87,62 39 88,91 40 91,22 41 92,91 42 95,94 43 (99) 44 101,07 45 102,91 46 106,40 47 107,87 48 112,41 49 114,82 50 118,69 51 121,75 52 127,60 53 126,90 54 131,30 Rb 0,82 Sr 0,95 Y 1,22 Zr 1,33 Nb 1,60 Mo 2,16 Tc 1,90 Ru 2,20 Rh 2,28 Pd 2,20 Ag 1,93 Cd 1,69 In 1,78 Sn 1,96 Sb 2,05 Te 2,10 I 2,66 Xe 2, 60 5 Rubidium Strontium Yttrium Zirconium Niobium Molybdenum Technetium Ruthenium Rhodium Paladium Silver Cadmium Indium Tin Antimony Tellurium Iodine Xenon [Kr]5s1 [Kr]5s2 [Kr]4d15s2 [Kr]4d25s2 [Kr]4d45s1 [Kr]4d55s1 [Kr]4d55s2 [Kr]4d75s1 [Kr]4d85s1 [Kr]4d105s0 [Kr]4d105s1 [Kr]4d105s2 [Kr]4d105s25p1 [Kr]4d105s25p2 [Kr]4d105s25p3 [Kr]4d105s25p4 [Kr]4d105s25p5 [Kr]4d105s25p6 1 2 3 [2], [3], 4 [2], [3], [4], 5 2, 3, 4, [5], 6 3, 4, [5], [6], 7 2, 3, 4, [5, 6], 8 2, 3, 4 2, [3], 4 1, [2] 2 1, 3 2, 4 -3, 3, [4], 5 -2, [2], 4, 6 -1, 1, 3, 5, 7 2, 4, 6 55 132,91 56 137,31 72 178,49 73 180,95 74 183,85 75 186,20 76 190,20 77 192,20 78 195,09 79 196,97 80 200,59 81 204,37 82 207,20 83 208,98 84 (209) 85 (210) 86 (222) Cs 57 – 71 0,79 Ba 0,89 Hf 1,30 Ta 1,50 W 2,36 Re 1,90 Os 2,20 Ir 2,20 Pt 2,28 Au 2,54 Hg 2,00 Tl 1,80 Pb 1,80 Bi 1,90 Po 2,00 At 2,20 Rn 6 Caesium Barium Lanthanides Hafnium Tantalum Tungsten Rhenium Osmium Iridium Platinum Gold Mercury Thallium Lead Bismuth Polonium Astatine Radon [Xe]6s1 [Xe]6s2 [Xe]4f145d26s2 [Xe]4f145d36s2 [Xe]4f145d46s2 [Xe]4f145d56s2 [Xe]4f145d66s2 [Xe]4f145d76s2 [Xe]4f145d96s1 [Xe]4f145d106s1 [Xe]4f145d106s2 [Xe]4f145d106s26p1 [Xe]4f145d106s26p2 [Xe]4f145d106s26p3 [Xe]4f145d106s26p4 [Xe]4f145d106s26p5 [Xe]4f145d106s26p6 1 2 [2], [3], 4 [2], [3], [4], 5 2, [3], [4], [5], 6 [2], 3, 4, [5], [6], 7 2, 3, 4, [6], 8 2, 3, 4, [6] 2, [3], 4, [6] 1, 3 1, 3 1, 3 2, 4 3, 5 -2, 2, 4, 6 -1, 1, 3, 5, 7 [4] 87 (223) 88 (226) 104 (267)* 105 (268)* 106 (269)* 107 (270)* 108 (277)* 109 (278)* 110 (281)* 111 (282)* 112 (285)* 113 (286)* 114 (289)* 115 (290)* 116 (293)* 117 (294)* 118 (294)* Fr 89 – 103 0,70 Ra 0,90 Rf Db Sg Bh Hs Mt Ds Rg Cn Nh Fl Mc Lv Ts Og 7 Francium Radium Actinides Rutherfordium Dubnium Seaborgium Bohrium Hassium Meitnerium Darmstadtium Roentgenium Copernicium Nihonium Flerovium Moscovium Livermorium Tennessium Oganesson [Rn]7s1 [Rn]7s2 [Rn]5f146d27s2 [Rn]5f146d37s2 [Rn]5f146d47s2 [Rn]5f146d57s2 [Rn]5f146d67s2 [Rn]5f146d77s2 [Rn]5f146d87s2 [Rn]5f146d97s2 [Rn]5f146d107s2 [Rn]5f146d107s27p1 [Rn]5f146d107s27p2 [Rn]5f146d107s27p3 [Rn]5f146d107s27p4 [Rn]5f146d107s27p5 [Rn]5f146d107s27p6 1 2 Các nguyên tố s Các nguyên tố d Các nguyên tố p Các nguyên tố f Kim Phi loại kim 57 138,91 58 140,12 59 140,91 60 144,24 61 (147) 62 150,35 63 151,96 64 157,25 65 158,93 66 162,50 67 164,93 68 167,26 69 168,93 70 173,04 71 174,97 La 1,10 Ce 1,12 Pr 1,13 Nd 1,14 Pm 1,13 Sm 1,17 Eu 1,20 Gd 1,20 Tb 1,10 Dy 1,22 Ho 1,23 Er 1,24 Tm 1,25 Yb 1,10 Lu 1,27
- Lưu ý: Số oxi hóa Lanthanum Cerium Praseodymium Neodymium Promethium Samarium Europium Gadolinium Terbium Dysprosium Homium Erbium Thulium Ytterbium Lutetium [Xe]5d16s2 [Xe]4f25d06s2 [Xe]4f35d06s2 [Xe]4f45d06s2 [Xe]4f55d06s2 [Xe]4f65d06s2 [Xe]4f75d06s2 [Xe]4f75d16s2 [Xe]4f95d06s2 [Xe]4f105d06s2 [Xe]4f115d06s2 [Xe]4f125d06s2 [Xe]4f135d06s2 [Xe]4f145d06s2 [Xe]4f145d16s2 dương trong bảng 3 3, 4 3, 4 [2], 3, 4 3 2, 3 2, 3 3 3, 4 3, 4 3 3 (2), 3 2, 3 3
