











Preview text:
VIETNAM NATIONAL UNIVERSITY – HO CHI MINH CITY
INTERNATIONAL UNIVERSITY
In vitro and in vivo investigation of antiurolithiatic activity of Ensete glaucum
(Roxb.) Cheesman seeds extract A thesis submitted to
The School of Biotechnology, International University
In partial fulfillment of the requirements for the degree of B.S. in Applied Biochemistry
Student name: Nguyen Thi Ngoc Linh – BTBCIU17041
Supervisor: Dr. Le Van Minh September 2022 ACKNOWLEGMENT
First of all, I would like to thank to the Research Center of Ginseng and Medicinal Materials Ho Chi
Minh City for the opportunities and accommodations given.
Especially, I would express my sincere gratitude Dr. Le Van Minh, who has been an inspirational teacher,
mentor, and thesis supervisor. I am so proud of being his student. He always guided me so positively
and made me feel more confident in my abilities.
Secondly, I would like to extend my deep thanks to my site-supervisor MSc. Ly Hai Trieu for the
continuous support, for the patience and motivation, he helped me a lot during my thesis time. He was
enthusiasm to guide me how to conduct my project properly.
Besides, I would like to say thank you to Ms. Le Thi Kim Oanh, who was an important person in my
project, she was very kind to teach me everything in lab, especially with the practical section. My project
could not be finished without the help of them. Their guidance helped me all the time of researching and writing of this report.
It was impossible to extend enough thanks to my family, my friends, my lab-mate, who gave me the
encouragement I needed throughout this process. CONTENT
Abstract............................................................................................1
1. Introduction.................................................................................2
2. Materials and methods..................................................................3
2.1 Research object and location..........................................3
2.2 Experimental design......................................................3 2.2.1
Plant material – Aqueous extract......................................3 2.2.2
Chemicals and equipment................................................3 2.2.3
In vitro test investigating the inhibition of CaOx
crystallization and aggregation.................................................4 2.2.3.1
Nucleation assay......................................................4 2.2.3.2
Aggregation assay...................................................4 2.2.4
In vivo test by animal model of sodium glyoxylate
induced urolithiasis in mice......................................................4 2.2.4.1
Animals...................................................................4 2.2.4.2
Urolithiasis mice induced by using sodium
glyoxylate..........................................................................5 2.3 Data
analysis.................................................................6
3. Results.........................................................................................7
3.1 Aqueous extract inhibited the formation of calciumoxalate
crystals...................................................................7
3.2 Aqueous extract inhibited the aggregation of calciumoxalate
crystals.................................................................10
3.3 Effect of E. glaucum seed aqueous extract on animal model sodium glyoxylate induced in
mice...........................13
4. Discussion..................................................................................20
5. Conclusion..................................................................................22
6. Reference...................................................................................22
7. Appendix....................................................................................24 LIST OF ABBREATION ABBREVIATION EXPLANATION AE Aqueous extract CaOx Calcium oxalate COD Calcium oxalate dihydrate COM Calcium oxalate monohydrate E. glaucum Ensete glaucum ESWL
Extracorporeal shock wave lithotripsy HPF High power field Ig Intragastric administration Ip Intraperitoneal injection NaGOx Sodium glyoxylate S.E.M Standard error of the mean URS Ureteroscopy LIST OF TABLES
Table 1: Experimental group design on urolithiasis mouse model induced by sodium
glyoxylate.................................................................................................6
Table 2: Effect of E. glaucum seed aqueous extract on the biochemical parameter of mouse serum at
day 7...................................................................14
Table 3: Effect of E. glaucum seed aqueous extract on the biochemical parameter of mouse urine at
day 7.....................................................................15
Table 4: Effect of E. glaucum seed aqueous extract on the biochemical parameter of mouse serum at
day 14.................................................................16
Table 5: Effect of E. glaucum seed aqueous extract on the biochemical parameter of mouse urine at
day 14...................................................................17 LIST OF FIGURES
Figure 1: Effect of aqueous extract and Cystone in reducing crystal size............7
Figure 2: The percentage of crystals size reduction according to each concentration of tested sample
compared to its control........................................8 Figure 3: Effect of aqueous extract on CaOx
crystallization.................................9
Figure 4: Effect of Cystone on CaOx crystallization............................................10
Figure 5: Effect of aqueous extract and Cystone in inhibiting crystal
aggregation.........................................................................................................11
Figure 6: The percentage of crystal aggregation reduction according to each concentration of tested
sample compared to its control......................................11 Figure 7: Effect of aqueous extract on crystal
aggregation...............................12
Figure 8: Effect of Cystone on crystal aggregation............................................13
Figure 9: Total volume of drinking water and excreted urine of mice at day 7 (A) and day 14
(B).....................................................................................................18 Figure 10: Photomicrograph of kidney tissue section with original 100X
magnification......................................................................................................20 Figure 11: Body
weight of mice at day 7 and day 14.........................................24 In vitro and in vivo investigation of
anti-urolithiatic activity of Ensete glaucum (Roxb.) Cheesman seeds extract
Nguyen Thi Ngoc LinhLinh N.T. Nguyena, Le Van Minh Minh V.Leb a
School of Biotechnology, International University – Vietnam National Universityin HCMC b
Research Center of Ginseng and Medicinal Materials Ho Chi Minh city – NationalInstitute of Medicinal Materials Abstract
Ensete glaucum (Roxb.) Cheesman, is known as one of traditional herbs, commonly used for the
treatment of kidney stone related problems in folk medicine. This study investigated the anti-
urolithiatic activity of E. glaucum seeds aqueous extract through in vitro nucleation and aggregation
assays as well as urolithiasis mouse model induced by sodium glyoxylate. The aqueous extract of E.
glaucum seeds at concentration 1.25 mg/mL showed prominent inhibition of the initial phase
nucleation by reducing the crystal size down to 6.19 µm2 compared to 13.28 µm2 of control crystal, also
the extract could transform the crystal morphology from COM to COD properly. Besides, the extract
reduced the number of crystals aggregations as the concentration increased. In vivo results showed
that serum parameters consist of uric acid, creatinine, phosphorous, and urea at both day 7 and day
14 were significantly lower in pathological mice received the extract dose 400 mg/kg compared to
pathological mice. Urolithiasis mice received the extract 400 mg/kg had low concentration of urine
phosphorous, and urine calcium, while the level of magnesium was high at day 7 and 14.
Histopathological examination showed that mice received dose 200 mg/kg did not improve the
inflammatory cells and there was some calcification in the kidney. While dose 400 mg/kg administrated
to mice for 14 days got a significant improvement to the kidney cell. These outcomes showed the
efficiency of the aqueous extract of E. glaucum seeds in the prevention and treatment of renal stone
disease. However, more research needed to be performed to clearly demonstrate the effectiveness of
E. glaucum seeds in the management of urolithiasis disease.
Keywords: Ensete glaucum seeds, aqueous extract, urolithiasis, nucleation, aggregation, sodium glyoxylate. 1. Introduction
Urolithiasis is the most common urologic diseases in Vietnam. It is varying according to ages and
genders, but it occurs more frequently in men than in women within the age of 20–49 years who are
mostly in the working-age (Edvardsson et al., 2013). Nowadays, the diseases prevalence is rising
significantly because of a very complex etiology, it is a multifactorial process involving intrinsic factors
(age, sex, heredity) and extrinsic factors (food intake) (Alelign & Petros, 2018). This growing trend is
believed to be associated with changes in lifestyle modifications, specifically for the extrinsic factor,
there are some common risk factors for stone formation include lower dietary intake of vegetables or
fruit, higher consumption of animal proteins, high oxalate intake (found in foods such as beans,
spinach) high salt intake, and inadequate fluid intake (Thakore & Liang, 2022). Urolithiasis is a big
challenge for doctors and medical industry, because it is not only incidence but also the lifetime
recurrent is at high rate (Afsar et al., 2016). The symptoms of urologic stone are related to their location
whether it is in the kidney, ureter, or urinary bladder. Initially, stone formation does not cause any
symptom. Later, signs and symptoms of the stone disease consist of intense cramping pain, pain in the
back side, hematuria, urinary tract infections, blockage of urine flow, and hydronephrosis (Alelign & Petros, 2018).
