
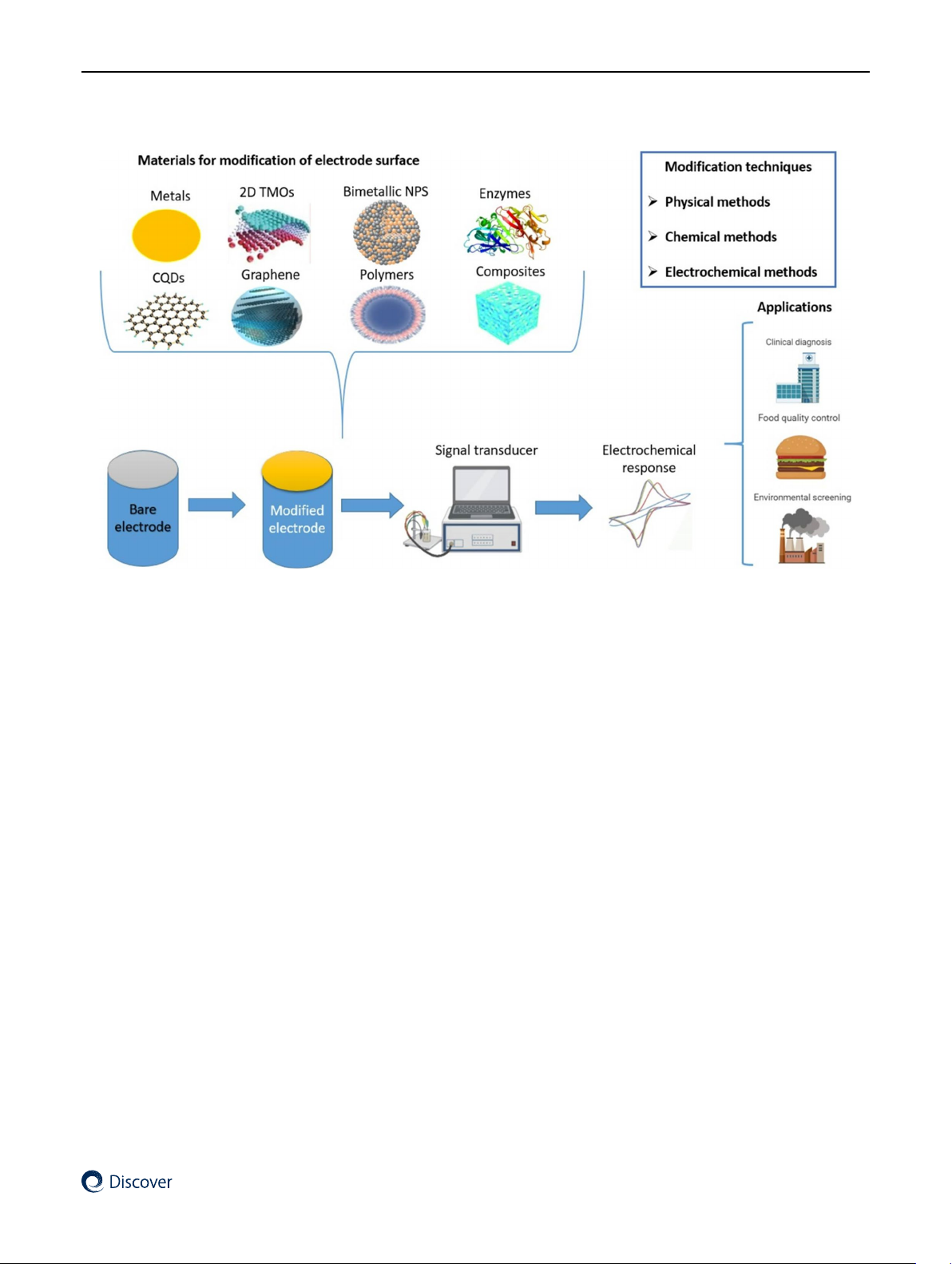















Preview text:
Discover Electrochemistry Review
A brief review on methods and materials for electrode modification:
electroanalytical applications towards biologically relevant compounds Mariya Pimpilova1
Received: 10 September 2024 / Accepted: 12 November 2024 © The Author(s) 2024 OPEN Abstract
This review provides an overview of the advancements in electrochemical sensors and biosensors, along with their appli-
cations. The review covers the methods and materials used for modifying the surface of electrodes, and also discusses the
use of electrochemical sensors for quantitative analysis of biologically relevant compounds, such as hydrogen peroxide,
dopamine, serotonin, glucose, and other markers of oxidative stress and neurotransmitters. Various electrochemical
characterization methods have also been highlighted. Recently, there has been a growing interest in combining recogni-
tion elements with electronic elements to establish electrochemical sensors and biosensors. These devices have proven
to be effective in detecting chemical and biological targets through changes in electrochemical activity at electrode
interfaces. The use of nanomaterials has significantly improved the sensitivity and selectivity of electrochemical sensing
platforms. Electrode materials are critical to the construction of high-performance sensors for detecting target molecules.
The integration of functional nanomaterials can enhance catalytic activity, conductivity, and biocompatibility, leading to
more accurate and sensitive biosensing. Overall, the development of functional electrode materials, along with various
electrochemical methods, has greatly expanded the potential applications of electrochemical devices.
* Mariya Pimpilova, mariq.pimpilova@gmail.com | 1Laboratory of Biologically Active Substances, Institute of Organic Chemistry With
Centre of Phytochemistry, Bulgarian Academy of Sciences, 139 Ruski Blvd., 4000 Plovdiv, Bulgaria.
Discover Electrochemistry (2024) 1:12
| https://doi.org/10.1007/s44373-024-00012-8 Vol.:(0123456789) Review
Discover Electrochemistry (2024) 1:12
| https://doi.org/10.1007/s44373-024-00012-8 Graphical Abstract
Keywords Electrochemistry · Electrocatalysts · Biosensors · Nanoparticles 1 Introduction
One of the main goals of the world’s scientific efforts is to improve the quality of life. Achieving this aim is directly related
to the availability of methods for rapid diagnosis of common diseases, food quality control, and environmental monitor-
ing. Several scientific studies are aimed at developing analytical methods that meet these requirements. There are optical
(spectrophotometry, fluorometry, and surface plasmon resonance) [1], piezoelectric, and magnetoelectric [2], as well as
electrochemical methods available based on their detection principles [3].
In the past few decades, electroanalytical methods have attracted the attention of many researchers due to their
experimental simplicity, relatively low cost, and low detection limits, typically ranging from nanomolar (nM) to micro-
molar (µM) [4]. Other important prerequisites for their great popularity are their easy digitization and the compact size
of the equipment, the latter allowing in situ monitoring. For these reasons, they are widely used in areas such as clinical,
industrial, environmental, and agricultural analysis.
