











Preview text:
lOMoAR cPSD| 59085392 Vessel Structures
• Three tissue layers (tunica’)
• Tunica Intima: innermost, thinnest layer lining the vascular lumen.
• Tunica Media: middle, relatively thick layer with substantial smooth muscles and elastic fibers.
• Tunica Externa: outermost layer with elastic and collagen fibers, may contain nerves and smaller blood vessels. Arteries
• Function: conducting blood away from the ventricles of the heart.
• Conducting arteries (aorta, pulmonary arteries, etc.) contains high percentage of elastic fibers*.
• Medium-sized arteries contains an increased percentage of smooth muscle with relatively thick walls. Capillaries
• Smallest vessels, branched and inter-connected channels, forming a capillary bed between the
arterioles (‘little arteries’) and venules (‘little veins’)
• ONLY one-cell thick endothelium and basement membrane. lOMoAR cPSD| 59085392
• Gases, nutrients, and waste exchange between blood and interstitial fluids. Veins
• Function: Leading blood back away from the capillary bed.
• Veins are highly distensible, having large lumens, thin walls, and little smooth muscles.
• Venules and Veins also function as blood reservoir (64%) (venoconstriction when needed)
• Valves: flaps of the tunica intima that prevents blood backflow. Venous Return
• Low pressure in veins may be insufficient to transport blood to atria. (especially from lower limbs) • Skeletal Muscle Pump
❖Veins are usually close to skeletal muscles
❖Contraction of muscles compresses veins
❖Valves ensure blood is pushed in 1 direction →Physical
exercises improve venous return. Pericardium
The heart is encased within a multilayer membrane called pericardium
• Fibrous pericardium: tough, inelastic, dense connective tissues.
→Preventing overstretching. Protecting the heart from external trauma.
• Serous pericardium: thinner, double layer
→Parietal layer: lining the inner side of fibrous pericardium.
→Visceral layer: lining the wall of the heart
→Fluid-filled pericardial cavity: lubrication
Malfunctioning Pericardium
• Normally pericardial fluid layer is only 1-2 mm.
• Pericarditis: no known cause, but often associated with bacterial or viral infections • Acute pericarditis
Increased friction leading to chest pain lOMoAR cPSD| 59085392