Stones is various in size, shape, and chemical compositions. According to the chemical composition,
urologic stones are classified into four main groups which include calcium stone, struvite stone, uric
acid stone, and cystine stone (Alelign & Petros, 2018). Among these four types, calcium stone is the
most predominant, it is further categorized into two small groups that are calcium oxalate and calcium
phosphate. Calcium oxalate (CaOx) is found in the majority of kidney stones and exists in the form of
calcium oxalate monohydrate (COM, CaC2O4·H2O), and calcium oxalate dihydrate (COD, CaC2O4·2H2O)
(Alelign & Petros, 2018). In clinical urolithiasis, COM is more frequently observed than COD. However,
COM crystals are the most important factors contribute to the urologic stone formation. Because COM
crystals are the most thermodynamically stable stones, and they have a greatest adsorptive capability,
therefore it can bind to macromolecules like proteins, glycoproteins on renal tubular epithelial cell
surfaces of urinary tract. Additionally, individual COM crystals can aggregate to create the
agglomeration, hence COM plays the most important role in kidney stone formation. So, in this study,
the research about a management of COM crystals by nucleation assay and aggregation assay is an
ideal solution to prevent kidney stone incidence and recurrent.
Recently, synthetic drugs and some interventional procedures such as extracorporeal shock wave
lithotripsy (ESWL), and ureteroscopy (URS) have been utilized to treat urolithiasis. Nonetheless, there
are no effective medicines to use in clinical therapy properly because stone removal cannot be
completed, or stone recurrence is still a possibility. Besides, exposure to shock waves in therapeutic
doses lead to acute renal damage, renal impairment, a reduction in renal functions, and an increase in
stone recurrence (Butterweck & Khan, 2009). Due to many side effects of urologic stone treatment,
phytotherapeutic agents could be useful as either an alternative or an adjunctive therapy in the
management of urolithiasis. Vietnam has a great source of medicinal plants, and our country also have
a long history of using these herbs as treatments for some diseases. Medicinal plants are regarded as
an acceptable, cheap, easily available, and safe source of active compounds for pharmaceutical (Bahmani et al., 2016).
Ensete glaucum (Roxb.) Cheesman, also known as Snow Banana belongs to the Musaceae family, it
widely distributed in Phuoc Binh National Park (Ninh Thuan province) and Bu Gia Map National Park
(Binh Phuoc province) (Ly et al., 2022). Snow Banana has been commonly used as traditional medicine
for many years ago in Asia. Specifically, in Vietnam, people use all part of the tree as fresh ingredients,
dried materials like leaves, or seeds used as phytotherapeutic medicine to treat various disease like
diuretic, kidney stone, diabetes. In traditional medicine of Vietnam, the therapeutic seeds are mainly
used in the treatment of urinary disorders like urolithiasis. There are several studies of various species
in the same genus show anti-urolithiatic activity. For instance, Ensete superburn (Roxb) Cheesman (N.
K. Sethiya et al., 2017), Musa acuminate (Abu Zarin et al., 2020a; Umamaheswari et al., 2017) , Musa
balbisiana (Abu Zarin et al., 2020b) has been proven its efficiency in inhibit the formation of calcium
oxalate crystallization. The Research Center of Ginseng and Medicinal Materials Ho Chi Minh City has
been screening phytochemical of E. glaucum and analyzing these chemical components, the result
shows that E. glaucum seeds contain various main compounds such as flavonoid, tannin, saponin (Ly
et al., 2022). E. glaucum is a potential medicinal plant, which needs to be further exploited and
evaluated for its effect in management of urolithiasis.
In Vietnam until now, the use of E.glaucum seeds in the treatment of urinary stones is through
experience and word of mouth, the anti-urolithiatic activity has not been proven effectively by scientific
experiments. Therefore, this study is performed to evaluate the potential of aqueous extract in the
management of urolithiasis and prevention of kidney stones. This project is going to provide more
scientific evidence about anti-urolithiatic actitvity of Ensete glaucum (Roxb.) Cheesman seeds aqueous
extract. Moreover, this plant extract can be further developed as pharmaceutical medicines to support
the patient during and after the treatment of urolithiasis.
2. Materials and methods 2.1
Research object and location
The aim of this research is investigating the ability of E. glaucum seeds aqueous extract to inhibit
calcium oxalate crystallization and aggregation, because these two processes are very crucial in the
kidney stone formation. Besides, evaluating potential in anti-urolithiatic activity carrying out the animal
model sodium glyoxylate – induced in mice. Furthermore, the research is going to provide more
scientific evidence about E. glaucum (Roxb.) Cheesman in the role as antiurolithiasis plant material and
opens a new research direction on natural medicinal herbs.
All the experiments were performed at the Research Center of Ginseng and Medicinal Materials in Ho Chi Minh City. 2.2 Experimental design
2.2.1 Plant material – Aqueous extract
The sample aqueous extract was provided by the Research Center of Ginseng and Medicinal Materials
in Ho Chi Minh City. Aqueous extract was collected by using decoction method.
2.2.2 Chemicals and equipment
The main chemicals used in the experiment was tris-buffer saline pH 7.4, sodium oxalate, calcium
chloride, sodium glyoxylate monohydrate (Sigma Co. Ltd, USA), and Cystone (Himalaya – Apollo Pharmacy, USA).
The main equipment used in the experiment was Nikon inverted microscope ECLIPSE Ts2 (Nikon, Tokyo,
Japan), water bath (DK-8D, China).
2.2.3 In vitro test investigating the inhibition of CaOx crystallization and aggregation
2.2.3.1 Nucleation assay
Firstly, the solution of calcium chloride 10 mM and sodium oxalate 1 mM was prepared in crystallization
buffer which containing 10 mM Tris–HCl and 90 mM NaCl (pH 7.4). The experiment was done in
triplicate in 24 well-plate. As described briefly, 190 µL of 10 mM CaCl2 was added into 24 well-plate
before adding 20 µL of tested extract with various concentrations (5, 2.5, 1.25, 0.625, 0.3125 mg/mL).
Cystone played roles as positive control group, it was dissolved in distilled water to give concentrations
of 5, 2.5, 1.25, 0.625, 0.3125 mg/mL. Besides, for the control of each time, an equal volume of the
basic buffer was added into the well. To induce the crystallization reaction, 190 µL of 1 mM Na2C2O4
was added into each well. Thereafter, the mixture was incubated at 37 °C in water bath for 30 minutes
(Chaiyarit & Thongboonkerd, 2021). Crystal images were captured randomly from 9 high-power fields
(HPFs) with 400X magnification under Nikon inverted phase-contrast light microscope ECLIPSE Ts2
(Nikon; Tokyo, Japan). Crystal sizes were measured using NIS Element D software (Nikon).