Electrode materials play a major role in the development of highly sensitive electroanalytical methods for the deter-
mination of target analytes. The modification of electrode surfaces is carried out with several main goals, including
increasing the sensitivity of the determination, improving the electrode selectivity, decreasing the limits of detection
and quantification, and inhibiting the electrode surface fouling phenomena. This is achieved either by increasing the
electrochemically active surface or by applying catalysts to it [5].
This review covers research conducted over the past two decades and mainly summarizes techniques and materials
for modifying electrode surfaces, their characterization using various electrochemical methods, and their successful
application to the quantitative analysis of biologically relevant compounds. Although several reviews have been pub-
lished on electrochemical methods and electrode modifications, this work presents a more recent overview, with an
emphasis on the use of nanomaterials and novel electrochemical approaches that were not covered in earlier reviews. Vol:.(1234567890)
Discover Electrochemistry (2024) 1:12
| https://doi.org/10.1007/s44373-024-00012-8 Review
1.1 Methods for modifying electrode surfaces
The most widely used working electrodes are carbon-based, characterized by relatively low cost, low background cur-
rent, good electrical conductivity, and chemical inertness [6]. In the past few years, glassy carbon has been a preferred
electrode material in electroanalysis due to its electrochemical stability over a wide range of potentials (from −1.5 V to
1.5 V) [3], impermeability to liquids and gases, and easy surface modification. It is characterized by high thermal and
chemical resistance, which makes it superior to other forms of carbon used as working electrodes. However, the glassy
carbon electrode (GCE) has several disadvantages such as easy surface contamination associated with adsorption pro-
cesses or unwanted precipitation, low rate of electrochemical reactions (i.e. oxidation and reduction processes occur at
high overpotential), which affects the sensitivity and selectivity of electroanalytical determinations. Another significant
drawback that limits its practical use is the occurrence of non-specific oxidation–reduction reactions at the electrode
surface [7]. With repeated use of GCE, the active centres on the surface may be partially or completely blocked, which
would lead to a negative impact on the reproducibility of the results. 1.2 Physical methods
In recent decades, there has been an intensive development of electrode modification techniques for various applica-
tions, such as solar energy conversion and storage [8], selective electroorganic synthesis [9], molecular electronics [10],
electrochromic display devices [11], corrosion protection [12] and electroanalysis [13]. In general, methods for modifying
electrode surfaces can be classified as physical, chemical, and electrochemical.
Physical methods include processes in which the modifier binds to the electrode surface through electrostatic forces,
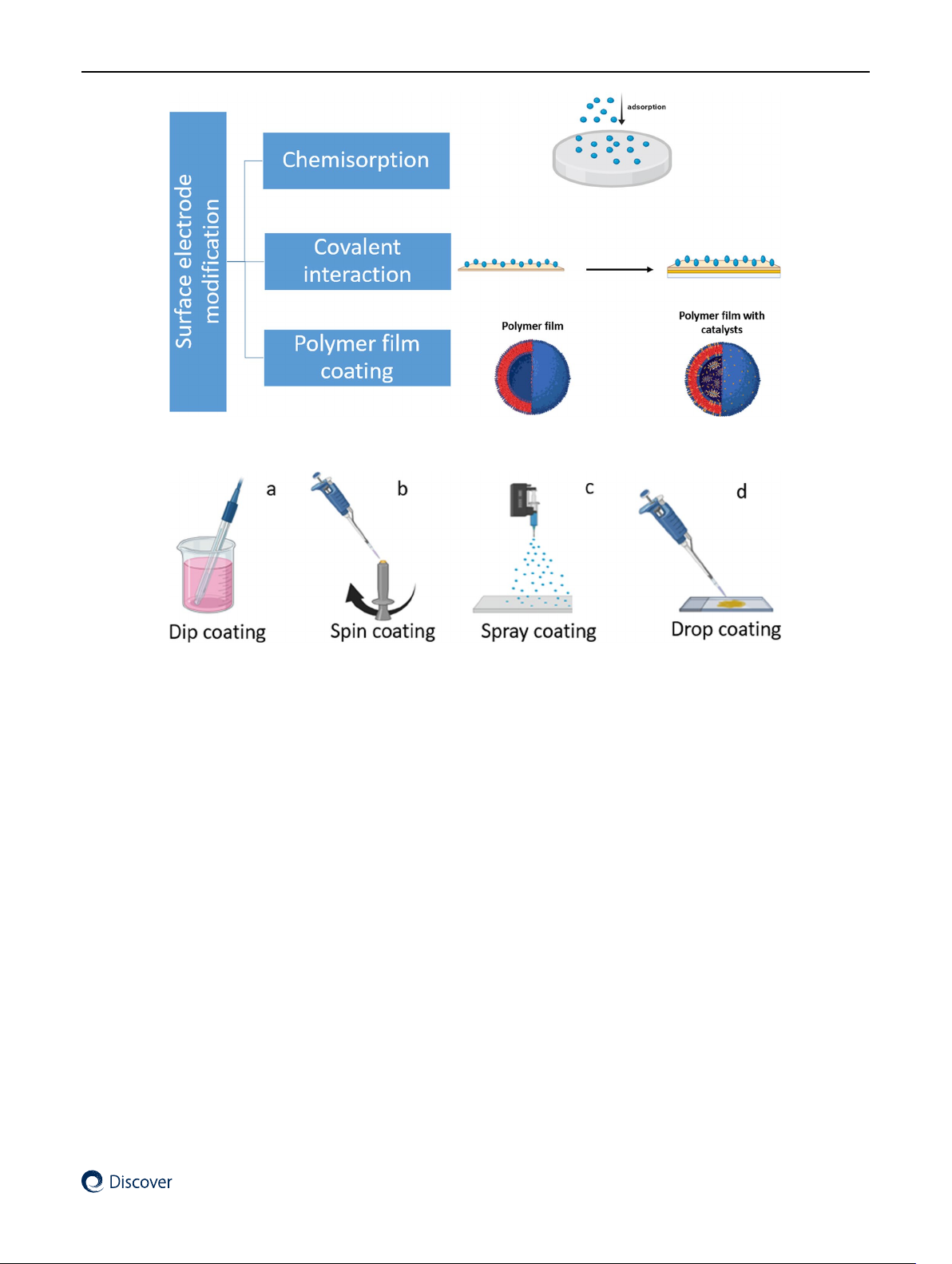
hydrogen bonds, π-π-interactions, and Van Der Waals forces [14] (Fig. 1). One of the most applied physical techniques is
encapsulation, the application of polymer coatings and adsorption of surface-active substances [15]. The main disad-
vantages of physical methods are the strong anisotropy of the modifying phase, the uneven coating of the electrode
surface, the poor mechanical stability of the coatings, and the non-reproducible surface. 1.3 Chemical methods
Chemical methods such as chemisorption, covalent interactions, and polymer film coatings with or without catalyst/s
are often used for surface modification of conductive matrices. The adsorption of a modifier in a solution on an
electrode surface depends on the hydrophobicity of both the electrode and the modifier. For example, hydrophobic
Fig. 1 Schematic of physical methods for modification Vol.:(0123456789) Review
Discover Electrochemistry (2024) 1:12
| https://doi.org/10.1007/s44373-024-00012-8
Fig. 2 Schematic of chemical methods for modification
Fig. 3 Schematic of the four coating-based methods for deposition onto electrode surfaces
organic molecules absorb more readily on a hydrophilic platinum electrode, as show in [16]. Owing to the forma-
tion of covalent bonds between the functional groups on the electrode surface and the modifying reagent, the
modifying phase is strongly and irreversibly adsorbed onto the electrode surface, resulting in the formation of one
or several monomolecular layers [17]. In some cases, bifunctional reagents such as glutaraldehyde, ethylene glycol,
and isothiocyanates can be used to further stabilize the modifier on the surface by forming strong covalent cross-
links between the modifier and the surface, increasing the durability and adhesion of the film. A frequently applied
approach to obtain electrodes with high catalytic activity is modification with polymer films. Electron-conductive
and non-conductive polymer films are adsorbed on the electrode surface by a combination of chemisorption and
low solubility in the electrolyte solution. The polymer can be organic, organometallic, or inorganic, containing the
desired modifier or added at a later stage of the modification [18]. Polymer-modified electrode surfaces can be clas-
sified according to the technique used to deposit the film. Figure 2 summarises chemical methods for modification.