Scratchy sound (pericardial friction rub) • Chronic pericarditis
Fluid buildup, compressing the heart
Force of contraction depends on the ability of the heart to stretch Electrocardiogram
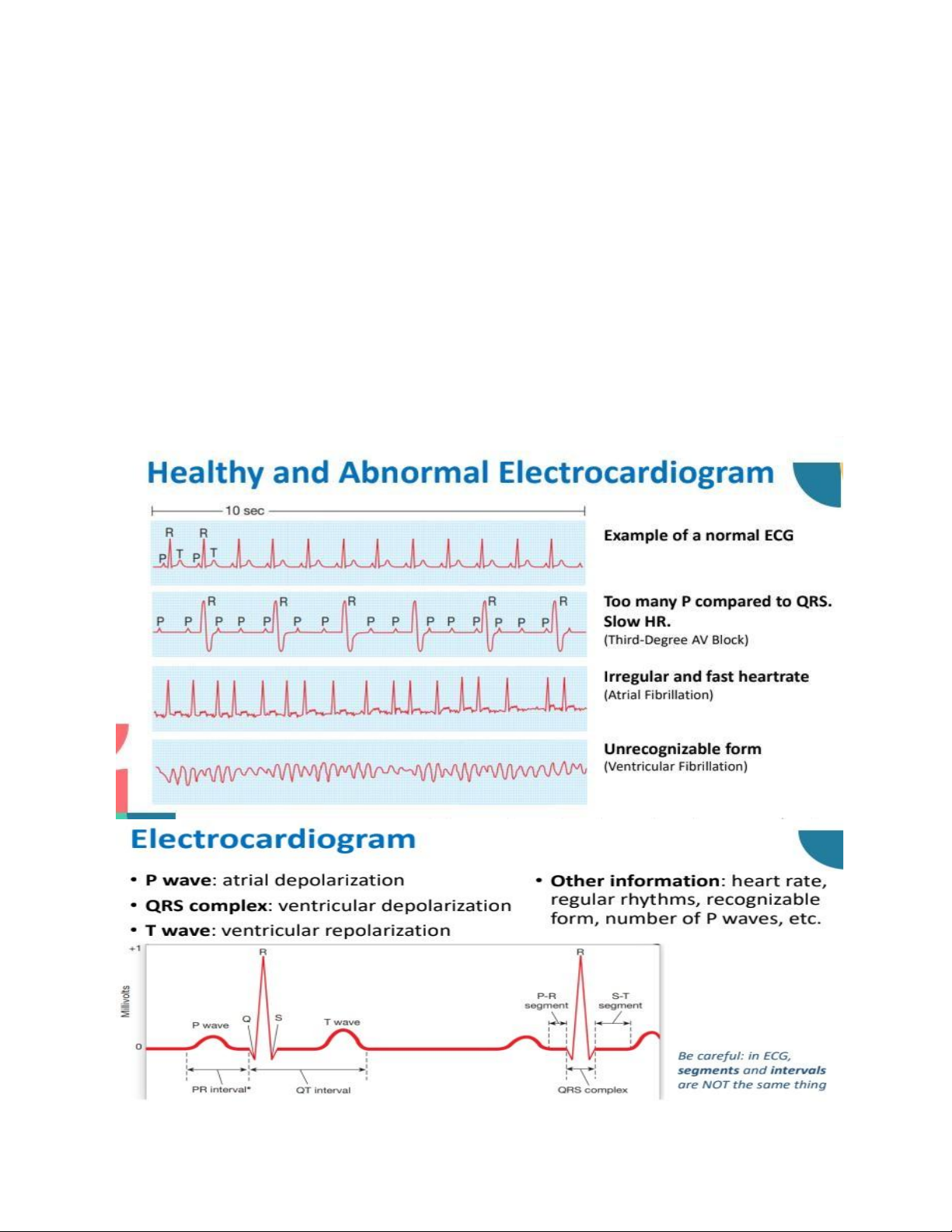
• Action potentials in the heart are conducted to the body surface and can be measured.
• Electrocardiogram is a voltage versus time graph using record from various combinations of limb and/or chest electrodes. Cardiac Functions lOMoAR cPSD| 59085392
• Cardiac Output (CO): volume of bloodpushed out from the left ventricles perminute.
→ Depends on Heart Rate (HR) and Stroke Volume (SV) CO = HR x SV
Overview of Hypertension • “Silent killer”
• Healthy BP <130 (systolic) and <85 (diastolic) (Vietnamese Society of Hypertension)
• HOWEVER, the BP thresholds for hypertensives are arbitrary and other co- existing
cardiovascular risk factors (e.g. diabetes) must be considered.
Degenerative Changes in Hypertension
• Stiffening and narrowing blood vessels due to damaged endothelium.
→Risk of stroke and aortic rupture
• Left ventricle hypertrophy to maintain cardiac outputs against increased peripheral resistance.
→Higher risk of Heart Failure
Management of Hypertension
• Lifestyle interventions are important:
Weight loss (in overweight people) Restricting sodium intake Aerobic exercise Moderating alcohol intake Smoking cessation
• Drug treatments aim to reduce MAP. Common mechanisms:
Reduce cardiac output (e.g. diuretics)
Reduce vascular resistance (e.g. calcium channel blockers) Question for cardiovascular:
1. Baby Aaron was six months old when he was hospitalised due to lifethreatening lung
inflammation and recurrent bacterial infections. A blood test shows a marked decrease lOMoAR cPSD| 59085392
in all types of antibodies. The level of T lymphocytes, however, was normal. Baby
Aaron was later found to have a kind of hereditary immunodeficiency.” (a) Explain the
cardinal signs of inflammations and how they arise. (b) Name and explain the two
forms of adaptive immune responses. Which form of adaptive immune response is
affected in this case and why? (c) Bone marrow transplantation was suggested as a
treatment option for baby Aaron. Briefly explain the importance of selecting a suitable donor.