2.2.3.2 Aggregation assay
Firstly, individual COM crystals were prepared by mixing calcium chloride 10 mM and sodium oxalate
1 mM in buffer with ratio 1:1 (v/v). Then the solution was incubated at 25 °C for at least one hour. The
supernatant was discarded by a centrifugation, whereas COM crystals were harvested and washed
three times with methanol. After another centrifugation, methanol was discarded, and the crystals
were air dried by evaporation overnight at room temperature. The COM crystals are resuspended in
Tris-buffer saline at a concentration of 800 µg/mL. Briefly, 150 µL of COM crystal solution was added
to 50 µL of extract in different concentrations (5, 2.5, 1.25, 0.625, 0.3125 mg/mL). As the same
procedure for positive control Cystone (5, 2.5, 1.25, 0.625, 0.3125 mg/mL). Besides, for the control of
each time, an equal volume of the basic buffer was added into the well. Then the plate was
continuously shaken on a rotary shaking machine at 25 °C for one hour (Chaiyarit & Thongboonkerd,
2021; Kanlaya et al., 2019). Thereafter, observing the images about the formation of CaOx crystals
aggregations under the Nikon inverted phase-contrast light microscope ECLIPSE Ts2. Number of CaOx
crystal aggregates were counted from 3 randomized HPFs per well. The experiment was done in triplicate in 96 well-plate.
2.2.4 In vivo test by animal model of sodium glyoxylate induced urolithiasis in mice 2.2.4.1 Animals
The mature Swiss-albino mice aged 4 to 5 weeks, weighing from 20 ± 2 g were used in the animal model
of anti-urolithiasis. All mice were purchased from the Institute of Vaccine and Medical Biologicals in
Nha Trang City. They were housed at the department facility and acclimatized to the laboratory
environment under standard conditions at 25 ± 2 oC with relative humidity at 50 – 70%, and 12-hour
light/dark cycle for a week before carrying out the experiments. The mice were kept in polypropylene
cages with paddy husk bedding maintained in hygienic condition. Besides, all healthy mice were fed
with tablets of food provided by the Institute of Vaccine and Medical Biologicals in Nha Trang City and
had free access to drinking water. The animal studies followed the recommendations of “Guide for
Care and Use of Laboratory Animals” and Decision 141/QĐ-K2ĐT on October 27th, 2015, of the Ministry of Health.
2.2.4.2 Urolithiasis mice induced by using sodium glyoxylate
The animal model was carried out within 14 days. After conditioned housing for one week, 56 mice
were equally divided into 7 groups of 8 mice. To induce the renal calculi CaOx, sodium glyoxylate (NaGOx,
100 mg/kg) was administered by successive intraperitoneal injections for 7 consecutive days (from day
1 to day 7). While for the normal group, saline NaCl 0.9% was injected intraperitoneally from day 1 to
day 7. For groups that received the treatment with aqueous extract (AE) would drink the extract for 14
days, while Cystone only drank from day 7 to day 14.
Table 1: Experimental groups design on urolithiasis mouse model induced by sodium glyoxylate Groups Name Treatment
ip (intraperitoneal injection) of saline NaCl 0.9% I Normal mice
ig (intragastric administration) of distilled water ip of NaGOx II Pathological mice ig of distilled water Normal mice ip of saline NaCl 0.9% III
received the extract ig of extract – dose 200 mg/kg (day 1-14) dose 1 Normal mice ip of saline NaCl 0.9% IV
received the extract ig of extract – dose 400 mg/kg (day 1-14) dose 2 Pathological mice V
received the extract ip of NaGOx ig of extract – dose 200 mg/kg (day 1-14) dose 1 Pathological mice VI
received the extract ip of NaGOx ig of extract – dose 400 mg/kg (day 1-14) dose 2 Pathological mice ip of NaGOx VII received Cystone
ig of Cystone – dose 750 mg/kg (day 7-14)
Evaluation criteria - Mice body weight
- Analysis phosphate, calcium, magnesium contents in urine (Urine was collected after 24-hour at day 7 and 14).
- Analysis phosphate, calcium, urea, and uric acid in blood (Blood was collected at day 7 and day 14).
- Kidney histopathological evaluation. ● Serum analysis
On the 7th and 14th day, animal blood was withdrawn by gently milking the tail from each mouse. For
evaluation of serum parameter, serum was separated by centrifugation machine at 10,000× g for 10
min and analyzed for the content of creatinine, urea nitrogen, uric acid, calcium, and phosphate by biochemical kits. ● Urine analysis
On the 7th and 14th day, animals were kept in individual metabolic cages and 24h urine samples were
collected. Animals had free access to drinking water during the urine collection period. The urine
samples were analyzed for level of phosphate, calcium, magnesium with the help of diagnostic
biochemical kits. ● Histopathological analysis
On the 15th day, all alive mice were sacrificed. The abdomen will be cut open to remove both kidneys
from each animal and isolated kidneys were cleaned from extraneous tissues and rinsed with room
temperature physiological saline. Both kidneys were further fixed in 10% neutral buffer formalin
solution (pH 7.4) for histopathological analyses. Kidney tissue section of 5 µm was stained with
hematoxylin and eosin for evaluation of histopathological changes and calcium oxalate deposits. The
kidney specimens were observed under a light microscope and captured the images of tissues with an optical x100 magnification. 2.3 Data analysis
The data were expressed in terms of Mean ± SEM (standard error of the mean). The Graphpad Prism
software version 9.3.1 (Inc., La Jolla, CA, USA) and Microsoft Excel would be used for analyzing data
by applying t-test and ANOVA one-way with pvalue < 0.05 applied for significant level.