“Dip and Dry or Dip coating”. This represents a simple approach to modifying electrodes by incubating the elec-
trode in a polymer suspension for a sufficiently long time until a film is formed by adsorption processes on the
electrode surface (Fig. 3a). Polymer film thickness can be controlled by incubation time, particle concentration in
suspension, and mass transfer rate [19]. Despite its cost effectiveness and low material consumption rate, dip coating
suffers from a main disadvantage of being a low process, leading to partial or inhomogeneous electrode coverage.
An alternative method to overcome the inhomogeneity problem is spin coating.
„Spin coating”. In this method, thin and uniform films are formed on an electrode surface (Fig. 3b). The methodol-
ogy consists of placing an optimum amount (µl) of the composite phase (a polymer containing a catalyst) on the
surface of the electrode and of centrifuging a desired volume ∼ 2000 rpm, before gradually slowing the rotation
speed down and allowing evaporation of the solvent. Depending on the properties of the modifying phase, the accel-
eration, and the angular velocity of rotation, the film thickness varies in the range of several nanometers to several Vol:.(1234567890)
Discover Electrochemistry (2024) 1:12
| https://doi.org/10.1007/s44373-024-00012-8 Review
micrometers [20]. This is a suitable method for depositing thin films on screen-printed electrodes, which requires
special and expensive equipment.
„Spray coating”. This is another method that can facilitate the deposition of a uniform and thin film with a large elec-
trochemically active surface area (Fig. 3c) [21]. The modifier suspension is sprayed onto the electrode surface with a jet
apparatus using a carrier gas or a nozzle with subsequent evaporation of the solvent. The automated process provides
homogeneous and reproducible surfaces by adjusting the distance between the nozzle and the electrode, thus the
particle size can be controlled. The main disadvantages of this method include a large consumption of materials and expensive equipment.
„Drop coating”. This method is widely used to prepare the surface of chemically modified electrodes by applying a
desired volume of a modifier-containing suspension to an electrode surface, followed by drying under UV [22], under a
N2 [23], or merely room conditions (Fig. 3d) [24]. This method offers the advantages of short production time (∼1 min.
[25]), simplicity, and reusability. The amount of drop-cast modifier can be quantitatively related to the number of mon-
olayers formed on the electrode surface. Disadvantages are related to the possibility of agglomeration, inhomogeneous
coating of particles on the surface, and observation of the "coffee-ring" effect, which refers to the phenomenon where
a droplet of liquid containing suspended particles leaves a ring-shaped stain upon evaporation. This effect occurs due
to the uneven distribution of particles as the solvent evaporates, causing the particles to concentrate at the edge of the
droplet. It is particularly problematic in applications where uniform coating is desired, as it can lead to inconsistencies
in the distribution of catalyst particles after drying. It is related to the distribution of catalyst particles after drying. In a
publication by Kaliyaraj Selva Kumar et al. [26], two approaches are proposed were proposed to address this problem,
such as electrowetting or the use of highly hydrophobic surfaces. Electrowetting enhances the mobility of the catalyst
particles by modifying the wetting properties of the surface, allowing for better dispersion during the drying process.
This results in a more uniform distribution of particles, which is crucial for optimizing catalytic performance. On the other
hand, highly hydrophobic surfaces minimize the adhesion of catalyst particles, preventing agglomeration and ensuring
that they remain well-distributed after drying.