(a) The cardinal signs of inflammation are redness, heat, swelling, pain , and sometimes
loss of function . These signs arise as part of the body’s natural defense response to
injury or infection and are driven by the activation of the immune system and the release
of inflammatory mediators. Here's how they occur: Redness:
o Cause: Increased blood flow (hyperemia) to the affected area due to the dilation
of blood vessels (vasodilation).
o Mechanism: Chemicals like histamine and prostaglandin released by damaged
cells and immune cells cause the surrounding blood vessels to widen, allowing
more blood to flow to the site. Heat:
o Cause: Increased blood flow to the site of inflammation.
o Mechanism: The warmer temperature of the blood combined with the metabolic
activity of immune cells generates localized heat. Swelling:
o Cause: Accumulation of fluid in the tissues (edema).
o Mechanism: Increased permeability of blood vessels allows plasma, proteins,
and immune cells to leak into the interstitial spaces to combat infection or heal tissue. Pain:
o Cause: Stimulation of nerve endings by inflammatory mediators.
o Mechanism: Substances like bradykinin, prostaglandins, and serotonin sensitize
or directly activate nociceptors (pain receptors). Additionally, swelling can
increase pressure on nearby nerves, amplifying the sensation of pain. Loss of Function:
o Cause: Combination of pain, swelling, and tissue damage. lOMoAR cPSD| 59085392
o Mechanism: Pain may inhibit movement to prevent further injury, while swelling
and tissue damage can physically impair normal function.
(b) Humoral Immune Response:
Mediated by: B lymphocytes (B cells) and the antibodies they produce.
Mechanism: B cells recognize antigens (pathogens or foreign substances) and are activated.
Upon activation, B cells differentiate into plasma cells that secrete antibodies. Antibodies bind
to specific antigens, neutralizing pathogens or marking them for destruction by other immune cells.
Target: Primarily extracellular pathogens (e.g., bacteria, toxins, and viruses outside cells). Cell-
Mediated Immune Response:
Mediated by: T lymphocytes (T cells), including helper T cells (CD4⁺) and cytotoxic T cells (CD8⁺).
Mechanism: Helper T cells coordinate the immune response by releasing cytokines that activate
other immune cells. Cytotoxic T cells directly kill infected or abnormal cells (e.g., virus-infected cells, tumor cells).
Target: Intracellular pathogens (e.g., viruses within cells) and abnormal cells (e.g., cancer cells).
In Baby Aaron's case, the humoral immune response is affected. Here's why:
Marked decrease in antibodies: Indicates a dysfunction in B cell function, as antibodies are
produced by B cells and their plasma cell derivatives.
Normal T lymphocyte levels: Suggest that the cell-mediated immune response (mediated by T cells) is intact.
Recurrent bacterial infections: These infections are typically controlled by antibodies that
neutralize bacteria and promote their clearance. The inability to produce sufficient antibodies
leads to increased susceptibility.
(c) Immune Compatibility
The donor's tissue type must closely match the recipient's to prevent immune rejection.
Graft-Versus-Host Disease (GVHD) Risk
A suitable donor minimizes the risk of GVHD by ensuring the immune cells from the graft
are less likely to perceive the recipient’s tissues as foreign.
Ideal Donor Characteristics
HLA Match: A full match (10/10 HLA alleles) is ideal, usually found in siblings with identical HLA typing.
Close Genetic Relationship: Siblings are preferred donors because they have a 25%
chance of being a perfect match. lOMoAR cPSD| 59085392
Unrelated Matches: If no sibling is a match, unrelated donors or cord blood from a public
registry may be used, with careful HLA matching.
Other Factors: The donor’s overall health, absence of transmissible diseases, and age also influence suitability.
2. Describe electrical and mechanical events that occur during a cardiac cycle. Electrical Events
Electrical events are regulated by the heart's conduction system, which generates and
propagates electrical impulses to coordinate the cardiac cycle.
Sinoatrial (SA) Node Activation (Pacemaker): o
The cycle begins when the SA node
in the right atrium generates an electrical impulse.
o This impulse spreads through the atria, causing atrial depolarization.
o Electrocardiogram (ECG): Represented by the P wave.
Atrioventricular (AV) Node Delay: o The impulse reaches the AV node, where
it is delayed briefly to allow complete atrial contraction and ventricular filling.