3. Results 3.1 Aqueous extract inhibited the formation of calcium oxalate crystals
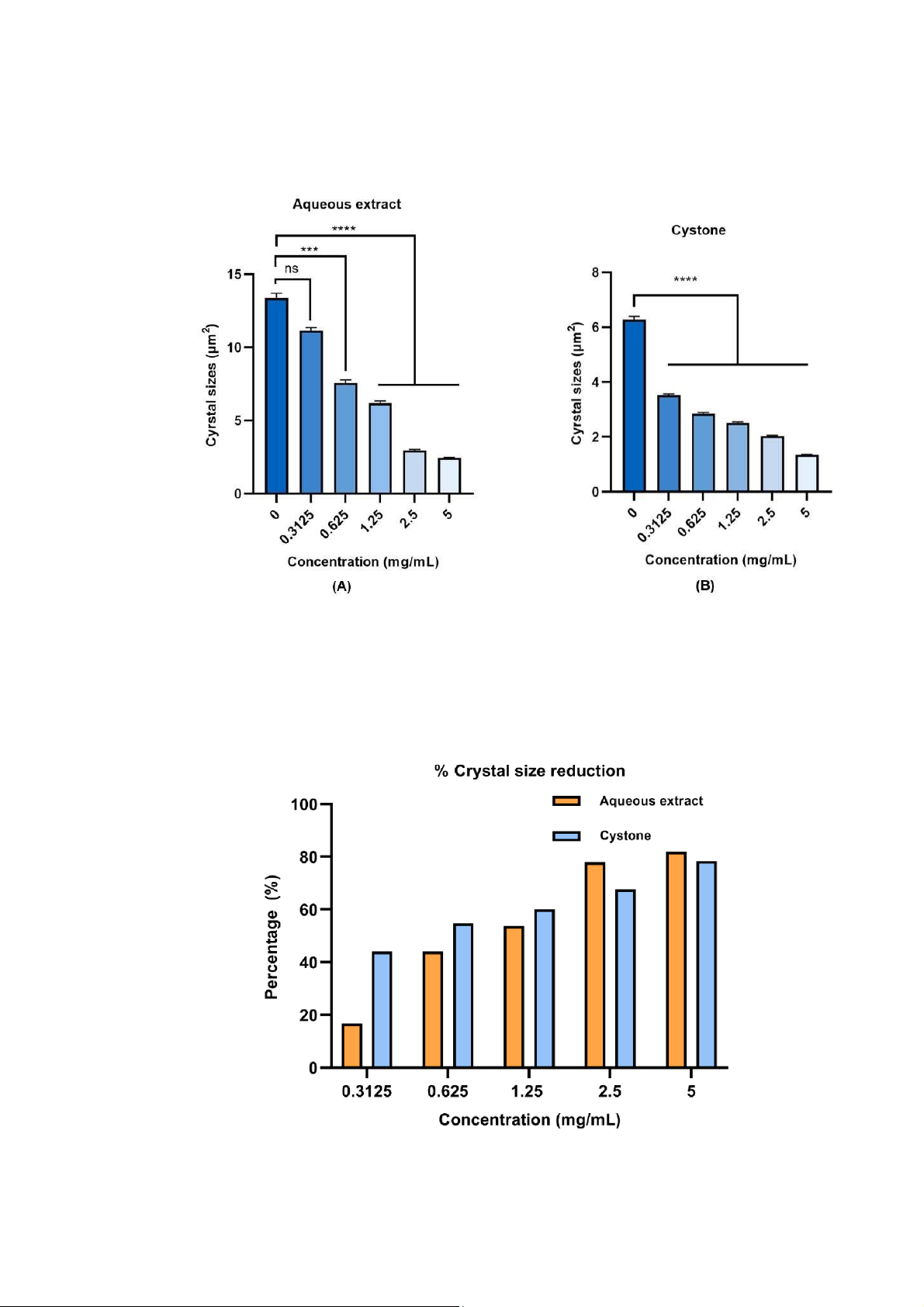
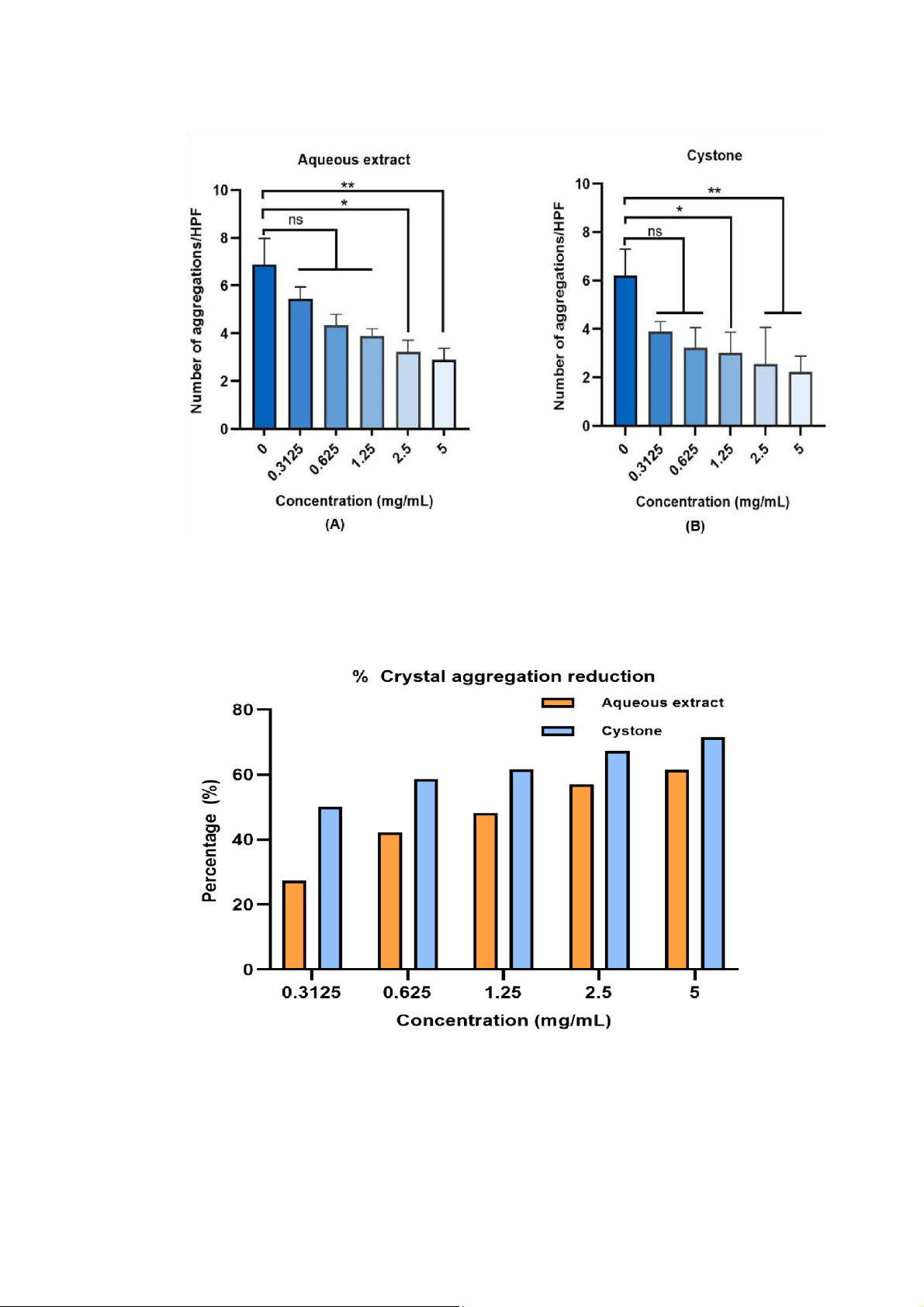
Figure 1: Effect of aqueous extract and Cystone in reducing crystal size. Crystal number was counted
from 9 HPFs and crystal size was measured by NIS Element D software (Nikon) from at least 20 crystals
from each HPFs. Each bar represents mean ± SEM of the data derived from 3 independent experiments.
Data was analyzed by one way analysis of variance test (ANOVA) with nsp > 0.05 no significant
difference; ***p < 0.001 and ****p < 0.0001 significant difference.
Figure 2: The percentage of crystals size reduction according to each concentration of tested sample
compared to its control.
The average crystals size of the extract control (0 mg/mL) (Figure 1A) was 13.28 μm2, while at the
concentration 1.25 mg/mL, the crystal size was 6.19 μm2. This value was significantly decreased up to
50 percent (Figure 2). In the other hand, control Cystone had the average crystal (Figure 1B) was 6.28
μm2, by comparing that value with 2.51 μm2 crystal size of Cystone at concentration of 1.25 mg/mL. As
the result from the Figure 2, Cystone got ability to reduce the crystal size up to 60 percent at the
concertation 1.25 mg/mL, this number was quite greater compared to the extract. The results indicated
that aqueous extract and Cystone had ability to reduce crystal sizes according to concentration increased.