tuần hoàn không kèm 89 (227) 90 232,04 91 231,04 92 238,03 93 (237) 94 (244) 95 (243) 96 (247) 97 (247) 98 (251) 99 (252) 100 (257) 101 (258) 102 (259) 103 (266) thêm dấu Ac 1,10 Th 1,30 Pa 1,50 U 1,38 Np 1,36 Pu 1,28 Am 1,13 Cm 1,28 Bk 2,20 Cf 1,30 Es 1,30 Fm 1,30 Md 1,30 No 1,30 Lr Actinium Thorium Protactinium Uranium Neptunium Plutonium Americium Curium Berkelium Califormium Einsteinium Fermium Mendelevium Nobelium Lawrencium
- (*): Đồng vị bền [Rn]6d17s2 [Rn]5f06d27s2 [Rn]5f26d17s2 [Rn]5f36d17s2 [Rn]5f46d17s2 [Rn]5f66d07s2 [Rn]5f76d07s2 [Rn]5f76d17s2 [Rn]5f96d07s2 [Rn]5f106d07s2 [Rn]5f116d07s2 [Rn]5f126d07s2 [Rn]5f136d07s2 [Rn]5f146d07s2 [Rn]5f146d17s2 3 4 4, 5 [3], 4, [5], 6 [3], 4, 5, 6 [3], 4, 5, 6 [3], 4, 5, 6 3 3, 4 3 3 3 2, 3 2, 3 3
BẢNG TUẦN HOÀN CÁC NGUYÊN TỐ HÓA (IA) HỌC
Design: ThS. Nguyễn (VIIIA) (1) Phú Hoạt (18) 1 1,008 12 24,31 2 4,003 H 2,20 He 1 Hydrogen (IIA) (IIIA) (IVA) (VA) (VIA) (VIIA) Helium Mg 1,31 1s1 1s2 (2) (13) (14) (15) (16) (17) -1, 1 3 6,94 4 9,01 5 10,81 6 12,01 7 14,007 8 15,999 9 18,998 10 20,18 Magnesium Li 0,98 Be 1,57 B 2,04 C 2,55 N 3,04 O 3,44 F 3,98 Ne 2 Lithium Beryllium Boron Carbon Nitrogen Oxygen Fluorine Neon [Ne]3s2 1s22s1 1s22s2 AUD A I UD O I 1s22s22p1 1s22s22p2 1s22s22p3 1s22s22p4 1s22s22p5 1s22s22p6 1 2 3 -4,-3,-2,-1,0,1,2,3,4 -3, 1, 2, 3, 4, 5 -2,-1, [-1/2,-1/3,1],2 -1 2 11 22,989 12 24,31 Mg 1,31 Mg M 13 26,98 14 28,09 15 30,97 16 32,06 17 35,45 18 39,95 Na 0,93 Al 1,61 Si 1,90 P 2,19 S 2,58 Cl 3,16 Ar 3 Sodium Magnesium (IIIB) (IVB) (VB) (VIB) (VIIB) (VIIIB) (VIIIB) (VIIIB) (IB) (IIB) Aluminium Silicon Phosphorus Sulfur Chlorine Argon [Ne]3s1 [Ne]3s2 [Ne]3s23p1 [Ne]3s23p2 [Ne]3s23p3 [Ne]3s23p4 [Ne]3s23p5 [Ne]3s23p6 (3) (4) (5) (6) (7) (8) (9) (10) (11) (12) 1 2 3 4 -3, [1], 3, [4], 5 -2, -1, [1, 2] 4, 6 -1, 1, 3, [4], 5, 7 19 39,098 20 40,08 21 44,96 22 47,90 23 50,94 24 51,996 25 54,94 26 55,85 27 58,93 28 58,71 29 63,54 30 65,38 31 69,72 32 72,64 33 74,92 34 78,96 35 79,91 36 83,80 K 0,82 Ca 1,00 Sc 1,36 Ti 1,54 V 1,63 Cr 1,66 Mn 1,55 Fe 1,83 Co 1,88 Ni 1,91 Cu 1,90 Zn 1,65 Ga 1,81 Ge 2,01 As 2,18 Se 2,55 Br 2,96 Kr 3,00 4 Potassium Calcium Scandium Titanium Vanadium Chromium Manganese Iron Cobalt Nickel Copper Zinc Gallium Germanium Arsenic Selenium Bromine Krypton [Ar]4s1 [Ar]4s2 [Ar]3d14s2 [Ar]3d24s2 [Ar]3d34s2 [Ar]3d54s1 [Ar]3d54s2 [Ar]3d64s2 [Ar]3d74s2 [Ar]3d84s2 [Ar]3d104s1 [Ar]3d104s2 [Ar]3d104s24p1 [Ar]3d104s24p2 [Ar]3d104s24p3 [Ar]3d104s24p4 [Ar]3d104s24p5 [Ar]3d104s24p6 1 2 3 2, 3, 4 2, [3], 4, 5 2, 3, 4, 6 2, 3, 4, [5], 6, 7 2, 3, [4, 5, 6] 2, [3], [4] 2, [3], [4] 1, 2 2 3 2, 4 -3, 3, 5 -2, 4, 6 -1, 1, [3], [4], 5, 7 2, 4 37 85,47 38 87,62 39 88,91 40 91,22 41 92,91 42 95,94 43 (99) 44 101,07 45 102,91 46 106,40 47 107,87 48 112,41 49 114,82 50 118,69 51 121,75 52 127,60 53 126,90 54 131,30 Rb 0,82 Sr 0,95 Y 1,22 Zr 1,33 Nb 1,60 Mo 2,16 Tc 1,90 Ru 2,20 Rh 2,28 Pd 2,20 Ag 1,93 Cd 1,69 In 1,78 Sn 1,96 Sb 2,05 Te 2,10 I 2,66 Xe 2, 60 5 Rubidium Strontium Yttrium Zirconium Niobium Molybdenum Technetium Ruthenium Rhodium Paladium Silver Cadmium Indium Tin Antimony Tellurium Iodine Xenon [Kr]5s1 [Kr]5s2 [Kr]4d15s2 [Kr]4d25s2 [Kr]4d45s1 [Kr]4d55s1 [Kr]4d55s2 [Kr]4d75s1 [Kr]4d85s1 [Kr]4d105s0 [Kr]4d105s1 [Kr]4d105s2 [Kr]4d105s25p1 [Kr]4d105s25p2 [Kr]4d105s25p3 [Kr]4d105s25p4 [Kr]4d105s25p5 [Kr]4d105s25p6 1 2 3 [2], [3], 4 [2], [3], [4], 5 2, 3, 4, [5], 6 3, 4, [5], [6], 7 2, 3, 4, [5, 6], 8 2, 3, 4 2, [3], 4 1, [2] 2 1, 3 2, 4 -3, 3, [4], 5 -2, [2], 4, 6 -1, 1, 3, 5, 7 2, 4, 6 55 132,91 56 137,31 72 178,49 73 180,95 74 183,85 75 186,20 76 190,20 77 192,20 78 195,09 79 196,97 80 200,59 81 204,37 82 207,20 83 208,98 84 (209) 85 (210) 86 (222) Cs 57 – 71 0,79 Ba 0,89 Hf 1,30 Ta 1,50 W 2,36 Re 1,90 Os 2,20 Ir 2,20 Pt 2,28 Au 2,54 Hg 2,00 Tl 1,80 Pb 1,80 Bi 1,90 Po 2,00 At 2,20 Rn 6 Caesium Barium Lanthanides Hafnium Tantalum Tungsten Rhenium Osmium Iridium Platinum Gold Mercury Thallium Lead Bismuth Polonium Astatine Radon [Xe]6s1 [Xe]6s2 [Xe]4f145d26s2 [Xe]4f145d36s2 [Xe]4f145d46s2 [Xe]4f145d56s2 [Xe]4f145d66s2 [Xe]4f145d76s2 [Xe]4f145d96s1 [Xe]4f145d106s1 [Xe]4f145d106s2 [Xe]4f145d106s26p1 [Xe]4f145d106s26p2 [Xe]4f145d106s26p3 [Xe]4f145d106s26p4 [Xe]4f145d106s26p5 [Xe]4f145d106s26p6 1 2 [2], [3], 4 [2], [3], [4], 5 2, [3], [4], [5], 6 [2], 3, 4, [5], [6], 7 2, 3, 4, [6], 8 2, 3, 4, [6] 2, [3], 4, [6] 1, 3 1, 3 1, 3 2, 4 3, 5 -2, 2, 4, 6 -1, 1, 3, 5, 7 [4] 87 (223) 88 (226) 104 (267)* 105 (268)* 106 (269)* 107 (270)* 108 (277)* 109 (278)* 110 (281)* 111 (282)* 112 (285)* 113 (286)* 114 (289)* 115 (290)* 116 (293)* 117 (294)* 118 (294)* Fr 89 – 103 0,70 Ra 0,90 Rf Db Sg Bh Hs Mt Ds Rg Cn Nh Fl Mc Lv Ts Og 7 Francium Radium Actinides Rutherfordium Dubnium Seaborgium Bohrium Hassium Meitnerium Darmstadtium Roentgenium Copernicium Nihonium Flerovium Moscovium Livermorium Tennessium Oganesson [Rn]7s1 [Rn]7s2 [Rn]5f146d27s2 [Rn]5f146d37s2 [Rn]5f146d47s2 [Rn]5f146d57s2 [Rn]5f146d67s2 [Rn]5f146d77s2 [Rn]5f146d87s2 [Rn]5f146d97s2 [Rn]5f146d107s2 [Rn]5f146d107s27p1 [Rn]5f146d107s27p2 [Rn]5f146d107s27p3 [Rn]5f146d107s27p4 [Rn]5f146d107s27p5 [Rn]5f146d107s27p6 1 2 Các nguyên tố s Các nguyên tố d Các nguyên tố p Các nguyên tố f Kim Phi loại kim 57 138,91 58 140,12 59 140,91 60 144,24 61 (147) 62 150,35 63 151,96 64 157,25 65 158,93 66 162,50 67 164,93 68 167,26 69 168,93 70 173,04 71 174,97 La 1,10 Ce 1,12 Pr 1,13 Nd 1,14 Pm 1,13 Sm 1,17 Eu 1,20 Gd 1,20 Tb 1,10 Dy 1,22 Ho 1,23 Er 1,24 Tm 1,25 Yb 1,10 Lu 1,27
- Lưu ý: Số oxi hóa Lanthanum Cerium Praseodymium Neodymium Promethium Samarium Europium Gadolinium Terbium Dysprosium Homium Erbium Thulium Ytterbium Lutetium [Xe]5d16s2 [Xe]4f25d06s2 [Xe]4f35d06s2 [Xe]4f45d06s2 [Xe]4f55d06s2 [Xe]4f65d06s2 [Xe]4f75d06s2 [Xe]4f75d16s2 [Xe]4f95d06s2 [Xe]4f105d06s2 [Xe]4f115d06s2 [Xe]4f125d06s2 [Xe]4f135d06s2 [Xe]4f145d06s2 [Xe]4f145d16s2 dương trong bảng 3 3, 4 3, 4 [2], 3, 4 3 2, 3 2, 3 3 3, 4 3, 4 3 3 (2), 3 2, 3 3
tuần hoàn không kèm 89 (227) 90 232,04 91 231,04 92 238,03 93 (237) 94 (244) 95 (243) 96 (247) 97 (247) 98 (251) 99 (252) 100 (257) 101 (258) 102 (259) 103 (266) thêm dấu Ac 1,10 Th 1,30 Pa 1,50 U 1,38 Np 1,36 Pu 1,28 Am 1,13 Cm 1,28 Bk 2,20 Cf 1,30 Es 1,30 Fm 1,30 Md 1,30 No 1,30 Lr Actinium Thorium Protactinium Uranium Neptunium Plutonium Americium Curium Berkelium Califormium Einsteinium Fermium Mendelevium Nobelium Lawrencium