Impulse Propagation via the Bundle of His and Purkinje Fibers: o The electrical
impulse travels through the Bundle of His, divides into right and left bundle
branches, and spreads to Purkinje fibers.
o This causes ventricular depolarization, triggering ventricular contraction.
o ECG: Represented by the QRS complex. Repolarization:
o The ventricles repolarize, leading to relaxation.
o ECG: Represented by the T wave. Mechanical Events
The mechanical events involve the contraction (systole) and relaxation (diastole) of the atria
and ventricles, driven by the electrical activity.
Atrial Systole (Atrial Contraction):
o Trigger: Atrial depolarization (P wave). o
Action: The atria contract, pushing
remaining blood into the ventricles. lOMoAR cPSD| 59085392
o Valves: Atrioventricular (AV) valves (mitral and tricuspid) are open; semilunar
valves (aortic and pulmonary) are closed.
Isovolumetric Ventricular Contraction:
o Trigger: Ventricular depolarization (QRS complex).
o Action: The ventricles begin to contract, but all valves are closed, so the volume
remains constant while pressure builds.
o Valves: All valves are closed.
Ventricular Ejection (Systole):
o Trigger: Ventricular pressure exceeds arterial pressure.
o Action: Semilunar valves open, and blood is ejected into the aorta and pulmonary artery.
o Valves: Semilunar valves are open; AV valves remain closed.
Isovolumetric Ventricular Relaxation:
o Trigger: Ventricular repolarization (T wave).
o Action: The ventricles relax, and pressure drops. All valves remain closed as the
ventricles prepare to fill again.
o Valves: All valves are closed.
Ventricular Filling (Diastole):
o Action: The ventricles fill passively as blood flows from the atria. o Phases:
▪ Rapid Filling: Initially rapid due to pressure differences.
▪ Diastasis: Slower filling as pressures equalize.
▪ Atrial systole contributes the final amount of blood.
o Valves: AV valves are open; semilunar valves are closed.
3. How is ventricular hypertrophy in hypertensive different from the ventricular
hypertrophy in athletes who train intensively?
Hypertensive hypertrophy is a pathological response to pressure overload, often leading to
impaired cardiac function and increased cardiovascular risk.
Athletic hypertrophy is a physiological and adaptive response to increased workload
demands, typically enhancing performance and rarely associated with adverse outcomes. lOMoAR cPSD| 59085392 Blood
An average adult human has ~ 5L of blood.
Whole blood consists of TWO components:
• Plasma: (55%) watery extracellular matrix.
• Formed elements: (45%) circulating blood cells and cell fragments, all of which originate
from bone marrows. Hematocrit
• Hematocrit: volume percentage of blood occupied by red blood cells. • Male 40%-54% • Female 38%-46%
• Gender difference may be due to testosterone and menstruation
• Abnormally low hematocrit can indicate anemia. Erythrocytes lOMoAR cPSD| 59085392 • Principal oxygen carriers
• Biconcave shape stabilized by cytoskeleton filaments.
• Highly deformable to enter narrow capillaries.
• No nucleus, mitochondria or other organelles.
Optimizes oxygen transport and delivery.