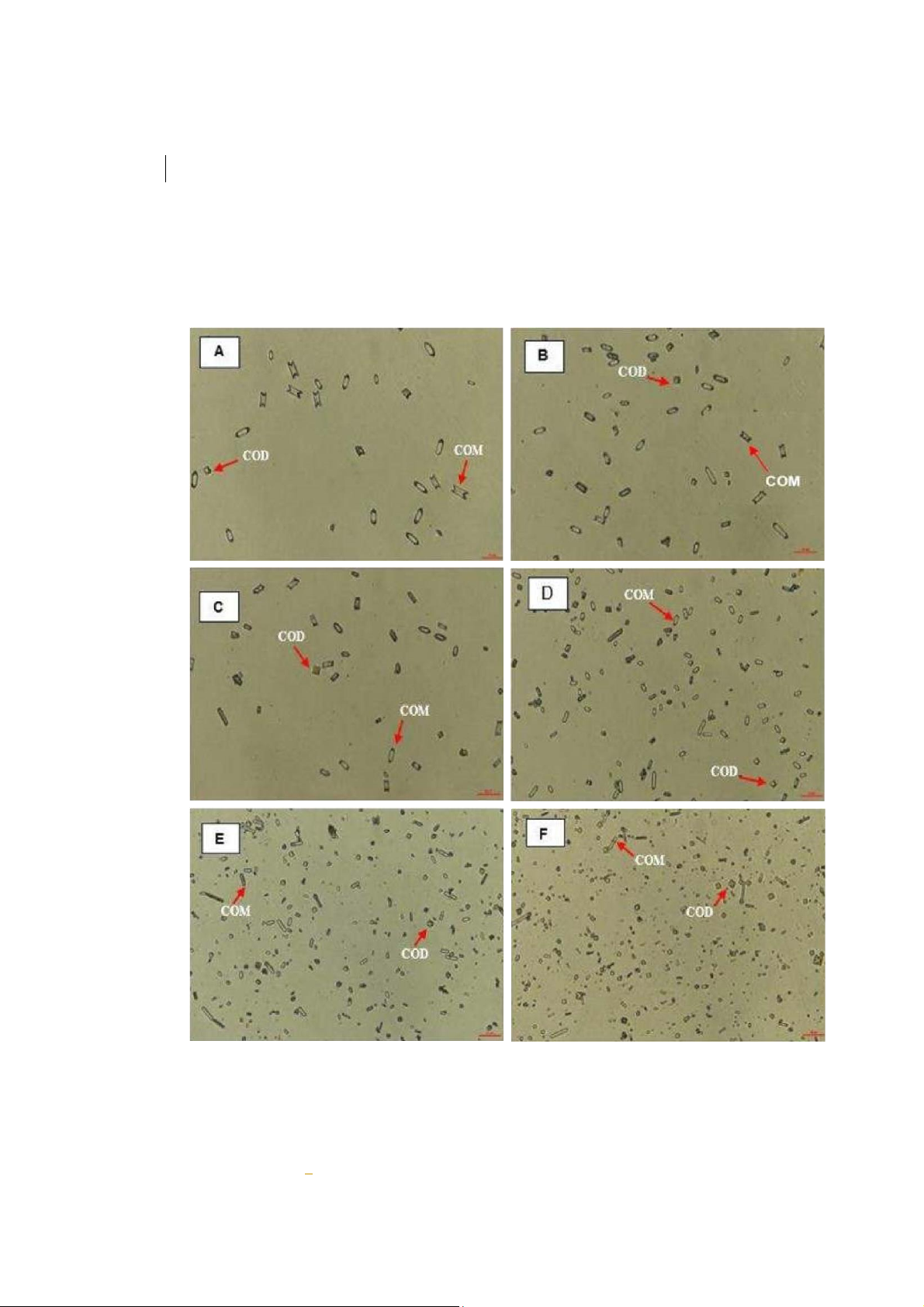
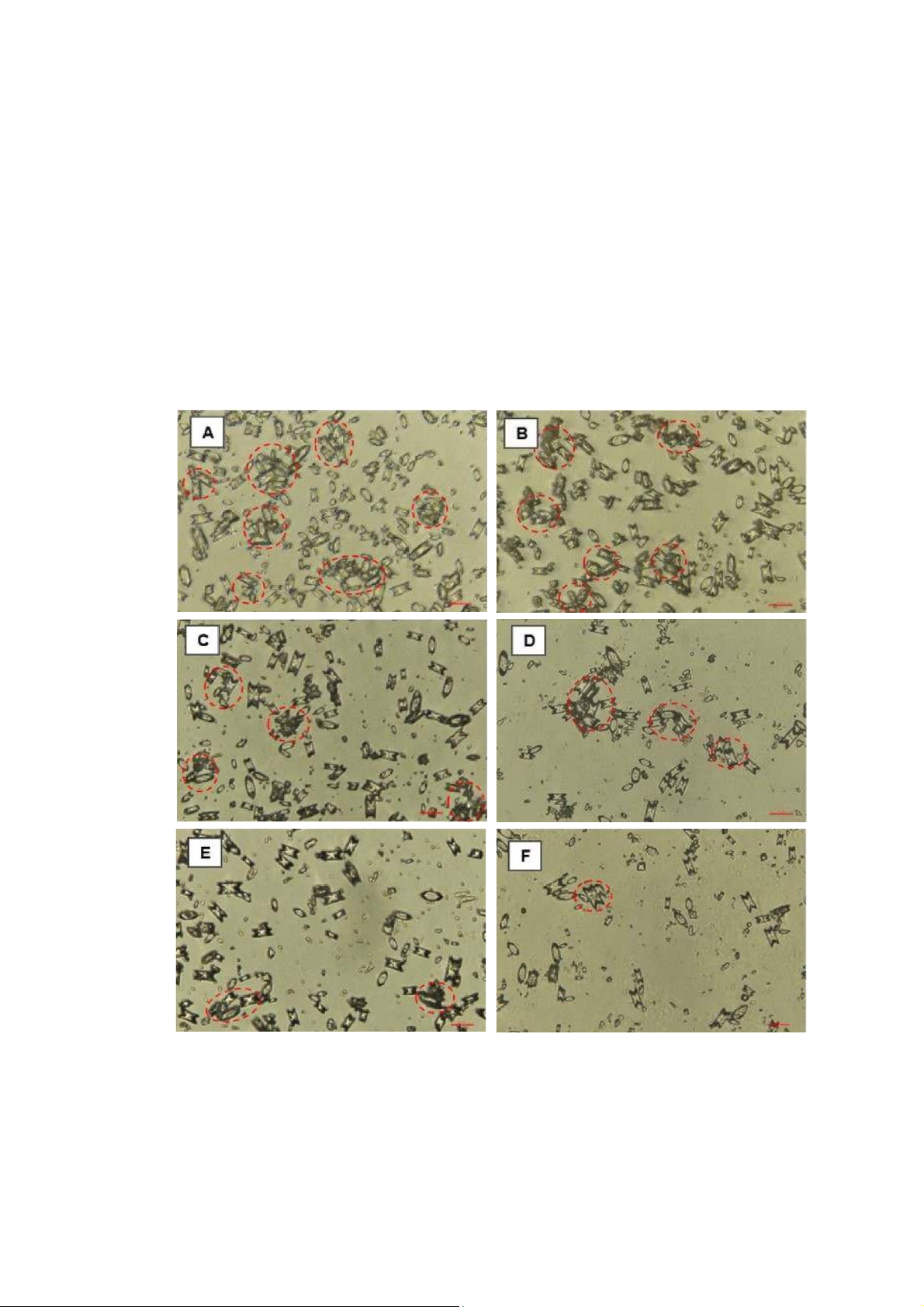
Figure 3: Effect of aqueous extract on CaOx crystallization. A (Negative control, 0 mg/mL); B (0.3125
mg/mL); C (0.625 mg/mL); D (1.25 mg/mL); E (2.5 mg/mL); F (5 mg/mL). COM (Calcium oxalate
monohydrate) and COD (Calcium oxalate dihydrate) crystals were indicated by a red arrow. COM crystal
had a hexagonal lozenge elongated shape, while the COD crystal got a square envelope shape.