- (*): Đồng vị bền [Rn]6d17s2 [Rn]5f06d27s2 [Rn]5f26d17s2 [Rn]5f36d17s2 [Rn]5f46d17s2 [Rn]5f66d07s2 [Rn]5f76d07s2 [Rn]5f76d17s2 [Rn]5f96d07s2 [Rn]5f106d07s2 [Rn]5f116d07s2 [Rn]5f126d07s2 [Rn]5f136d07s2 [Rn]5f146d07s2 [Rn]5f146d17s2 3 4 4, 5 [3], 4, [5], 6 [3], 4, 5, 6 [3], 4, 5, 6 [3], 4, 5, 6 3 3, 4 3 3 3 2, 3 2, 3 3
BẢNG TUẦN HOÀN CÁC NGUYÊN TỐ HÓA (IA) HỌC
Design: ThS. Nguyễn (VIIIA) (1) Phú Hoạt (18) 1 1,008 17 35,45 2 4,003 H 2,20 He 1 Hydrogen (IIA) (IIIA) (IVA) (VA) (VIA) (VIIA) Helium Cl 3,16 1s1 1s2 (2) (13) (14) (15) (16) (17) -1, 1 3 6,94 4 9,01 5 10,81 6 12,01 7 14,007 8 15,999 9 18,998 10 20,18 Chlorine Li 0,98 Be 1,57 B 2,04 C 2,55 N 3,04 O 3,44 F 3,98 Ne 2 Lithium Beryllium Boron Carbon Nitrogen Oxygen Fluorine Neon [Ne]3s23p5 1s22s1 1s22s2 1s22s22p1 1s22s22p2 1s22s22p3 AUD A I UD O I 1s22s22p4 1s22s22p5 1s22s22p6 1 2 3 -4,-3,-2,-1,0,1,2,3,4 -3, 1, 2, 3, 4, 5 -2,-1, [-1/2,-1/3,1],2 -1 -1, 1, 3, [4], 5, 7 11 22,989 12 24,31 13 26,98 14 28,09 15 30,97 16 32,06 Cl 17 35,45 18 39,95 Na 0,93 Mg 1,31 Al 1,61 Si 1,90 P 2,19 S 2,58 Cl 3,16 Ar 3 Sodium Magnesium (IIIB) (IVB) (VB) (VIB) (VIIB) (VIIIB) (VIIIB) (VIIIB) (IB) (IIB) Aluminium Silicon Phosphorus Sulfur Chlorine Argon [Ne]3s1 [Ne]3s2 [Ne]3s23p1 [Ne]3s23p2 [Ne]3s23p3 [Ne]3s23p4 [Ne]3s23p5 [Ne]3s23p6 (3) (4) (5) (6) (7) (8) (9) (10) (11) (12) 1 2 3 4 -3, [1], 3, [4], 5 -2, -1, [1, 2] 4, 6 -1, 1, 3, [4], 5, 7 19 39,098 20 40,08 21 44,96 22 47,90 23 50,94 24 51,996 25 54,94 26 55,85 27 58,93 28 58,71 29 63,54 30 65,38 31 69,72 32 72,64 33 74,92 34 78,96 35 79,91 36 83,80 K 0,82 Ca 1,00 Sc 1,36 Ti 1,54 V 1,63 Cr 1,66 Mn 1,55 Fe 1,83 Co 1,88 Ni 1,91 Cu 1,90 Zn 1,65 Ga 1,81 Ge 2,01 As 2,18 Se 2,55 Br 2,96 Kr 3,00 4 Potassium Calcium Scandium Titanium Vanadium Chromium Manganese Iron Cobalt Nickel Copper Zinc Gallium Germanium Arsenic Selenium Bromine Krypton [Ar]4s1 [Ar]4s2 [Ar]3d14s2 [Ar]3d24s2 [Ar]3d34s2 [Ar]3d54s1 [Ar]3d54s2 [Ar]3d64s2 [Ar]3d74s2 [Ar]3d84s2 [Ar]3d104s1 [Ar]3d104s2 [Ar]3d104s24p1 [Ar]3d104s24p2 [Ar]3d104s24p3 [Ar]3d104s24p4 [Ar]3d104s24p5 [Ar]3d104s24p6 1 2 3 2, 3, 4 2, [3], 4, 5 2, 3, 4, 6 2, 3, 4, [5], 6, 7 2, 3, [4, 5, 6] 2, [3], [4] 2, [3], [4] 1, 2 2 3 2, 4 -3, 3, 5 -2, 4, 6 -1, 1, [3], [4], 5, 7 2, 4 37 85,47 38 87,62 39 88,91 40 91,22 41 92,91 42 95,94 43 (99) 44 101,07 45 102,91 46 106,40 47 107,87 48 112,41 49 114,82 50 118,69 51 121,75 52 127,60 53 126,90 54 131,30 Rb 0,82 Sr 0,95 Y 1,22 Zr 1,33 Nb 1,60 Mo 2,16 Tc 1,90 Ru 2,20 Rh 2,28 Pd 2,20 Ag 1,93 Cd 1,69 In 1,78 Sn 1,96 Sb 2,05 Te 2,10 I 2,66 Xe 2, 60 5 Rubidium Strontium Yttrium Zirconium Niobium Molybdenum Technetium Ruthenium Rhodium Paladium Silver Cadmium Indium Tin Antimony Tellurium Iodine Xenon [Kr]5s1 [Kr]5s2 [Kr]4d15s2 [Kr]4d25s2 [Kr]4d45s1 [Kr]4d55s1 [Kr]4d55s2 [Kr]4d75s1 [Kr]4d85s1 [Kr]4d105s0 [Kr]4d105s1 [Kr]4d105s2 [Kr]4d105s25p1 [Kr]4d105s25p2 [Kr]4d105s25p3 [Kr]4d105s25p4 [Kr]4d105s25p5 [Kr]4d105s25p6 1 2 3 [2], [3], 4 [2], [3], [4], 5 2, 3, 4, [5], 6 3, 4, [5], [6], 7 2, 3, 4, [5, 6], 8 2, 3, 4 2, [3], 4 1, [2] 2 1, 3 2, 4 -3, 3, [4], 5 -2, [2], 4, 6 -1, 1, 3, 5, 7 2, 4, 6 55 132,91 56 137,31 72 178,49 73 180,95 74 183,85 75 186,20 76 190,20 77 192,20 78 195,09 79 196,97 80 200,59 81 204,37 82 207,20 83 208,98 84 (209) 85 (210) 86 (222) Cs 57 – 71 0,79 Ba 0,89 Hf 1,30 Ta 1,50 W 2,36 Re 1,90 