Provides flexibility for navigation through capillaries. Hemoglobin
• Polypeptides with the 2 alpha plus 2 beta chains in adult (or 2 alpha and 2 gamma chains in fetus)
• Four heme groups containing Fe2+ that bind oxygen*
• Carbon monoxide affinity to Hb is 200-300 times higher than O2 → CO poisoning
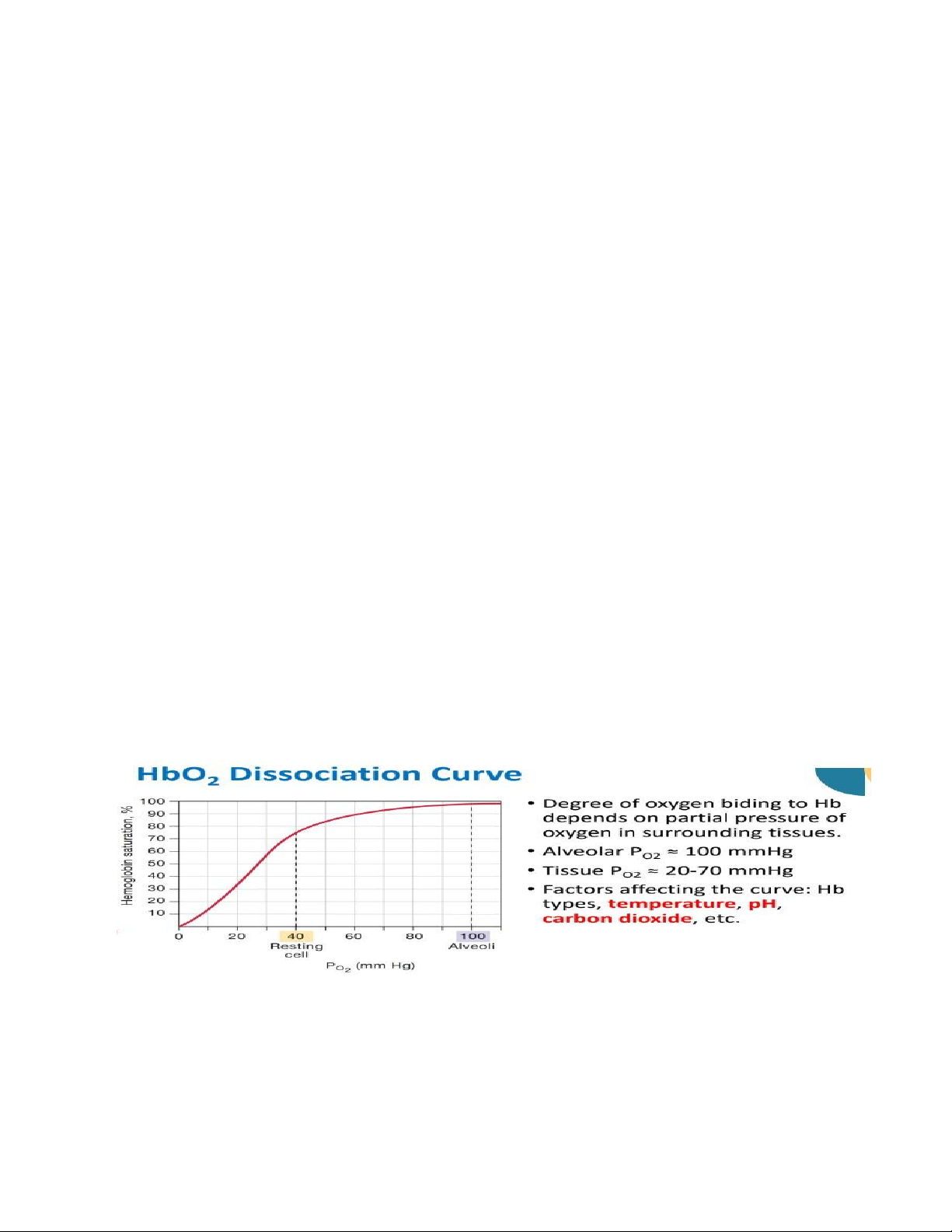
Oxygen dissociation from hemoglobin is enhanced in:
A. High pH, high temperature, high CO2 partial pressure
B. Low pH, high temperature, low CO2 partial pressure
C. High pH, low temperature, high CO2 partial pressure
D. Low pH, high temperature, high CO2 partial pressure
Factors Affecting HbO2 Dissociation
Dissociation is enhanced at low pH, high temperature, or high CO2 partial pressure.
Erythrocytes assist CO2 transportation
• Tissues produce far more CO2 than the plasma can dissolve.
• 7% dissolved in plasma; 93% carried by erythrocytes by two mechanisms: lOMoAR cPSD| 59085392
1. Hemoglobin: NOT AT HEME GROUPS. But at exposed amine groups.
2. Carbonic Anhydrase: converts CO2 into H2CO3 and HCO3. Platelets
• Cell fragments budding from megakaryocytes in bone marrows.
• No nucleus; still contains mitochondria, and smooth endoplasmic reticulum.
• Many vesicles of cytokines and growth factors.
• Function: stop blood loss; mediate immune responses including inflammation. Hemostasis
• Hemostasis is the response to keep blood within damaged vessels.
