Preview text:
Internaonal University, Vietnam Naonal University - HCMC 1
ANALYTICAL CHEMISTRY LABORATORY EXPERIMENT 4 –
SPECTROPHOTOMETRIC DETECTION OF IRON 1. OBJECTIVES
Familiar with the use of spectrophotometric technique to determine the concentraon of an unknown soluon. 2. INTRODUCTION
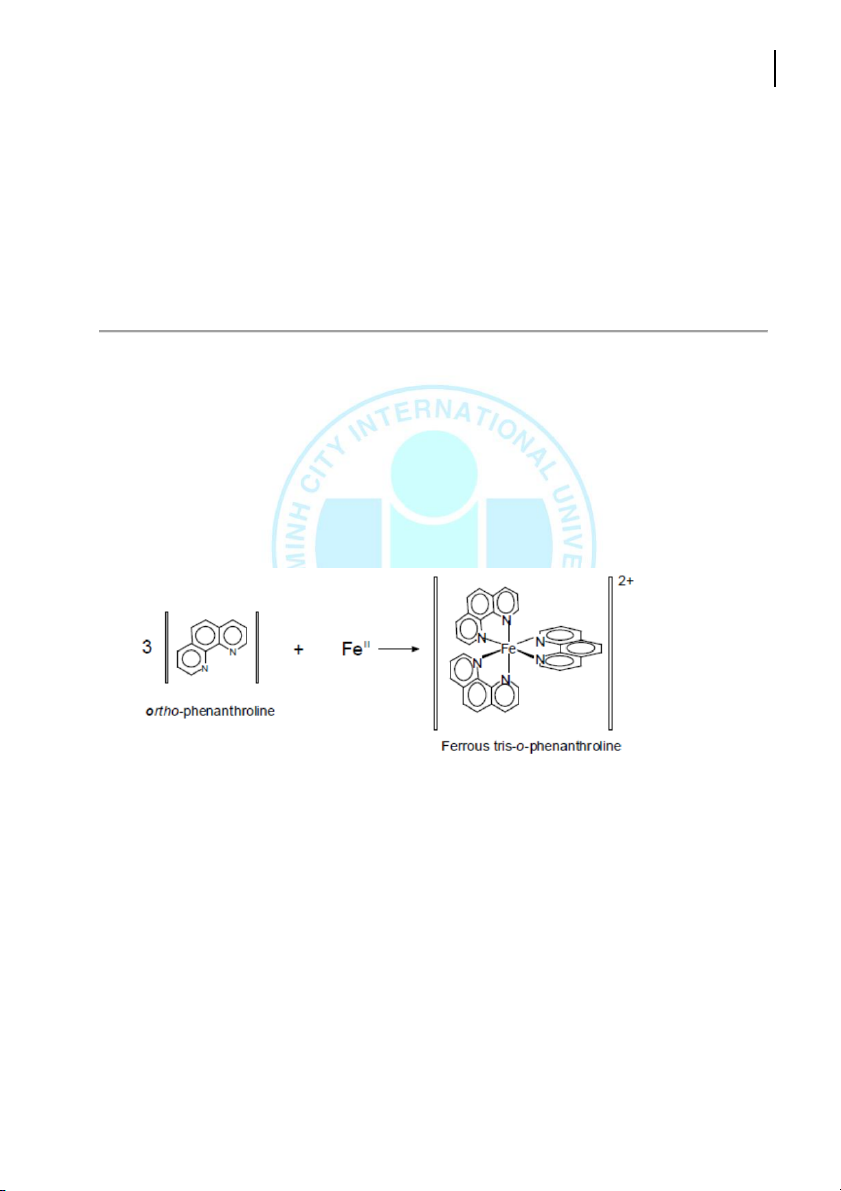
Fe+2 is reacted with o-phenanthroline to form a orange-red complex ion. The intensity of the colored
complex is linearly proporonal to the concentraon of Fe+2. This compound absorbs light in the visible
region with a maximum absorbance occurring around 515 nm. Orange-red
A calibraon curve (absorbance versus concentraon) is constructed for known concentraon Fe+2 soluon
and the concentraon of the unknown iron sample is determined.