Os 2,20 Ir 2,20 Pt 2,28 Au 2,54 Hg 2,00 Tl 1,80 Pb 1,80 Bi 1,90 Po 2,00 At 2,20 Rn 6 Caesium Barium Lanthanides Hafnium Tantalum Tungsten Rhenium Osmium Iridium Platinum Gold Mercury Thallium Lead Bismuth Polonium Astatine Radon [Xe]6s1 [Xe]6s2 [Xe]4f145d26s2 [Xe]4f145d36s2 [Xe]4f145d46s2 [Xe]4f145d56s2 [Xe]4f145d66s2 [Xe]4f145d76s2 [Xe]4f145d96s1 [Xe]4f145d106s1 [Xe]4f145d106s2 [Xe]4f145d106s26p1 [Xe]4f145d106s26p2 [Xe]4f145d106s26p3 [Xe]4f145d106s26p4 [Xe]4f145d106s26p5 [Xe]4f145d106s26p6 1 2 [2], [3], 4 [2], [3], [4], 5 2, [3], [4], [5], 6 [2], 3, 4, [5], [6], 7 2, 3, 4, [6], 8 2, 3, 4, [6] 2, [3], 4, [6] 1, 3 1, 3 1, 3 2, 4 3, 5 -2, 2, 4, 6 -1, 1, 3, 5, 7 [4] 87 (223) 88 (226) 104 (267)* 105 (268)* 106 (269)* 107 (270)* 108 (277)* 109 (278)* 110 (281)* 111 (282)* 112 (285)* 113 (286)* 114 (289)* 115 (290)* 116 (293)* 117 (294)* 118 (294)* Fr 89 – 103 0,70 Ra 0,90 Rf Db Sg Bh Hs Mt Ds Rg Cn Nh Fl Mc Lv Ts Og 7 Francium Radium Actinides Rutherfordium Dubnium Seaborgium Bohrium Hassium Meitnerium Darmstadtium Roentgenium Copernicium Nihonium Flerovium Moscovium Livermorium Tennessium Oganesson [Rn]7s1 [Rn]7s2 [Rn]5f146d27s2 [Rn]5f146d37s2 [Rn]5f146d47s2 [Rn]5f146d57s2 [Rn]5f146d67s2 [Rn]5f146d77s2 [Rn]5f146d87s2 [Rn]5f146d97s2 [Rn]5f146d107s2 [Rn]5f146d107s27p1 [Rn]5f146d107s27p2 [Rn]5f146d107s27p3 [Rn]5f146d107s27p4 [Rn]5f146d107s27p5 [Rn]5f146d107s27p6 1 2 Các nguyên tố s Các nguyên tố d Các nguyên tố p Các nguyên tố f Kim Phi loại kim 57 138,91 58 140,12 59 140,91 60 144,24 61 (147) 62 150,35 63 151,96 64 157,25 65 158,93 66 162,50 67 164,93 68 167,26 69 168,93 70 173,04 71 174,97 La 1,10 Ce 1,12 Pr 1,13 Nd 1,14 Pm 1,13 Sm 1,17 Eu 1,20 Gd 1,20 Tb 1,10 Dy 1,22 Ho 1,23 Er 1,24 Tm 1,25 Yb 1,10 Lu 1,27
- Lưu ý: Số oxi hóa Lanthanum Cerium Praseodymium Neodymium Promethium Samarium Europium Gadolinium Terbium Dysprosium Homium Erbium Thulium Ytterbium Lutetium [Xe]5d16s2 [Xe]4f25d06s2 [Xe]4f35d06s2 [Xe]4f45d06s2 [Xe]4f55d06s2 [Xe]4f65d06s2 [Xe]4f75d06s2 [Xe]4f75d16s2 [Xe]4f95d06s2 [Xe]4f105d06s2 [Xe]4f115d06s2 [Xe]4f125d06s2 [Xe]4f135d06s2 [Xe]4f145d06s2 [Xe]4f145d16s2 dương trong bảng 3 3, 4 3, 4 [2], 3, 4 3 2, 3 2, 3 3 3, 4 3, 4 3 3 (2), 3 2, 3 3
tuần hoàn không kèm 89 (227) 90 232,04 91 231,04 92 238,03 93 (237) 94 (244) 95 (243) 96 (247) 97 (247) 98 (251) 99 (252) 100 (257) 101 (258) 102 (259) 103 (266) thêm dấu Ac 1,10 Th 1,30 Pa 1,50 U 1,38 Np 1,36 Pu 1,28 Am 1,13 Cm 1,28 Bk 2,20 Cf 1,30 Es 1,30 Fm 1,30 Md 1,30 No 1,30 Lr Actinium Thorium Protactinium Uranium Neptunium Plutonium Americium Curium Berkelium Califormium Einsteinium Fermium Mendelevium Nobelium Lawrencium
- (*): Đồng vị bền [Rn]6d17s2 [Rn]5f06d27s2 [Rn]5f26d17s2 [Rn]5f36d17s2 [Rn]5f46d17s2 [Rn]5f66d07s2 [Rn]5f76d07s2 [Rn]5f76d17s2 [Rn]5f96d07s2 [Rn]5f106d07s2 [Rn]5f116d07s2 [Rn]5f126d07s2 [Rn]5f136d07s2 [Rn]5f146d07s2 [Rn]5f146d17s2 3 4 4, 5 [3], 4, [5], 6 [3], 4, 5, 6 [3], 4, 5, 6 [3], 4, 5, 6 3 3, 4 3 3 3 2, 3 2, 3 3
BẢNG TUẦN HOÀN CÁC NGUYÊN TỐ HÓA (IA) HỌC