• Damage to the endothelium leads to exposure of collagen
• Platelet adherence and activation, releasing granules containing signaling molecules. →1st step: Vasoconstriction
→2nd step: Recruitment and aggregation of platelets to form a PLUG
• 3rd step Blood clot: a gel containing various blood cells (inc. erythrocytes) entangled in fibrin threads.
• Two complex reaction cascades starting from either (1) collagen or (2) released tissue factor →Formation of Thrombin
→Conversion of Fibrinogen → Fibrin (insoluble) →
Entrapping erythrocytes to create a clot. Hematopoiesis
• Erythrocytes are produced from hematopoietic stem cells in red bone marrow Hematopoietic
stem cells differentiate in red bone marrow until nucleus ejection
Reticulocytes enter bloodstream for 1 day before fully maturing into erythrocytes.
Enhanced by the hormone erythropoietin produced mostly in kidney (adults) and liver (fetuses).
Erythrocyte production is regulated by erythropoietin (EPO), a hormone secreted primarily from the kidneys.
• Stimuli: reduced tissue oxygenation: Low blood volume Anemia lOMoAR cPSD| 59085392 Low hemoglobin Poor blood flow Pulmonary disease
Removal of Red Blood Cells
• Erythrocytes lifetime: 120±20 hours
• Increasing fragility prevents passing through small capillaries → rupture or phagocytosis by
macrophage in liver and spleen. • Processing:
Hemoglobin> Amino acids> New proteins
Heme group> Bilirubin> Bile pigment, urine
Iron> Release into blood> Stored in liver and bone Sickle cell anemia
• Genetic disorder with a single amino acid substitution in β-globin
• When deoxygenated, the defective hemoglobin has a high tendency to polymerize. • Initially
reversible upon oxygenation, but repeated deformation damages the cytoskeleton irreversibly.
• Sickle RBC tends to get entangled and blocked blood vessels →Tissue damage and pain from hypoxia.
• Enlargement and fibrosis of the spleen: due to congestion by sickle cells → higher infection risk
• Heterozygous individuals generally show no symptoms and lead a normal life. Sickle cell anemia patients:
A. Experience pain and hypoxia due to blood vessel occlusion
B. Have a mutation in the alpha-chains of hemoglobin
C. Have to no anatomical change in the spleen D. All the above are correct
Which of the following is incorrect about the human red blood cells:
A. Healthy red blood cells have a biconcave shape
B. Red blood cells generate their energy from mitochondria lOMoAR cPSD| 59085392
C. Red blood cells contain no ribosomes nor endoplasmic reticulum.
D. Mature red blood cells cannot undergo mitosis
Antigen-presenting cells (APC) display peptides fragments of the antigens on their surface using: A. Antibody B. Cytokine C. T-cell receptor