1.4 Electrochemical methods
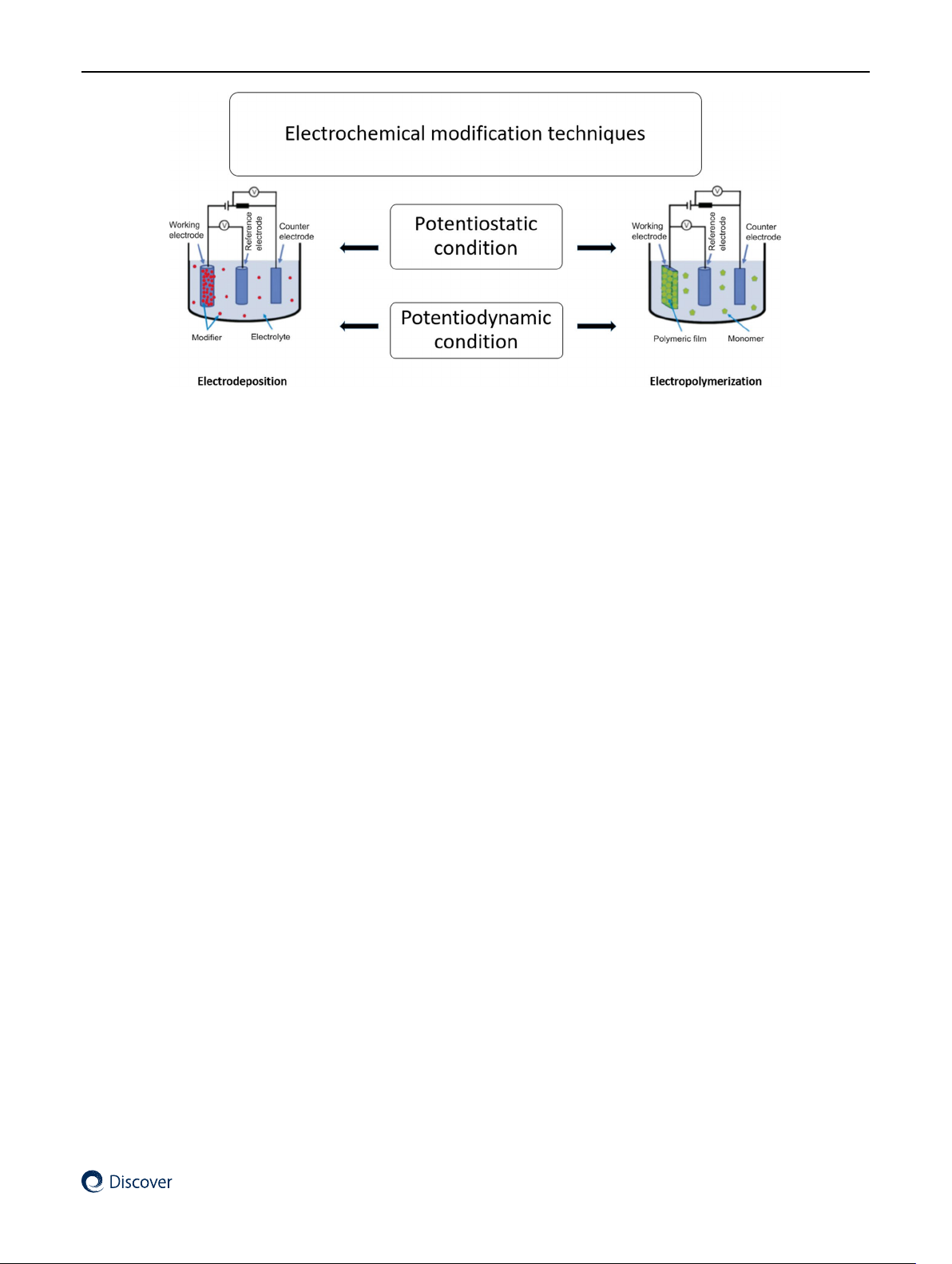
Electrochemical deposition is one of the most useful approaches for the fabrication of layers on electrode surfaces from
metal nanostructures or polymers. Electrochemical modification techniques can be performed in two different modes,
i.e. under potentiodynamic or potentiostatic conditions, which can be carried out in both aqueous and non-aqueous
media [27, 28]. In the potentiostatic technique, a constant potential is applied between the working electrode and ref-
erence electrode for a desired duration. The applied voltage must be at least 0.15 V more negative than the reduction
peak potential of a targeted metal ion to promote deposition of the surface of the working electrode. Very often, the
sites with defects (e.g., edges, pores) on the electrode surface are where deposition occurs. Depending on the condi-
tions, for example, the type and concentration of the electrolyte bath, the deposition time can vary from a few seconds
to minutes [29]. In contrast, potentiodynamic techniques require a scanning from an initial potential to a final potential
at a particular rate [30]. One usually chooses an initial potential to be at which no electroreduction of metal ions from
the electrolyte occurs on the electrode. Similarly, a final potential is usually where the reduction reaction takes place
at a significant rate. Alternatively, the reduction can proceed at a high speed at the chosen initial potential, and at the
final potential, the metal ions are not reduced on the working electrode. The potentiodynamic modes can be conducted
with voltammetric methods such as cyclic and differential pulse voltammetry [31]. Figure 4 represent electrochemical
methods for modification of electrode surfaces.
Modification of electrode surfaces can also be performed by electropolymerization directly from a monomer solu-
tion added to an inert electrolyte that has been initially deaerated [32]. Usually, the polymerization is carried out in a
potentiodynamic mode in an aqueous medium at a certain pH value [33]. This allows the deposition of a dense thin film
with high adhesion to the electrode surface. Potentiostatic electrolysis is used to a lesser extent in the development of
electrochemically modified electrodes, but is preferable from the point of view of the solubility of the monomer and
polymer form and because of the possibility of obtaining porous coatings [34]. The polymerization medium is also essen-
tial because organic solvents can either enhance or deteriorate the reproducibility of the resulting layer, depending on
their specific properties and interactions during the polymerization process [35].
Electrodeposition of metallic micro- or nanostructures can be performed in two modes of operation, as mentioned
above. The solution used contains salts of the deposited metal (e.g. AgNO3 for Ag) with a very low concentration, typi-
cally in range of 0.1 to 1 mM, which is essential for achieving controlled deposition. Crucial factors for controlling the Vol.:(0123456789) Review
Discover Electrochemistry (2024) 1:12
| https://doi.org/10.1007/s44373-024-00012-8
Fig. 4 Electrochemical methods for modification of electrodes surface
size and shape of the resulting nanostructures during modification are the applied potential, current density, solution
concentration, deposition time, and scan rate [36]. With the use of electrochemical techniques, nanostructures with
targeted (1 nm – 100 nm) sizes, well-defined composition and morphology can be obtained. The advantages are the
absence of unwanted by-products, shorter synthesis time (for example, 3 s [37]), and the negated use of a binder to fix
the particles, which is usually required in the "drop coating" method.
The rapid progress of modern technologies necessitates the inclusion of nanomaterials in the design of electrochemi-
cal devices used for batteries [38], fuel cells [39], water purification [40] and biomedicine [41]. This revolution in scientific
achievements is based on the specific characteristics of nanoparticles such as efficient catalysis, improved mass transfer,
and enlarged electrode surface, arising from the large surface-to-volume ratio. Significant progress has been made in
the synthesis of nanomaterials with controllable morphologies such as spherical, rod-like, and sheet like structures;
sizes ranging from few nanometres to several micrometers; tunable surface charges (positive, negative or neutral); and
adjustable physicochemical properties such as porosity, crystallinity, and surface area [42–44]. Their use as modifiers of
conductive matrices enables the development of new sensitive and selective electrochemical systems [45–50].