Design: ThS. Nguyễn (VIIIA) (1) Phú Hoạt (18) 1 1,008 18 39,95 2 4,003 H 2,20 He 1 Hydrogen (IIA) (IIIA) (IVA) (VA) (VIA) (VIIA) Helium Ar 1s1 1s2 (2) (13) (14) (15) (16) (17) -1, 1 3 6,94 4 9,01 5 10,81 6 12,01 7 14,007 8 15,999 9 18,998 10 20,18 Argon Li 0,98 Be 1,57 B 2,04 C 2,55 N 3,04 O 3,44 F 3,98 Ne 2 Lithium Beryllium Boron Carbon Nitrogen Oxygen Fluorine Neon [Ne]3s23p6 1s22s1 1s22s2 1s22s22p1 1s22s22p2 1s22s22p3 AUD A I UD O I 1s22s22p4 1s22s22p5 1s22s22p6 1 2 3 -4,-3,-2,-1,0,1,2,3,4 -3, 1, 2, 3, 4, 5 -2,-1, [-1/2,-1/3,1],2 -1 11 22,989 12 24,31 13 26,98 14 28,09 15 30,97 16 32,06 Ar 17 35,45 18 39,95 Na 0,93 Mg 1,31 Al 1,61 Si 1,90 P 2,19 S 2,58 Cl 3,16 Ar 3 Sodium Magnesium (IIIB) (IVB) (VB) (VIB) (VIIB) (VIIIB) (VIIIB) (VIIIB) (IB) (IIB) Aluminium Silicon Phosphorus Sulfur Chlorine Argon [Ne]3s1 [Ne]3s2 [Ne]3s23p1 [Ne]3s23p2 [Ne]3s23p3 [Ne]3s23p4 [Ne]3s23p5 (3) (4) (5) (6) (7) (8) (9) (10) (11) (12) [Ne]3s23p6 1 2 3 4 -3, [1], 3, [4], 5 -2, -1, [1, 2] 4, 6 -1, 1, 3, [4], 5, 7 19 39,098 20 40,08 21 44,96 22 47,90 23 50,94 24 51,996 25 54,94 26 55,85 27 58,93 28 58,71 29 63,54 30 65,38 31 69,72 32 72,64 33 74,92 34 78,96 35 79,91 36 83,80 K 0,82 Ca 1,00 Sc 1,36 Ti 1,54 V 1,63 Cr 1,66 Mn 1,55 Fe 1,83 Co 1,88 Ni 1,91 Cu 1,90 Zn 1,65 Ga 1,81 Ge 2,01 As 2,18 Se 2,55 Br 2,96 Kr 3,00 4 Potassium Calcium Scandium Titanium Vanadium Chromium Manganese Iron Cobalt Nickel Copper Zinc Gallium Germanium Arsenic Selenium Bromine Krypton [Ar]4s1 [Ar]4s2 [Ar]3d14s2 [Ar]3d24s2 [Ar]3d34s2 [Ar]3d54s1 [Ar]3d54s2 [Ar]3d64s2 [Ar]3d74s2 [Ar]3d84s2 [Ar]3d104s1 [Ar]3d104s2 [Ar]3d104s24p1 [Ar]3d104s24p2 [Ar]3d104s24p3 [Ar]3d104s24p4 [Ar]3d104s24p5 [Ar]3d104s24p6 1 2 3 2, 3, 4 2, [3], 4, 5 2, 3, 4, 6 2, 3, 4, [5], 6, 7 2, 3, [4, 5, 6] 2, [3], [4] 2, [3], [4] 1, 2 2 3 2, 4 -3, 3, 5 -2, 4, 6 -1, 1, [3], [4], 5, 7 2, 4 37 85,47 38 87,62 39 88,91 40 91,22 41 92,91 42 95,94 43 (99) 44 101,07 45 102,91 46 106,40 47 107,87 48 112,41 49 114,82 50 118,69 51 121,75 52 127,60 53 126,90 54 131,30 Rb 0,82 Sr 0,95 Y 1,22 Zr 1,33 Nb 1,60 Mo 2,16 Tc 1,90 Ru 2,20 Rh 2,28 Pd 2,20 Ag 1,93 Cd 1,69 In 1,78 Sn 1,96 Sb 2,05 Te 2,10 I 2,66 Xe 2, 60 5 Rubidium Strontium Yttrium Zirconium Niobium Molybdenum Technetium Ruthenium Rhodium Paladium Silver Cadmium Indium Tin Antimony Tellurium Iodine Xenon [Kr]5s1 [Kr]5s2 [Kr]4d15s2 [Kr]4d25s2 [Kr]4d45s1 [Kr]4d55s1 [Kr]4d55s2 [Kr]4d75s1 [Kr]4d85s1 [Kr]4d105s0 [Kr]4d105s1 [Kr]4d105s2 [Kr]4d105s25p1 [Kr]4d105s25p2 [Kr]4d105s25p3 [Kr]4d105s25p4 [Kr]4d105s25p5 [Kr]4d105s25p6 1 2 3 [2], [3], 4 [2], [3], [4], 5 2, 3, 4, [5], 6 3, 4, [5], [6], 7 2, 3, 4, [5, 6], 8 2, 3, 4 2, [3], 4 1, [2] 2 1, 3 2, 4 -3, 3, [4], 5 -2, [2], 4, 6 -1, 1, 3, 5, 7 2, 4, 6 55 132,91 56 137,31 72 178,49 73 180,95 74 183,85 75 186,20 76 190,20 77 192,20 78 195,09 79 196,97 80 200,59 81 204,37 82 207,20 83 208,98 84 (209) 85 (210) 86 (222) Cs 57 – 71 0,79 Ba 0,89 Hf 1,30 Ta 1,50 W 2,36 Re 1,90 Os 2,20 Ir 2,20 Pt 2,28 Au 2,54 Hg 2,00 Tl 1,80 Pb 1,80 Bi 1,90 Po 2,00 At 2,20 Rn 6 Caesium Barium Lanthanides Hafnium Tantalum Tungsten Rhenium Osmium Iridium Platinum Gold Mercury Thallium Lead Bismuth Polonium Astatine Radon [Xe]6s1 [Xe]6s2 [Xe]4f145d26s2 [Xe]4f145d36s2 [Xe]4f145d46s2 [Xe]4f145d56s2 [Xe]4f145d66s2 [Xe]4f145d76s2 [Xe]4f145d96s1 [Xe]4f145d106s1 [Xe]4f145d106s2 [Xe]4f145d106s26p1 [Xe]4f145d106s26p2 [Xe]4f145d106s26p3 [Xe]4f145d106s26p4 [Xe]4f145d106s26p5 [Xe]4f145d106s26p6 1 2 [2], [3], 4 [2], [3], [4], 5 2, [3], [4], [5], 6 [2], 