D. Major histocompatibility complex Which is not a function of antibodies: A. Neutralization of toxins
B. Generating reactive oxidative species to eliminate pathogens. C. Opsonization of pathogens.
D. Activation of complement cascades lOMoAR cPSD| 59085392 lOMoAR cPSD| 59085392 1.
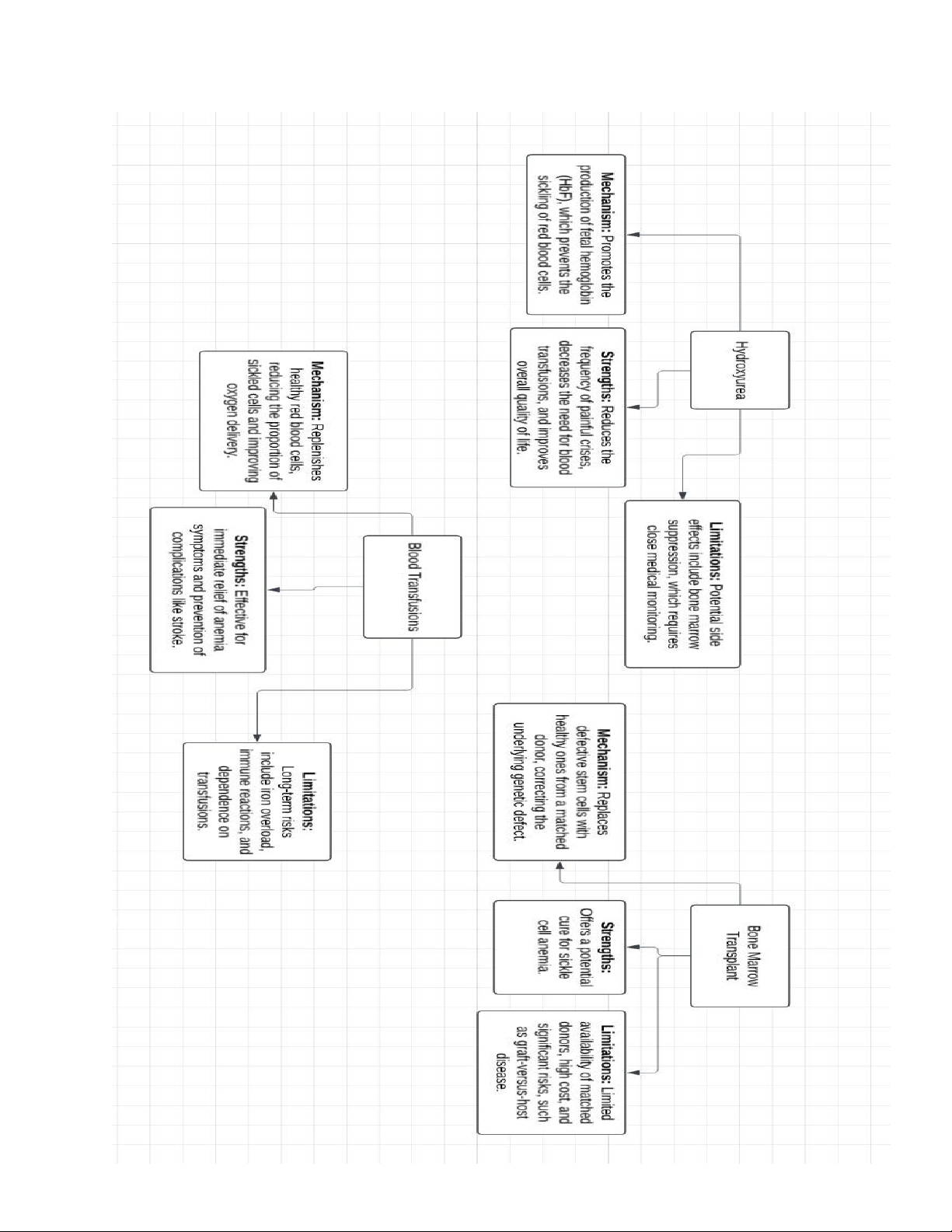
Hydroxyurea: This medication increases fetal hemoglobin levels, which reduces sickling
of red blood cells. Strengths: Widely available and effective in reducing pain crises and
hospitalizations. Limitations: May cause side effects like bone marrow suppression and require regular monitoring. 2.
Blood Transfusions: These replace sickled red blood cells with healthy ones from donors.
Strengths: Immediate relief from anemia and prevention of complications like stroke.
Limitations: Risk of iron overload and immune reactions; requires lifelong maintenance. 3.
Bone Marrow Transplant: A curative option that replaces defective stem cells with
healthy ones from a compatible donor. Strengths: Potential cure for sickle cell anemia.
Limitations: Limited by donor availability, high cost, and risks such as graft-versus-host disease.
Three Lines of Defense 1. BARRIERS •
Physical: skin, mucus, etc. •
Mechanical: mucociliary clearance, etc. •
Chemical: enzymes, gastric pH 2. INNATE IMMUNITY lOMoAR cPSD| 59085392 • Commence if barriers fail •
Rapid, Non-specific, no memory •
Cells: granulocytes, phagocytes, etc. •
Chemicals: complements, cytokines, etc. 3. ADAPTIVE IMMUNITY • Aided by innate immunity • Slow, specific, memory •
Cells: lymphocytes B, lymphocytes T •
Chemicals: antibodies
The human body is constantly exposed to both external and internal threats:
External: microbes, viruses, parasites, toxins, etc.
Internal: latent viruses, damaged cells, cancer cells, etc.
The immune systems need to distinguish SELF vs NON-SELF, and NORMAL vs ABNORMAL Often via surface molecules.