1.5 Materials for modifying electrode surfaces
1.5.1 Nanostructured metallic materials
Nanosized metallic materials have been extensively studied due to their characteristics: catalytic and antibacterial activ-
ity, and electronic and magnetic properties [51]. Their use offers additional advantages that are of great importance for
electrochemical research: excellent electrical conductivity, corrosion resistance, high catalytic activity in various reac-
tions, an increase in the electrochemically active surface, biocompatibility and functionalization of their surface with organic molecules [52–54].
Notably, nanostructured metals can be combined in the form of core–shell structures, where the core and shell are
made of different materials [55]. This combination of materials allows their physicochemical properties to be combined,
which in turn improves the electrochemical performance of the devices under development. Nanostructures of noble
metals such as gold (Au), silver (Ag), platinum (Pt), and palladium (Pd) and their bimetallic alloys are often used in the
development of electrochemical devices for various applications. This is due to their physical, chemical, and electrochemi-
cal properties, which vary depending on their sizes and shapes. For instance, smaller nanoparticles exhibit higher surface
area-to-volume ratios, which enhances their catalytic activity in electrochemical reactions. Additionally, the shape of
particles, such as rods, spheres, or cubes, influences their reactivity; rod-shaped particles have been shown to facilitate
faster electron transfer, improving electrochemical performance. Furthermore, specific crystal facets exposed by different
shapes can enhance catalytic efficiency, as demonstrated in studies involving platinum and gold nanoparticles [56–59].
There have hitherto been many publications on silver nanostructure-modified electrodes that exhibited electrocata-
lytic activity towards the electroreduction of various substances [60]. Vol:.(1234567890)
Discover Electrochemistry (2024) 1:12
| https://doi.org/10.1007/s44373-024-00012-8 Review
In the last two decades, researchers’ efforts have been focused on the development of new electroanalytical devices
for quantitative and qualitative determination of various substrates such as markers of severe diseases, presence of
biological and infectious agents for early-stage disease detection based on silver nanoparticles (AgNPs) [61] and their
nanocomposites [61]. For instance, AgNPs have been successful y integrated into electrochemical sensors for the detec-
tion of dopamine in human serum, achieving a detection of limit as low as 7 nM, demonstrating selectivity and stability
[56]. Furthermore, Ag-based nanocomposites have demonstrated significant potential in the detection of hydrogen
peroxide, exhibiting a high sensitivity of 8.9273 µA µM⁻1 cm⁻2 and low detection limit of 0.23 ± 0.012 µM. These proper-
ties make them highly valuable for applications in healthcare safety and environmental monitoring [62].
Carbon core and silver shell nanocomposite (Ag@C) doped with nickel (Ni) have been used to modify a glassy carbon
electrode [63]. The electrochemical behavior of the nanocomposite Ni/Ag@C demonstrated good electrocatalytic activity
in the reduction of H2O2, in a wide linear range from 0.03 mM to 17 mM. Another type of electrocatalyst for hydrogen
peroxide reduction was created based on AgNPs embedded on the surface of carbon quantum dots (CQDs). The modifica-
tion of a GCE was carried out by the "drop casting" method [64]. According to the authors, the achieved results show that
AgNPs embedded in CQDs are promising electrocatalysts for the reduction of H2O2, which can be attributed a 1.5-fold
larger quantity of AgNPs dispersed in the nanocomposite, compared to similar AgNPs—only systems.
Moreover, quantitative electrochemical investigations revealed that the AgNPs/CQDs nanohybrid sensor exhibited
a high sensitivity of 1.5 μA/μM, a detection limit of 80 nM, and a linear range from 0.2 to 27.0 μM for H2O2 detection.
These parameters clearly demonstrate the superior electrocatalytic performance of the AgNPs/CQDs nanocomposite compared to other materials.
In another study [65], tannic acid was coated on AgNPs—decorated magnetic Fe3O4, where tannic acid has been
used as a stabilizing and reducing agent for silver ions, via interaction of its hydroxyl and ketone groups with silver ions: C H O (tannic acid) + Ag+ 76 52 46
→ AgNPs + Oxidized tannic acid (1)
The modified electrode was used for the electrochemical determination of the ecologically toxic 4-nitrophenol. Accord-
ing to the authors, the high sensitivity of the signal during the reduction of the analyte is related to the effectiveness of
the nano-hybrid. The sensitivity of the electrode was measured with a detection limit of 33 nM and a wide working range
from 0.1 to 680.1 μM, indicating a significant improvement compared to other systems. These data demonstrate that the
nano-hybrid has high efficiency in electrochemical detection and catalytic reduction of 4-nitrophenol.
The development of electrochemical devices by modifying electrode surfaces with platinum nanomaterials has
attracted the attention of scientists due to their distinctive electronic and electrocatalytic properties [66]. Platinum
nanomaterials exhibit exceptional conductivity, high stability, and catalytic activity in various electrochemical reactions,
such as hydrogen or methanol oxidation and oxygen reduction [67]. For example, platinum nanoparticles demonstrate
significantly lower overpotentials in the hydrogen evolution reaction (HER) and the oxygen reduction reaction (ORR),
making them ideal for fuel cells and other electrochemical applications [68]. The uniform distribution of nanomaterials
on the electrode surface can be related to the stability of the colloidal suspension used. In this context, the colloidal
suspension refers to the dispersion nanoparticles in liquid medium, which is essential to achieve an even coating on
the electrode surface. Stabilizing reagents such as surfactants are used to prevent the agglomeration of PtNPs in the
colloid-dispersed systems, which would lead to uneven coatings [69]. Although PtNPs are characterized by selectivity and
sensitivity for many electrochemical reactions, they have two significant drawbacks—scarcity and high cost. Combining
them with other nanomaterials reduces cost and extends their electrocatalytic properties. Shahid et al. used the "drop
casting" technique to modify a glassy carbon electrode with a nanocomposite of reduced rGO, cobalt oxide (Co3O4)
nanocube, and platinum [70]. The studies showed that the modified electrode demonstrated excellent catalytic activity
in the electrochemical oxidation of nitric oxide, represent by the reaction: NO + H O + 2H+ + e− 2 → NO− 2 (2)
This catalytic performance was quantitatively demonstrated by the shift in oxidation potential to + 0.84 V and an
increased peak current of 119 µA, indicating superior electron-transfer kinetics at the modified electrode surface. The
authors attributed this enhanced activity to the synergistic effect between the Co3O4 nanocubes, platinum nanoparti-
cles, and rGO. Specifically, Co3O4 nanocubes provided a large surface area for the deposition of Pt nanoparticles, while
Pt nanoparticles enhanced electron transfer and reduced the overpotential. The rGO matrix further facilitated charge
transfer and prevented the agglomeration of Co3O4 nanocubes, thereby contributing to the overall improved catalytic performance. Vol.:(0123456789) Review
Discover Electrochemistry (2024) 1:12
| https://doi.org/10.1007/s44373-024-00012-8
Gold nanoparticles and their nanocomposites are widely used in the field of electrochemical research, due to the fol-
lowing advantages: (1) simple preparation methods; (2) increasing the electrochemically active surface area; (3) biocom-
patibility; (4) electrochemical stability over a wide range of potentials (typically from −0.2 V to + 1.2 V vs. Ag|AgCl), and
(5) high catalytic activity [71]. Additionally, their high catalytic activity is demonstrated through their ability to enhance
electron transfer rates in various electrochemical reactions. Al these properties provide the possibility of miniaturization
of electrochemical devices, fast response, and high sensitivity [72]. Au-based nanomaterials have hitherto been report-
edly used in the development of electrochemical devices for the detection of analytes, such as hydrogen peroxide [73],
metal ions [74], organic compounds [75], and others.