The scale bar was 100 µm for all captured images.COM
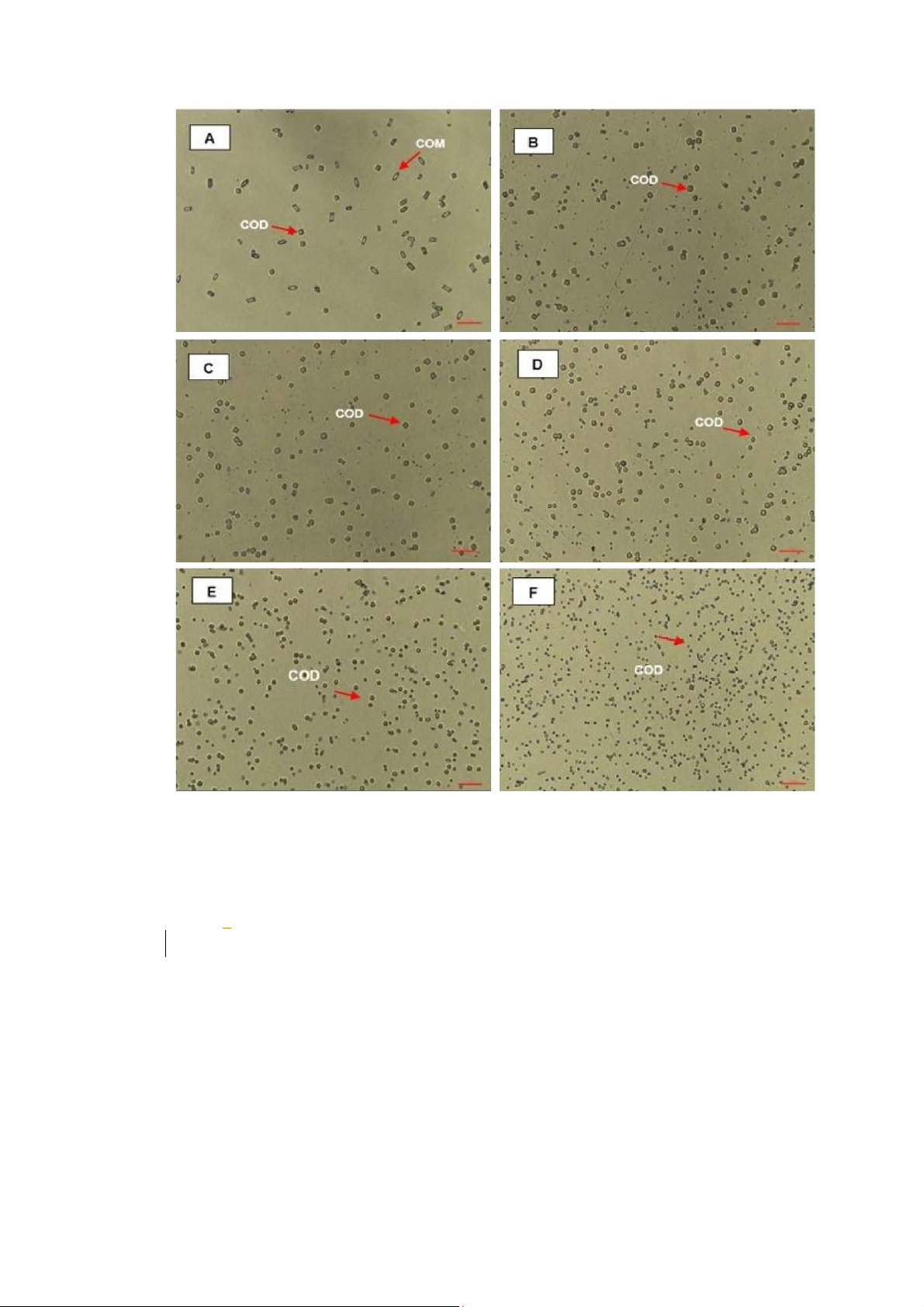
Figure 4: Effect of Cystone on CaOx crystallization. A (Negative control, 0 mg/mL); B (0.3125 mg/mL);
C (0.625 mg/mL); D (1.25 mg/mL); E (25 mg/mL); F (5 mg/mL). COM (Calcium oxalate monohydrate)
and COD (Calcium oxalate dihydrate) crystals was indicated by a red arrow. COM crystal had a
hexagonal lozenge elongated shape, while the COD crystal got a square envelope shape. The scale bar
was 100 µm for all captured images.
To demonstrate the ability in inhibition of CaOx crystallization, there were two main trends which were
a transformation from COM to COD and crystal sized reduction. Images of crystals were taken under
the microscope at 400X magnification. From the Figure 3 and 4, it was easily to see the trends of
aqueous extract, and Cystone was crystal morphology transformation and crystals area reduction.
3.2 Aqueous extract inhibited the aggregation of calcium oxalate crystals
Figure 5: Effect of aqueous extract and Cystone in inhibiting crystal aggregation. The number of
aggregations was counted from 9 HPFs manually. Each bar represented mean ± SEM of the data derived
from 3 independent experiments. Data was analyzed by one way analysis of variance test (ANOVA)
with nsp >0.05 no significant difference; *p < 0.05 and **p < 0.01 significant difference.
Figure 6: The percentage of crystal aggregation reduction according to each concentration of tested
sample compared to its control.
The control group of the extract (0 mg/mL) got an average number of crystal aggregation was 7.5,
however, it was just 3.2 aggregations at the concentration 2.5 mg/mL (Figure 5A). This value was
declined remarkably to 57 percent (Figure 6). The extract at the concentration 0.3125, 0.625, and 1.25
still had ability to inhibit crystal to adhere together, but these number was non-significant compared
to control. When the concentration of the extract increased to 2.5 and 5 mg/mL, the results were
statistically significant (Figure 5A). Meanwhile, the control group of Cystone (0 mg/mL) had
approximately 7.8 number of aggregations, by comparing that value with 2.56 crystal aggregation at
concentration of 1.25 mg/mL of Cystone (Figure 5B), it was reducing by 67% (Figure 6). It was showed
that Cystone inhibited crystal to aggregate at both 5 concentrations, but only the concentration at 1.25,
2.5, and 5 mg/mL expressed statistic significant. The results indicated that aqueous extract and Cystone
had ability to inhibit crystal aggregation according to concentration increased.