Immune cells that attack their own body are eliminated early or inhibited from action. lOMoAR cPSD| 59085392 Innate Immunity
• Early response to eliminate pathogen infections and damaged cells.
• Non-specific. No immunological memories.
• Two major types of innate reaction: inflammation and antiviral response. Inflammation
• Major stages of inflammation: 1. Vasodilation
2. Phagocyte migration (i.e. chemotaxis) 3. Tissue repair
What causes the pain, heat, redness, and swelling in inflammation?
Trigger: Injury, infection, or irritation activates immune cells (mast cells, macrophages, etc.).
Chemical Mediators Released: Histamine, prostaglandins, bradykinin, and cytokines (e.g., IL-1,
TNF-α) initiate the inflammatory response. lOMoAR cPSD| 59085392
Vasodilation and Permeability: These mediators increase blood flow and vascular permeability,
causing redness, heat, swelling, and pain.
Recruitment of Immune Cells: Neutrophils, monocytes, and lymphocytes are recruited to the
site to eliminate the cause of injury and initiate repair.
Inflammation Mediators • Cytokines:
Diverse groups of small proteins (many of which are produced by white blood cells) Mostly
acting locally to regulate immune response. Function:
❖ Initiate inflammation ❖ Recruit phagocytes.
❖ Stimulate proliferation and differentiation of lymphocytes (for adaptive immunity)
Eosinophils (Eosin-Loving)
• Morphology: stained dark red with acidic dye(eosin); nucleus with two lobes; granulated.
• Few in circulation; common in airway, digestive tract, connective tissues of skin, etc.
• Weak in phagocytosis. But bind to antibody-coated multicellular parasites
→ Release granules containing toxic enzymes and oxidative molecules
Basophils (Base-Loving)
• Morphology: stained dark blue with basic dye (hematoxylin), large number of granules obscuring the nuclei. • Very few in circulation.
• Related to mast cells in tissues. Both of which can release granules of histamine and heparin
Neutrophils (Neutral-Loving)
• Morphology: pink/purplish colour in H&E staining; multi-lobe nucleus; granules are less obvious.
• The most abundant WBC in the body lOMoAR cPSD| 59085392
• Function: ingesting pathogens and foreign particles (phagocytosis); releasing toxic and
inflammation-mediating molecules from granules. • The first responders.
• Involved in allergic reaction.
Macrophages (Big Eaters)
• Morphology: large size; round nucleus.
• Function: patrol the tissues to destroy pathogens and defective cells.
• More effective but slower respond.
• Macrophages (tissues) and its precursors monocytes (circulation) are attracted to chemicals
released from neutrophils and injured tissues ADAPTIVE IMMUNE RESPONSE Lymphocytes
• Morphology: round nucleus; less visible granules; large nucleus to cytoplasm ratio.
• Two major types, both starting from precursors in bone marrows • T Lymphocytes:
Maturation in the Thymus Roles in cell-mediated immunity • B Lymphocytes: Maturation in the Bone marrow
Roles in antibody-mediated immunity Lymphoid Tissues
Primary Lymphoid Tissues: where lymphocytes are produced from stem cells and mature. Bone marrows Thymus gland
Secondary Lymphoid Tissues: where lymphocytes and other immune cells monitor extracellular
fluids and initiate adaptive immunity Lymph nodes lOMoAR cPSD| 59085392 Spleen, tonsil, mucosa, etc. Lymph Nodes
• Antigens from tissues are drained into lymph nodes by lymphatic fluid or carried by specialized immune cells. → SURVEILLANCE
• Lymph nodes contain high concentration of lymphocytes and other immune cells
→ High probability for cell-antigen contacts and activation Adaptive Immunity
• Adaptive immunity are antigen-specific responses that develop after exposure to the antigens.
• Leading to formation of life-long immunological memories • Consisting of TWO major components:
Antibody-Mediated Immunity
• Antigen-recognizing molecules in B cells.
Proteins with four peptide chains.
2 heavy chains + 2 light chains
Antigen-binding sites: highly variable; each antibody highly specific for its antigen.
Fc region: stimulate other components of immune systems.
Clonal Expansion of B Cells
• During B cells development, each B cells is programmed to produce antibody against a specific antigen.