3, 4, [5], [6], 7 2, 3, 4, [6], 8 2, 3, 4, [6] 2, [3], 4, [6] 1, 3 1, 3 1, 3 2, 4 3, 5 -2, 2, 4, 6 -1, 1, 3, 5, 7 [4] 87 (223) 88 (226) 104 (267)* 105 (268)* 106 (269)* 107 (270)* 108 (277)* 109 (278)* 110 (281)* 111 (282)* 112 (285)* 113 (286)* 114 (289)* 115 (290)* 116 (293)* 117 (294)* 118 (294)* Fr 89 – 103 0,70 Ra 0,90 Rf Db Sg Bh Hs Mt Ds Rg Cn Nh Fl Mc Lv Ts Og 7 Francium Radium Actinides Rutherfordium Dubnium Seaborgium Bohrium Hassium Meitnerium Darmstadtium Roentgenium Copernicium Nihonium Flerovium Moscovium Livermorium Tennessium Oganesson [Rn]7s1 [Rn]7s2 [Rn]5f146d27s2 [Rn]5f146d37s2 [Rn]5f146d47s2 [Rn]5f146d57s2 [Rn]5f146d67s2 [Rn]5f146d77s2 [Rn]5f146d87s2 [Rn]5f146d97s2 [Rn]5f146d107s2 [Rn]5f146d107s27p1 [Rn]5f146d107s27p2 [Rn]5f146d107s27p3 [Rn]5f146d107s27p4 [Rn]5f146d107s27p5 [Rn]5f146d107s27p6 1 2 Các nguyên tố s Các nguyên tố d Các nguyên tố p Các nguyên tố f Kim Phi loại kim 57 138,91 58 140,12 59 140,91 60 144,24 61 (147) 62 150,35 63 151,96 64 157,25 65 158,93 66 162,50 67 164,93 68 167,26 69 168,93 70 173,04 71 174,97 La 1,10 Ce 1,12 Pr 1,13 Nd 1,14 Pm 1,13 Sm 1,17 Eu 1,20 Gd 1,20 Tb 1,10 Dy 1,22 Ho 1,23 Er 1,24 Tm 1,25 Yb 1,10 Lu 1,27
- Lưu ý: Số oxi hóa Lanthanum Cerium Praseodymium Neodymium Promethium Samarium Europium Gadolinium Terbium Dysprosium Homium Erbium Thulium Ytterbium Lutetium [Xe]5d16s2 [Xe]4f25d06s2 [Xe]4f35d06s2 [Xe]4f45d06s2 [Xe]4f55d06s2 [Xe]4f65d06s2 [Xe]4f75d06s2 [Xe]4f75d16s2 [Xe]4f95d06s2 [Xe]4f105d06s2 [Xe]4f115d06s2 [Xe]4f125d06s2 [Xe]4f135d06s2 [Xe]4f145d06s2 [Xe]4f145d16s2 dương trong bảng 3 3, 4 3, 4 [2], 3, 4 3 2, 3 2, 3 3 3, 4 3, 4 3 3 (2), 3 2, 3 3
tuần hoàn không kèm 89 (227) 90 232,04 91 231,04 92 238,03 93 (237) 94 (244) 95 (243) 96 (247) 97 (247) 98 (251) 99 (252) 100 (257) 101 (258) 102 (259) 103 (266) thêm dấu Ac 1,10 Th 1,30 Pa 1,50 U 1,38 Np 1,36 Pu 1,28 Am 1,13 Cm 1,28 Bk 2,20 Cf 1,30 Es 1,30 Fm 1,30 Md 1,30 No 1,30 Lr Actinium Thorium Protactinium Uranium Neptunium Plutonium Americium Curium Berkelium Califormium Einsteinium Fermium Mendelevium Nobelium Lawrencium
- (*): Đồng vị bền [Rn]6d17s2 [Rn]5f06d27s2 [Rn]5f26d17s2 [Rn]5f36d17s2 [Rn]5f46d17s2 [Rn]5f66d07s2 [Rn]5f76d07s2 [Rn]5f76d17s2 [Rn]5f96d07s2 [Rn]5f106d07s2 [Rn]5f116d07s2 [Rn]5f126d07s2 [Rn]5f136d07s2 [Rn]5f146d07s2 [Rn]5f146d17s2 3 4 4, 5 [3], 4, [5], 6 [3], 4, 5, 6 [3], 4, 5, 6 [3], 4, 5, 6 3 3, 4 3 3 3 2, 3 2, 3 3
PHẦN CHUNG SỨC (2 Phút)
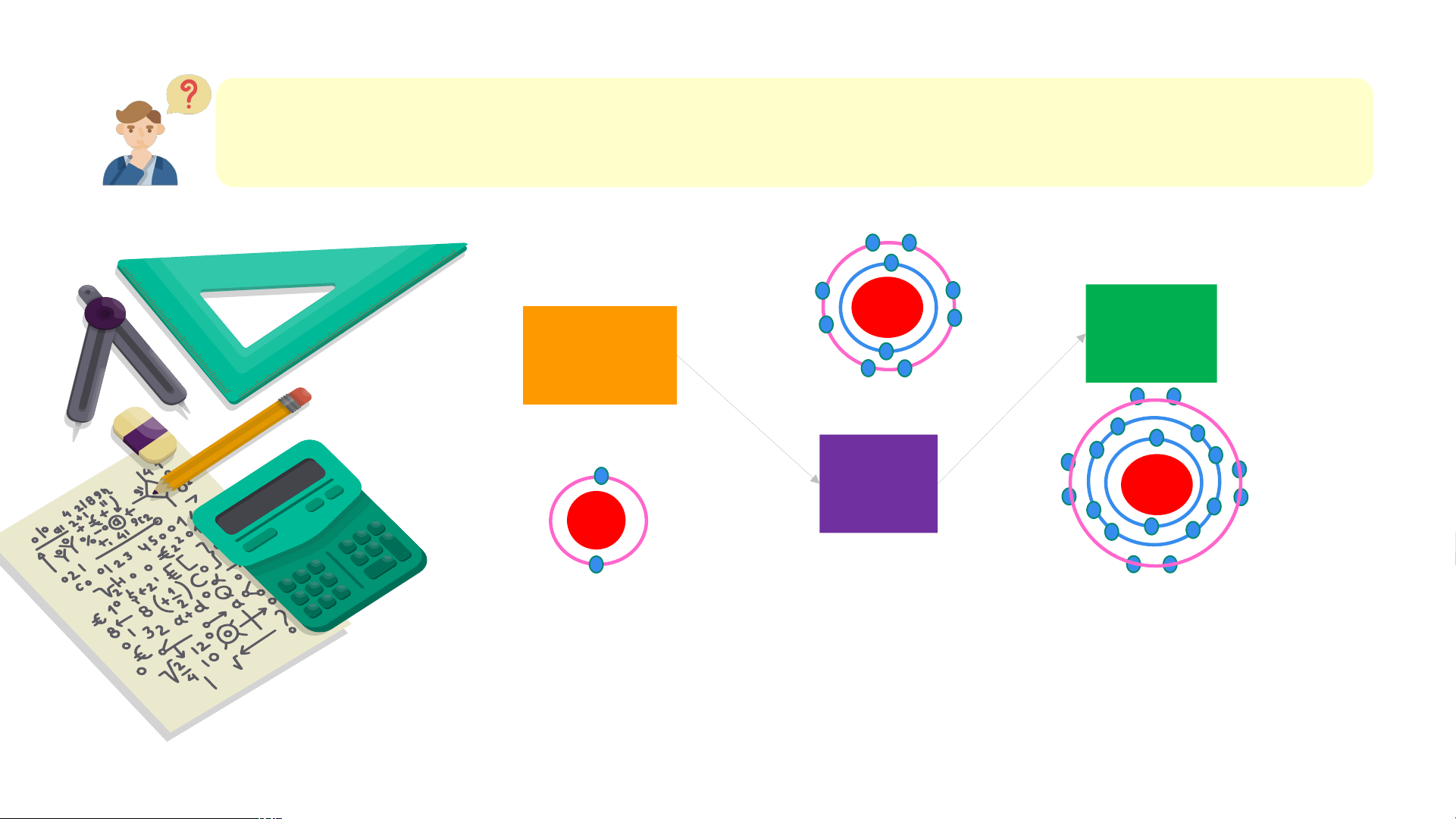
Quan sát mô hình hạt đại diện các chất ở điều kiện thường, trả lời câu hỏi: theo a, b, c,d
(a) Neon (b)Oxygen (c) Hydrogen (d) Nước
1. Chất .... Là đơn chất hay là hợp chất?
2. Cho biết số lượng nguyên tố tạo thành, số lượng nguyên tử trong các
hạt tương ứng mỗi chất. Bài 6: LIÊN KẾT HÓA HỌC NỘI DUNG BÀI HỌC
I. Cấu trúc electron bền
vững của khí hiếm II. Liên kết ion
III. Liên kết cộng hóa trị I. CẤU TRÚC ELECTRON
BỀN VỮNG CỦA KHÍ HIẾM Tại sao các nguyên tử khác luôn kết hợp với nhau? Ne
Trong tự nhiên, chỉ có các khí hiếm tồn tại
ở dạng đơn nguyên tử bền vững, còn O O
nguyên tử của các nguyên tố khác thường
có xu hướng kết hợp với nhau bằng các Na+ Cl-
liên kết hóa học. Các liên kết hóa học
được hình thành như thế nào? Tại sao khí hiếm như neon chỉ tồn tại độc lập?