As Fe3+ does not form the complex with o-phenanthroline, all of the iron must be reduced to its ferrous
state. In this experiment, the reducon is done by hydroxylamine hydrochloride (NH2OHHCl). 4Fe3+ + 2NH 2+ + 2OHHCl 4 Fe + N2O + 4H + H2O
Lecturer: Dr. Nguyen Thao Trang Semester I: 2013-2014
Internaonal University, Vietnam Naonal University - HCMC 2
ANALYTICAL CHEMISTRY LABORATORY Sodium acetate (CH 2+
3COONa) is used as a buer to maintain a constant pH at 3.5. Noted that Fe will be
oxidized to Fe3+ if the pH is high. When the pH is low, however, H+ will compete with Fe+2 for phenanthroline
and form phenanthroline -H+ complex. 3. PROCEDURE
3.1 Preparaon of standard Fe2+ soluon
Mohr’s salt (ferrous ammonium sulfate hexahydrate, Fe(NH4)2SO4.6H2O) at a concentraon of 0.07
mg/mL is used as the standard in this experiment. This standard soluon is made by dissolving 0.07 g
Fe(NH4)2SO4.6H2O in 300 mL DI water, then transferring to a 1000 mL volumetric ask. 25 mL H2SO4 2 M
is added into the ask and DI water is lled to the calibraon mark. This soluon is referred to the stock soluon. 3.2 Formaon of [Fe2
+ -o-phenanthroline] complex - Preparaon of 9 test tubes: B: is labeled for the blank
1-5 are labeled for the standard soluons
6-8: are labeled for the unknown sample (triplicate) - The formaon of the [Fe2
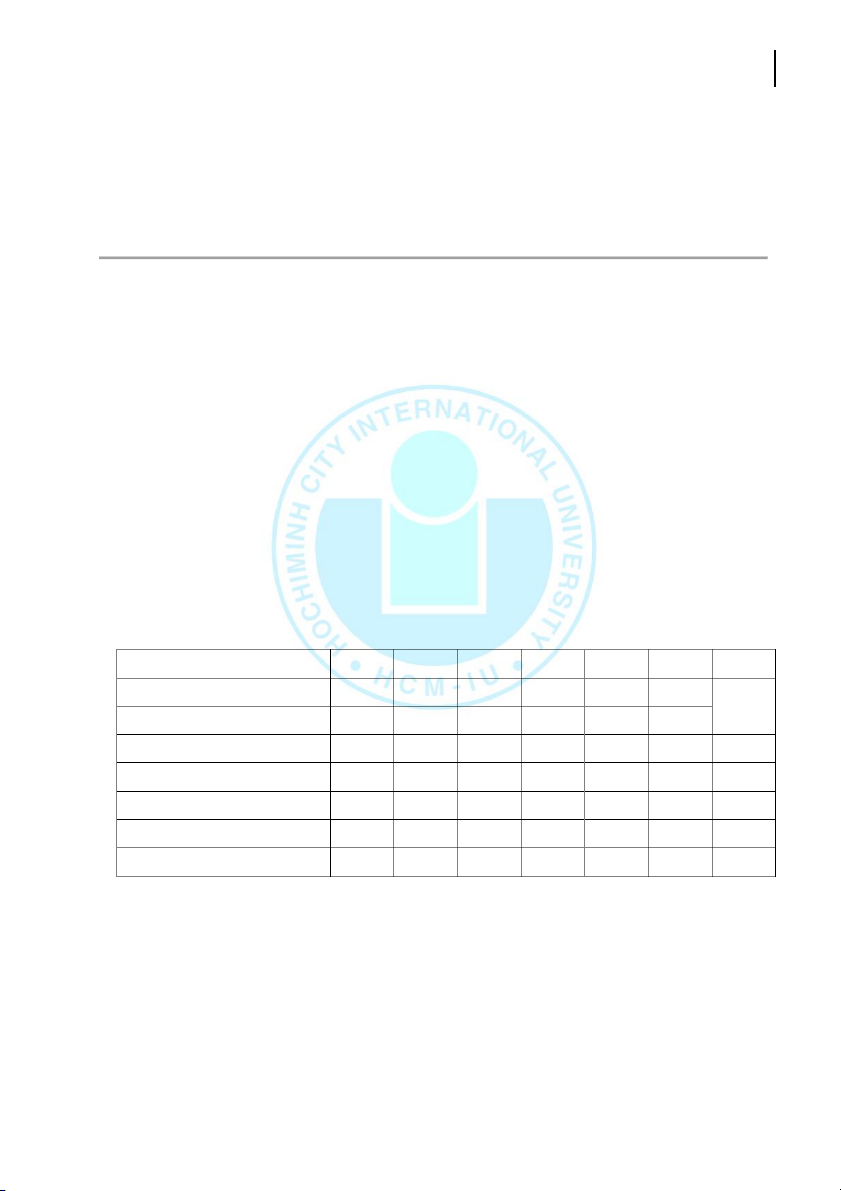
+ -o-phenanthroline] complex is carried out by the following procedure: Tube B 1 2 3 4 5 6-8 Diluon 1:100 1:50 1:20 1:10 1:5 1 mL Stock soluon [mL] 0 0.1 0.2 0.5 1 2 sample CH3COONa [ml] 1 1 1 1 1 1 1 NH2OH.HCl [mL] 1 1 1 1 1 1 1 Phenanthroline [mL] 1 1 1 1 1 1 1 DI water [mL] 7 6.9 6.8 6.5 6 5 6 Total volume [mL] 10 10 10 10 10 10 10 -
Then cover all test tubes with aluminum foil to prevent them from air exposure for 10 minutes for the color development. -
Performing a wavelength scan to determine the wavelength where the absorpon is maximum, called max.