Won et al. reported that the performance of electrochemical catalysts can be potentially affected by Au morphology
nanocrystals [76]. They found that electrodes modified with Au nanospheres and Au nanorods have similar characteristics
in terms of limit of detection of H2O2, but the Au nanorod-coated electrode exhibits a higher sensitivity (58.51μA mM−1)
due to the smaller number of interfacial boundaries between the nanorods. A Au nanocage-modified GCE also exhibited
a 4.4 times increase in electrochemically active surface area, which has in turn improved the detection sensitivity by
5.3 times [77]. In addition, there was a ~ 2-s response time, which is most likely due to the hollow structure of the cage
facilitating the diffusion of the analyte to the surface.
A synthetic method for obtaining bimetallic Au/Ag (core/shell) nanorods, embedded in an amino-functionalized sili-
cate sol–gel matrix (Au/Ag-N1-[3-(trimethoxysilyl)propyl]diethylene triamine nanords) in an aqueous medium, applied
in the electrochemical detection of hydrogen peroxide and nitrite ions, was developed. The amperometric electrode
was used to detect the analytes at physiological pH, without the need for a mediator, which the authors attributed to the
synergistic effect between the Au core and the Ag shell [78]. Specifically, Au provides excellent conductivity and stabil-
ity, while Ag enhances the electrocatalytic activity, particularly in the reduction of hydrogen peroxide. This combination
improves the electrode’s performance, allowing for efficient detection of these analytes under physiological conditions.
In addition to high catalytic activity and improved conductivity, metal nanoparticles are often able to retain a bioele-
ment in proximity close to the electrode surface [79]. Strategies to achieve this are based on (i) physical interactions,
such as electrostatic attraction, which can enhance the binding affinity between the nanoparticle and the bioelement;
(ii) chemisorption of sulfur-containing organic compounds on Au, Ag, and Pt nanostructures, which forms strong bonds
that improve the stability of the bioelement on the surface [80]; and (iii) the formation of covalent bonds, which provide
a robust attachment of the bioelement to the electrode, enhancing the overall detection sensitivity [81]. For instance,
studies have shown that using specific thiol compounds can significantly increase the retention of enzymes on gold
nanoparticles, resulting in improved catalytic performance.
Despite all the advantages, electrochemical devices face several limitations, such as moderate selectivity in the analysis
of some real-life samples and lack of reproducibility of measurements. In an attempt to overcome these drawbacks in
recent years the range of materials that are used to modify electrodes has been constantly expanding.
1.6 2D carbon nanomaterials
After the breakthrough discovery of single-layer graphene in 2004 [82], 2D carbon nanomaterials have attracted the atten-
tion of many researchers in various fields ranging from electronics to biomedicine [83]. Their unique catalytic properties
and high surface-to-volume ratio make them suitable for a wide range of applications, such as environmental catalysis
[84], drug delivery carriers [85], and sensing and energy applications [86]. These nanomaterials are often combined with
other materials to form nanocomposites that significantly improve the sensitivity (for example, x-fold, depending on the
system used [87]) and selectivity of modified surfaces, as a result of the synergistic effect between them. The enhanced
selectivity is primarily due to the specific surface functional groups and tunable electronic properties of 2D carbon
nanomaterials, such as graphene and its derivatives, which allow for targeted interaction with specific analytes [88]. In
addition, they can be functionalized with biomolecules, which effectively improves conductivity, mechanical stability, and direct electron transfer.
Graphene is a single-layer carbon material composed of carbon atoms in the sp2—hybridized state, which has a
large specific surface area (theoretically, it is 2630 m2 g−1), a high electron transfer rate (15,000 cm2 V−1 s−1), exceptional
mechanical strength (Young’s modulus ~ 1 TPa and tensile strength up to 130 GPa) and excellent thermal and electrical
conductivity (~ 5000 W/m·K for thermal conductivity and over 6000 S cm−1 for electrical conductivity) [89]. These proper-
ties make it an attractive material for the production of modified electrodes for various electroanalytical applications [90].
However, graphene is hydrophobic and tends to form agglomerates, which hinders its further application in electrochemi-
cal research. The absence of surface functionality and low solubility in water, as well as in most organic solvents, limit its Vol:.(1234567890)
Discover Electrochemistry (2024) 1:12
| https://doi.org/10.1007/s44373-024-00012-8 Review
practical application. To address these limitations and to improve the mechanical stability, electrochemical active surface,
and signal sensitivity, graphene is often combined with AuNPs or electrodeposited polymers such as polypyrrole [91].
Graphene oxide (GO) consists of a hexagonal carbon network with oxygen-containing functional groups combined
with non-oxidized regions where most of the carbon atoms are in sp2 hybridization. The high content of functional groups
in the form of hydroxyl and epoxy parts at the base of the plane and carboxyl groups at the edges of the sheet make it
highly hydrophilic [92]. Therefore, it is easily dispersed in water, thus facilitating the modification of electrode surfaces,
for example, by the “drop casting” method [93]. GO is an excellent platform for covalently immobilizing bio-receptors
due to its oxygen-containing functional groups and large surface area. However, predominant functional parts of GO
result in insulating properties, which is a significant disadvantage. In this respect, GO is often electrochemically reduced
to lower the number of oxygen-containing groups on the surface. This process enhances its electrical conductivity by
converting GO to rGO [85] through the following reaction: GO + H+ + e− → rGO + H O 2 (3)
Electrochemically prepared rGO sheets show a smaller O/C ratio (0.1–0.3) and are more conductive (500 – 10 000 mS
m−1) compared to those obtained by chemical methods (0.3–0.6 and 50–500 mS m−1) [86]. The oxygen-containing func-
tional groups must be properly balanced. To achieve the optimum effectiveness of the modifier, it is crucial to enhance
both its stability and conductivity. On the other hand, modification of electrode surfaces with composites of rGO and
nanoparticles of metal or/and metal oxides demonstrates excellent reproducibility (with relative standard deviation
values typically below 5% in repeated measurements) and electrical conductivity (with conductivity values reaching up
to ~ 104 S cm−1), for example, in the electrochemical determination of heavy metal ions [94].