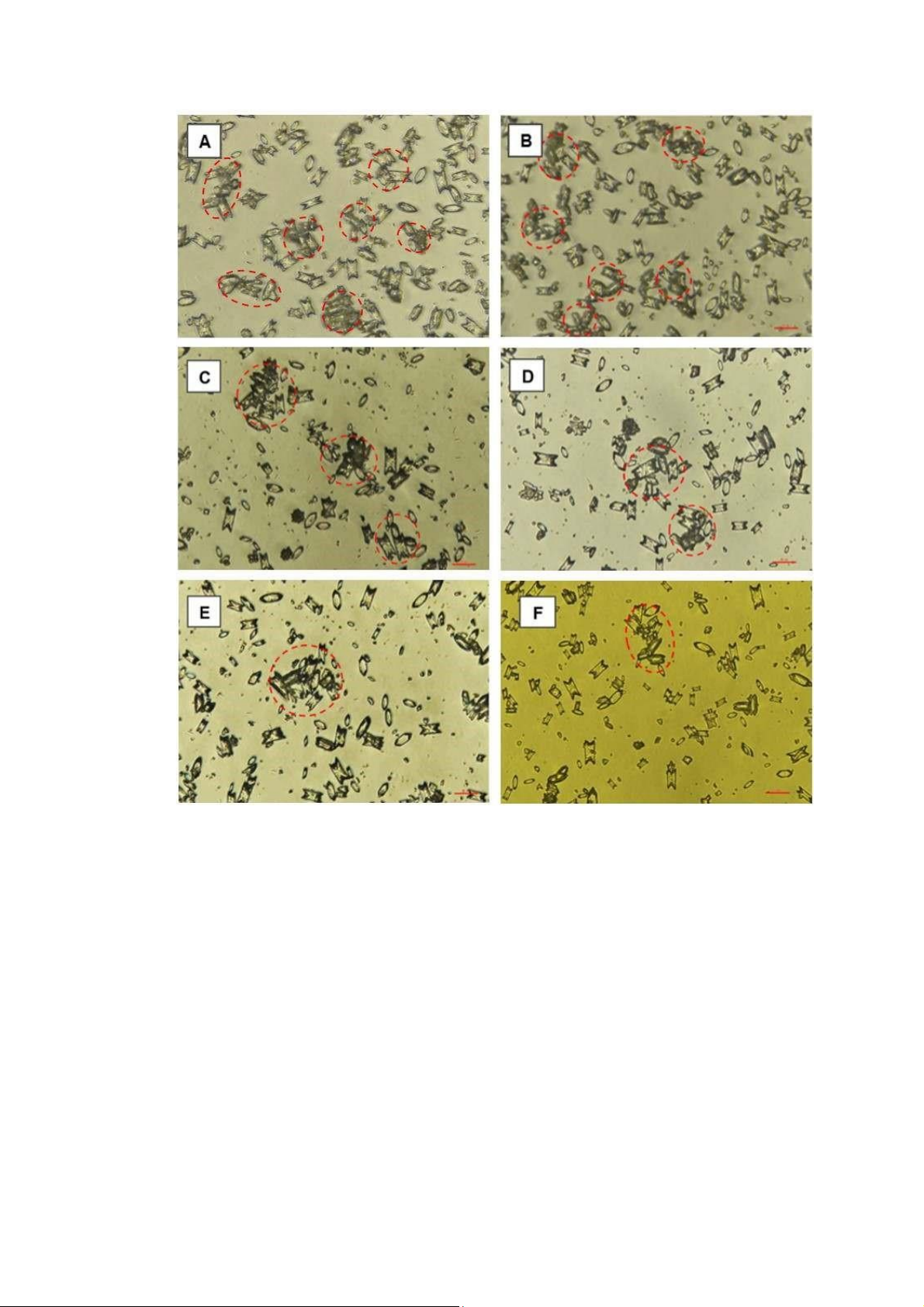
Figure 7: Effect of aqueous extract on crystal aggregation. A (Negative control, 0 mg/mL); B (0.3125
mg/mL); C (0.625 mg/mL); D (1.25 mg/mL); E (2.5 mg/mL); F (5 mg/mL). Aggregated COM crystals
which were derived from three or more individual crystals tightly joined together and it was indicated
by the dashed circle. The scale bar was 100 µm for all captured images.
Figure 8: Effect of Cystone on crystal aggregation. A (Negative Control, 0 mg/mL); B (0.3125 mg/mL);
C (0.625 mg/mL); D (1.25 mg/mL); E (2.5 mg/mL); F (5 mg/mL). Aggregated COM crystals which were
derived from three or more individual crystals tightly joined together and it was indicated by the
dashed circle. The scale bar was 100 µm for all captured images.
Micrographs of the aggregated COM crystals were taken under microscope with original magnification
was 400X (Figure 7 and 8). Both aqueous extract and Cystone worked effectively to prohibit the chance
for crystal to aggregate. This effectiveness was clearer when the concentration increased.
Overall, E. glaucum seed aqueous extract and Cystone showed its effectiveness in inhibition CaOx
crystallization and aggregation. Therefore, E. glaucum seed aqueous extract was a potential plant
material in urolithiasis management, further studies should be carried out.
3.3 Effect of E. glaucum seed aqueous extract on animal model sodium glyoxylate induced in mice
Table 2: Effect of E. glaucum seed aqueous extract on the biochemical parameter of mouse serum at day 7 Phosphoru s Uric acid Calcium Creatinine Grou p (mmol/L) Urea (mg/dL) (mg/dL) (mmol/L) (mg/dL) I 2.43 ± 4.66 ± 0.53 3.62 ± 0.26 51.68 ±3.89 0.39 ± 0.02 0.06 II 2.43 ± 67.63 ± 6.09 4.01 ± 0.29 3.60 ± 0.18 # 0.40 ± 0.02 0.04 III 2.37 ± 4.25 ± 0.49 3.23 ± 0.14 50.84 ± 4.19 0.43 ± 0.02 0.03 IV 2.45 ± 4.64 ± 0.23 3.37 ± 0.14 51.69 ± 3.55 0.36 ± 0.01 0.03 V 2.44 ± 3.75 ± 0.31 3.99 ± 0.27 65.36 ± 4.68 0.43 ± 0.02 0.06 VI 2.35 ± 43.44 ± 3.81 ± 0.32 3.27 ± 0.17 0.35 ± 0.008 0.02 2.88** VII 2.43 ± 3.81 ± 0.25 3.68 ± 0.23 55.07 ± 6.26 0.40 ± 0.03 0.04
All value were expressed as mean ± SEM (n = 8). Data were analyzed by t-test, one-way ANOVA
followed by Dunnette’s multiple comparison tests, # p < 0.05 statistically significant compared to group
I, **p < 0.01 statistically significant compared to group II. Group I: Normal mice; Group II: Pathological
mice; Group III: Normal mice received the extract 200 mg/kg; Group IV: Normal mice received the
extract 400 mg/kg; Group V: Pathological mice received the extract 200 mg/kg; Group VI: Pathological
mice received the extract 400 mg/kg; Group VII:
Pathological mice received Cystone 750 mg/kg.