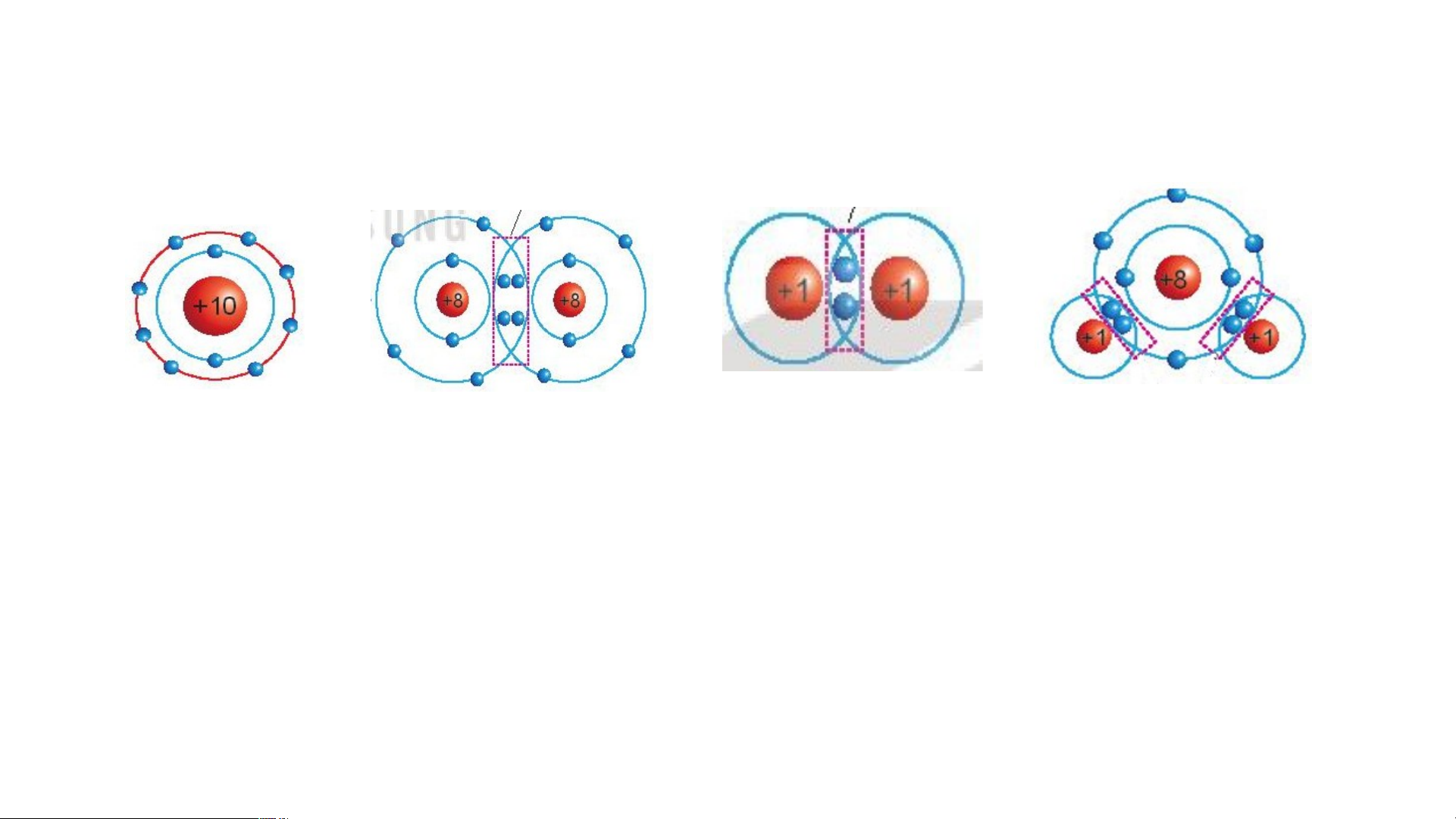
Quan sát Hình 6.1, so sánh số electron
lớp ngoài cùng của He, Ne và Ar. Nhận xét
chung về số e lớp ngoài cùng của khí hiếm (Thảo luận nhóm 5 phút) N + e 10 c. Ar a. He b. Ne A+r 18 +2
Hình 6.1. Mô hình sắp xếp e trong vỏ nguyên tử khí hiếm Lời giải
- Nguyên tử He có 2 electron ở lớp vỏ ngoài cùng
- Nguyên tử Ne có 8 electron ở lớp vỏ ngoài cùng
- Nguyên tử Ar có 8 electron ở lớp vỏ ngoài cùng
⇒ Nguyên tố He có số electron ở lớp vỏ ngoài cùng ít hơn
(Chỉ có 2 electron). Nguyên tố Ne và Ar có số electron ở lớp
vỏ ngoài cùng bằng nhau (đều bằng 8)
- Nguyên tử khí hiếm có lớp electron ngoài cùng bền vững,
khó bị biến đổi hóa học.
- Nguyên tử của các nguyên tố khác có thể đạt được lớp
electron ngoài cùng của khí hiếm bằng cách tạo thành liên kết hoá học. LUYỆN TẬP PHIẾU HỌC TẬP SỐ 2
1/ Nêu tên và kí hiệu hóa học của một số nguyên tố khí hiếm.
2/ Các nguyên tử khí hiếm có mấy lớp electron? So
sánh số electron lớp ngoài cùng của các nguyên tử khí hiếm? VẬN DỤNG PHIẾU HỌC TẬP SỐ 2
3/ Giải thích vì sao các khí hiếm tồn tại dưới dạng đơn nguyên tử bền vững?
Document Outline
- PHẦN KHỞI ĐỘNG
- Slide 2
- Slide 3
- Slide 4
- Slide 5
- Slide 6
- Slide 7
- Slide 8
- Slide 9
- Slide 10
- Slide 11
- Slide 12
- Slide 13
- Slide 14
- Slide 15
- Slide 16
- Slide 17
- Slide 18
- Slide 19
- Slide 20
- Slide 21