Lecturer: Dr. Nguyen Thao Trang Semester I: 2013-2014
Internaonal University, Vietnam Naonal University - HCMC 3
ANALYTICAL CHEMISTRY LABORATORY -
The absorbance will be measured at max. -
Record the absorbance values of the blank, standards and unknown sample. - Construct a calibraon curve. -
Determine the unknown concentraon.
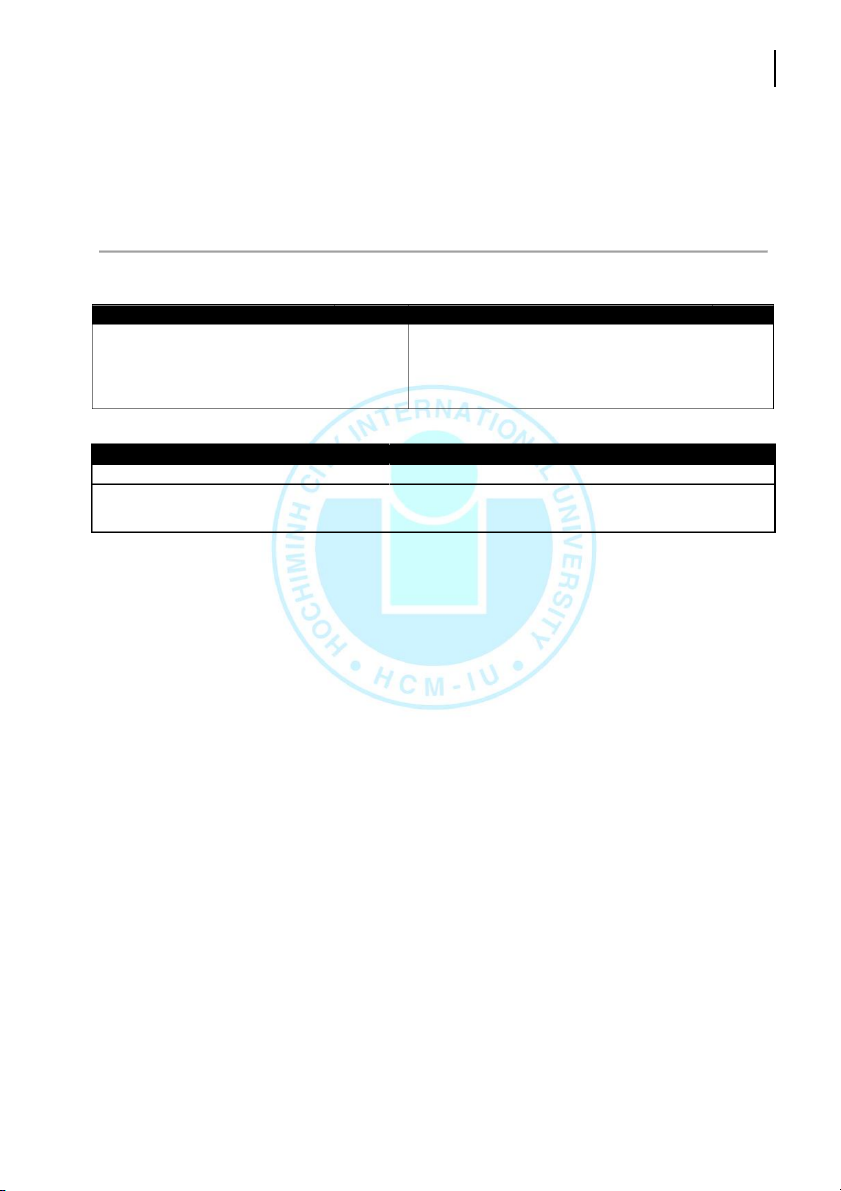
4. EQUIPMENT AND REAGENTS Equipment No. Equipment No. 100-mL beaker 01 1-mL pipet 01 50-mL beaker 01 5-mL pipet 01 Test tube 09 10-mL pipet 01 Srring rod 01 Reagents Locaon Reagents Locaon Fe(NH4)2SO4.6H2O assigned area NH2OH.HCl assigned area Phenanthroline assigned area Iron tablet assigned area CH3COONa assigned area
Lecturer: Dr. Nguyen Thao Trang Semester I: 2013-2014