In this study, mice serum parameters were evaluated after being injected with sodium glyoxylate for 7
consecutive days to induce kidney stone. Pathological mice (Group II) got a lower level of uric acid in
serum compared to normal mice (Group I), and the data was not statistically significant. It showed a
decreasing trend in serum uric acid among groups of pathological mice received the extract dose 200
mg/kg (Group V), 400 mg/kg (Group VI), and Cystone (Group VII) when comparing with pathological
mice (Group II). However, this downward trend was non-significant difference. The results showed that
the injection of sodium glyoxylate did not alter the output of serum calcium in comparison between
pathological mice (Group II) and normal mice (Group I). There was no difference in serum phosphorus
and serum creatinine between normal mice (Group I) and pathological mice (Group II). Nevertheless,
pathological mice received the extract dose 400 mg/kg (Group VI) got a lower value of serum
phosphorus and creatinine compared to pathological mice (Group II), although this slightly reduction
did not reach statistical significance. Intraperitoneal injection of sodium glyoxylate in dose 100 mg/kg
for 7 consecutive days caused a dramatical increase in serum urea of pathological mice (Group II)
compared to normal mice (Group I), and this resulted in statistically significant. The serum urea of
pathological mice received the extract 200 mg/kg (Group V) was not quite different from the
pathological mice (Group II), while pathological group received dose 400 mg/kg (Group VI) and Cystone
(Group VII) got a lower serum urea concentration than pathological mice (Group II). But the extract
400 mg/kg (group VI) gave a remarkable reduction than Cystone (Group VII).
Table 3: Effect of E. glaucum seed aqueous extract on the biochemical parameter of mouse urine at day 7. Phosphorus Magnesium Calcium Group (mmol/24h) (mmol/24h) (mmol/24h) I 13.08 ± 0.18 3.36 ± 0.93 0.66 ± 0.11 II 18.33 ± 4.11 4.74 ± 0.63 0.89 ± 0.12 III 13.14 ± 0.15 3.17 ± 0.80 0.55 ± 0.05 IV 13.60 ± 0.14 # 5.02 ± 0.75 0.62 ± 0.09 V 16.43 ± 3.03 1.32 ± 0.91 * 0.62 ± 0.12 VI 13.35 ± 0.12 3.41 ± 0.95 0.49 ± 0.04 * VII 14.05 ± 0.91 1.59 ± 0.80 * 0.63 ± 0.10
All value were expressed as mean ± SEM (n = 8). Data were analyzed by t-test, one-way ANOVA
followed by Dunnette’s multiple comparison tests. # p < 0.05 statistically significant compared to group
I, * p < 0.05 statistically significant compared to group II. Group I: Normal mice; Group II: Pathological
mice; Group III: Normal mice received the extract 200 mg/kg; Group IV: Normal mice received the
extract 400 mg/kg; Group V: Pathological mice received the extract 200 mg/kg; Group VI: Pathological
mice received the extract 400 mg/kg; Group VII:
Pathological mice received Cystone 750 mg/kg.
Besides, other urine parameter such as phosphorous, magnesium, and calcium also being appraised.
The results showed that the administration of sodium glyoxylate increased the concentration output
of urine phosphorous, magnesium, and calcium in comparison between pathological mice (Group II)
and normal mice (Group I), but these differences were statistical non-significant. The pathological mice
(Group II) had a higher concentration phosphorous in urine than normal mice (Group I), however this
result did not reach statistical significance because of high SEM. All three group pathological mice
received the extract 200 mg/kg, 400 mg/kg, and Cystone (Group V, VI, and VII, respectively) had a lower
value urine phosphorous compared to pathological mice (Group II), but this downward trend was
statistical non-significant. Meanwhile, normal group treated with extract 400 mg/kg was slightly
reduced in urine phosphorous level compared to normal mice (Group I), and this was statistically
significant difference. The urine magnesium level of pathological mice received extract 200 mg/kg
(Group V) and Cystone (Group VII) was reduced dramatically compared to the pathological mice
(Group II). The urine calcium of pathological mice received the extract 200 mg/kg (Group V), 400 mg/kg
(Group VI) and Cystone (Group VII) got a lower concentration than pathological mice (Group II). But
the extract 400 mg/kg (group VI) gave a remarkable reduction in urine calcium parameter than Cystone (Group VII).
Table 4: Effect of E. glaucum seed aqueous extract on the biochemical parameter of mouse serum at day 14 Phosphoru s Creatinin e Uric acid Calcium Urea Grou p (mmol/L) (mg/dL) (mg/dL) (mmol/L) (mg/dL) I 47.01 ± 0.38 ± 4.88 ± 0.35 2.48 ± 0.06 3.38 ± 0.16 2.48 0.02 II 3.75 ± 0.35 50.80 ± 0.40 ± # 2.45 ± 0.04 3.61 ± 0.25 3.88 0.02 III 46.39 ± 0.36 ± 4.73 ± 0.36 2.42 ± 0.04 3.08 ± 0.19 1.82 0.01 IV 57.63 ± 0.41 ± 3.94 ± 0.33 2.32 ± 0.02 # 3.39 ± 0.20 6.42 0.02 V 54.17 ± 0.41 ± 4.63 ± 0.29 2.44 ± 0.02 3.03 ± 0.10 2.11 0.01 VI 42.83 ± 0.37 ± 3.44 ± 0.24 2.29 ± 0.03 ** 3.22 ± 0.29 2.52 0.01 VII 43.02 ± 0.39 ± 3.88 ± 0.31 2.36 ± 0.03 3.38 ± 0.16 3.11 0.02
All value were expressed as mean ± SEM (n=8). Data were analyzed by t-test, one-way ANOVA followed
by Dunnette’s multiple comparison tests. # p < 0.05 statistically significant compared to group I, ** p <
0.01 statistically significant compared to group II. Group I: Normal mice; Group II: Pathological mice;
Group III: Normal mice received the extract 200 mg/kg; Group IV: Normal mice received the extract
400 mg/kg; Group V: Pathological mice received the extract 200 mg/kg; Group VI: Pathological mice
received the extract 400 mg/kg; Group VII:
Pathological mice received Cystone 750 mg/kg.
Pathological mice (Group II) got a lower level of uric acid in serum compared to normal mice (Group I),
and the data was statistically significant. There was an increase in serum uric acid among groups of
pathological mice received the extract dose 200 mg/kg (Group V), and Cystone (Group VII) when
comparing with pathological mice (Group II). However, this upward trend was non-significant
difference. There were only pathological mice received the extract 400 mg/kg reduced the level of uric
acid compared to the pathological mice (Group II). The serum calcium of pathological mice received
the extract 200 mg/kg (Group V) was not quite different from the pathological mice (Group II), while
pathological group received dose 400 mg/kg (Group VI) and Cystone (Group VII) got a lower serum
calcium concentration than pathological mice (Group II). However, the extract 400 mg/kg (group VI)
gave a remarkable reduction than Cystone (Group VII). Besides, normal group treated with extract 400
mg/kg was slightly reduced in serum calcium level compared to normal mice (Group I), and this was
statistically significant difference. The results showed that the injection of sodium glyoxylate caused an
increase level of serum phosphorous, magnesium, and calcium in comparison between pathological
mice (Group II) and normal mice (Group I), but it did not reach statistically significant. The pathological
mice received the extract dose 200 mg/kg (Group V), 400 mg/kg (Group VI) and Cystone (Group VII)
got a lower serum phosphorous and creatinine concentration than pathological mice (Group II), but