It has been established that the most effective approach to improving the electrocatalytic activity of carbon materials
is the addition of nitrogen atoms [95]. Carbon nitride-based compounds with a high N:C ratio (typically around 0.75 or
higher) are new-generation materials that are used to develop electrocatalysts [96]. Carbon nitrides reveal different crystal
structures, for example, tetrahedral carbon nitride (β-C3N4) and graphitic carbon nitride (g-C3N4). The β-C3N4 material
has high hardness of approximately 60 GPa and low compressibility, with a bulk modulus of about 400 GPa, similar to
diamond. In contrast, g-C3N4 is considered the most stable under ambient conditions and exhibits a lower hardness of
around 2–3 GPa and a bulk modulus of approximately 20–30 GPa [97]. g-C3N4 is a non-metallic, graphene-like 2D material
comprising mostly covalent bonds (CN), forming a π- π-conjugated polymer with a high molecular weight and a unique
electronic structure predetermining its semiconductor properties [98]. The 2D structure of g-C3N4 is composed of tri-s-
triazine units, possessing a high degree of condensation, which regulates its high thermal and chemical stability [99].
Therefore, g-C3N4 is regarded as a multifunctional, heterogeneous, non-metallic catalyst [100] whose main drawback is
the poorly developed specific surface area. Melamine has been reported to be successfully used as a precursor for they
synthesis of g-C3N4 [101] due to its low cost and toxicity while providing increased surface area and pore volume. Another
approach to increase the specific surface area of g-C3N4 is doping it with oxides of transition metals in the form of an
ultrafine dispersion, which are distributed between the nanosheets of g-C3N4 and prevent their agglomeration [102].
1.7 Transition metal oxide nanoparticles
Transition metal oxides (TMOs) possess good electrical and photocatalytic properties due to their size, shape, and larger
surface area [103]. The excessive scientific interest in developing electrocatalysts with TMOs nanoparticles is due to their
thermal stability, radiation resistance, and environmental safety [104]. The main drawback is the large energy bandgap
(Eg > 3 eV) makes them semiconductors and insulators [105]. This limitation can be minimised by conjugating the nano-
particles with a carbon matrix or other metallic nanostructures.
Among metal oxides, iron oxide (Fe3O4) nanoparticles have attracted attention in various electrochemical applica-
tions. They are highly desirable due to their biocompatibility, non-toxicity, thermal stability, and optical and magnetic
properties [106, 107]. These nanoparticles can effectively modify electrodes for the electrochemical detection of several
compounds. The exchange of electrons between Fe2+ and Fe3+ makes Fe3O4 nanoparticles an excellent electrical conduc-
tor even at room temperature [108]. To enhance their selectivity and sensitivity, they are often combined with metals
such as nickel [109], cobalt [110], silver [111] and others.
Iron oxide nanoparticles are dispersed in carbon matrices like graphene [112], GO [113], and carbon nanotubes [114],
to obtain different nanocomposites. The carbon matrix acts as a supporting medium for the homogeneous dispersion
of nanoparticles, prevents their aggregation, and gives a high surface/volume ratio (∼200 m2 g−1). Their use as electrode Vol.:(0123456789) Review
Discover Electrochemistry (2024) 1:12
| https://doi.org/10.1007/s44373-024-00012-8
modifiers increases the electroactive surface area, which improves signal selectivity and sensitivity. Another modifier
used is titanium dioxide (TiO2) nanoparticles, which are electrically conductive, chemically stable in both alkaline and
acidic medium, and are relatively cost effective [115]. Recent studies highlight that TiO2-based nanostructures, par-
ticularly non-stoichiometric variants like TiO2-X, demonstrate enhanced sensitivity and selectivity in gas sensors due to
their unique electronic properties [116]. Furthermore, TiO2-X heterostructures have been shown to reduce energy con-
sumption in sensors by operating in a ’self-heating’ mode, improving efficiency in gas and volatile organic compound
detection. The presence of surface active centers makes them a promising material for modifying various electrodes
for many electrochemical applications [117, 118]. The main disadvantages in developing electrochemical devices from
TiO2 alone are the low solubility of the nanoparticles and the instability of the film on the electrode surface, which often
leads to low sensitivity [108]. To overcome this issue, titanium oxide particles are combined with GO [119], AuNPs [120],
quantum dots, etc. This approach combines their catalytic properties to obtain new electrocatalysts with high selectiv-
ity and sensitivity in electroanalytical practical applications [121]. Selectivity of a TiO2-coated electrode refers to the
electrode’s ability to recognize and react with a specific analyte in the presence of other interfering substances. In the
context of electrochemical systems, this means that the TiO2 coating enhances the catalytic activity of the electrode
in a way that allows for selective detection of hydrogen peroxide (H2O2) or other target analytes, while minimizing the
effect of unwanted reactions with other compounds in the sample. The reaction of H2O2 on a TiO2-modified electrode can be represented as follows: TiO + H O + 2H+ + 2e− + O 2 2 2 → TiO2 2 (4)
Among the transition metal oxides, a very promising catalyst exhibiting high catalytic activity and stability in several
electrochemical reactions are the oxides of Co (II and/or III) with a spinel structure, particularly Co3O4 [122]. Different
synthesis techniques of cobalt oxide nanoparticles such as electrodeposition [123], chemical methods [70], green syn-
thesis [124], and calcination [125], have already discussed, which help control the size and shape of the nanoparticles.
A study [126] reported a H2O2 electrochemical sensor based on cobalt oxide nanoparticle | electrochemically rGO | GCE.
It was found that the inclusion of spinel Co3O4 between the sheets of g-C3N4 significantly affects their structure: the
specific surface area of carbon nitride doped with metal oxide is much higher than that of pristine g-C3N4, increasing
from 0.0025 cm2 to 0.097 cm2 [127], which may be due to the high dispersion of Co3O4 between the g-C3N4 layers, which
prevents particle agglomeration. The Co-g-C3N4 composite shows high activity and good results in the catalytic activa-
tion of peroxymonosulfate compared to pure Co3O4 [128].
1.8 Polymer films and enzymes
Conducting polymers such as polypyrrole (Ppy), polyaniline (PANI), polythiophene (PTH), and poly(3,4-ethylenedioxy-
thiophene) (PEDOT: PSS) are essential in the development of electrochemical sensors and biosensors due to their excel-
lent electrochemical properties, conductivity, and biocompatibility. These materials are widely recognized for their role
in improving the performance of analytical and bioanalytical systems, as outlined in reviews like [129]. The application
of conducting polymers, especially when electrochemically deposited on electrode surfaces, enhances sensor perfor-
mance by providing controlled film thickness, morphology, and high surface area, which significantly improves sensor
sensitivity and selectivity. Electrochemically deposited conducting polymer films can immobilize biological molecules
such as enzymes and antibodies, enhancing the electrochemical response of the sensor. As discussed by Ramanavicius
et al. [130], molecularly imprinted polymers (MIPs) enable the devolpment of highly selective sensor systems tailored
for specific analytical applications, providing an efficient and cost-effective alternative to conventional recognition ele-
ments such as antibodies and receptors by offering customizable recognition sites for target molecules. A major area
of advancement in electrochemical sensors is the improvement of charge transfer between redox-active enzymes and
electrodes, achieved through the use of conducting polymers. Polymers such as PEDOT: PSS and Ppy have demonstrated
their ability to facilitate efficient electron transfer in enzymatic systems like glucose oxidase (GOx)-based sensors. Dis-
cussions examining the aspects of charge transfer and the biocompatibility of conducting polymers used in enzymatic
biosensors and biofuel cells highlight key points regarding the charge transfer process. Additionally, emphasis is placed
on the biocompatibility of these materials, further supporting their application in fields such as biosensors and biofuel
cells [131]. These materials not only enhance electron transfer but also act as excellent immobilization matrices, improv-
ing the overall sensitivity and stability of enzymatic biosensors. Vol:.(1234567890)
Discover Electrochemistry (2024) 1:12
| https://doi.org/10.1007/s44373-024-00012-8 Review
Another important development in the field is the use of molecularly imprinted polymers (MIPs) for sensor design.
MIPs create specific recognition sites for target molecules, significantly improving sensor selectivity. A comprehensive
overview of how these polymers can be integrated into sensor systems to enhance analytical performance is provided
in the review discussing advances in MIPs-based affinity sensors. The combination of conducting polymers and MIPs
presents a promising approach for developing highly selective and sensitive electrochemical sensors.
The integration of electrochemically deposited conducting polymers and MIPs offers significant potential for improv-
ing the performance of electrochemical sensors and biosensors in a variety of fields, including biomedical diagnostics,
environmental monitoring, and food safety [132]. The researchers provide a solid foundation for future research and
development in this area, highlighting the ongoing advancements in charge transfer mechanisms, biocompatibility,
and material optimization that will continue to drive progress in sensor technology.
1.9 Electroanalytical applications
Globally, the development of sensor devices is developing dynamically, which is due to the diversity and constant tech-
nological improvements. Electrochemical sensors provide a practical solution for analyte detection and are widely used
in agriculture, food, and petroleum industries and environmental and biomedical applications (Fig. 5) [133]. For over
two decades, many nanomaterials with exceptional characteristics, such as metals, conducting polymers, metal oxides,
and carbon nanomaterials, have been used to construct electrochemical sensors to improve analytical results. Important
features determining the potential of electrochemical devices for practical application are low detection limits, typically
in the range of nanomalar concentrations (e.g. 1–10 nM), high sensitivity, often exceeding 100 µA mM of analyte con-
centration selectivity, and fast analytical response [134].
The development of electrochemical sensors has vast potential applications in the fields of nutrition and wellness.
Electrochemical sensors can be used to detect essential biomarkers related to nutritional status, such as glucose, vitamins,
minerals, and oxidative stress markers, which are critical in evaluating human health and wellness [135]. These sensors
provide quick, sensitive, and accurate quantification, making them ideal for monitoring dietary intake and metabolic health in real-time.
In food science, sensors are employed for the detection of contaminants, nutritional content analysis, and assessing
the freshness and safety of food products. For example, the detection of hydrogen peroxide in food matrices is crucial
for ensuring the safety and quality of perishable goods such as juices and dairy products [136]. The advancement of
electrochemical sensors can contribute significantly to personalized nutrition by offering tailored insights based on
individual biochemical feedback.
Peroxide compounds are used in many industries and can be found in various living cells, but they are dangerous
and toxic when the concentration exceed > 10–35% [137]. H2O2 is the simplest peroxide that exhibits oxidizing and
reducing properties at different pH values and is often used as a marker for oxidative stress analysis [138]. Oxidative
stress is believed to be a biomarker of many life-threatening diseases including atherosclerosis [139], diabetes [140],
cancer [141], acute myocardial infarction [142], and others [143]. In addition, H2O2 is a byproduct of reactions catalyzed
by most oxidase enzymes, such as glucose oxidase, cholesterol oxidase, and lactate oxidase [144, 145]. In the food and
paper industry, H2O2 is often utilized for its bactericidal and bleaching properties. However, it is important to note that
the strong oxidizing power of H2O2 can pose potential safety risks to human health. Prolonged exposure to H2O2 may
cause damage to the skin and organs, making it important to handle with care.
Fig. 5 Schematic representa- tion of the electrochemical applications Vol.:(0123456789) Review
Discover Electrochemistry (2024) 1:12
| https://doi.org/10.1007/s44373-024-00012-8
Waifalkar et al. [146], developed an electrocatalyst consisting of magnetic Fe3O4, graphene, H2O2 for the cyclic volta-
metric determination of hydrogen peroxide. The electroreduction of the analyte was observed at a potential of -0.8 V (vs.
SCE) with a very good sensitivity of 48.08 µA µM−1 cm−2 in the concentration range from 0 to 100 µM. By quantitatively
determining hydrogen peroxide in real-life orange juice samples, a recovery of ∼ 99% (relative standard deviation)
was estimated, indicating minimal interference by the orange juice matrix. The recovery was felicitated by the enzyme
horseradish peroxidase (HRP). The study was conducted on natural orange juice samples to verify the applicability of the
modified electrode for the determination of hydrogen peroxide. Studies were made in real samples of natural orange
juice for determination of hydrogen peroxide to verify the applicability of the modified electrode. The measured amounts
of H2O2 are very close to the values of the added concentrations, and according to the authors, the orange juice matrix
does not interfere with the hydrogen peroxide reading. In another study, a Nafion | Pd@Ag bimetallic nanoparticles |
(3-aminopropyl) triethoxysilane functionalized rGO-immobilised GCE used to amperometrical y detect hydrogen peroxide
was demonstrated to show a fast response time of 2 s and a sensitivity of 1307.46 µA µM−1 cm−2 [147]. Electrochemical
reduction of the analyte was carried out at a constant potential of −0.45 V with a linear range from 0.002 to 15.90 mM
and a detection limit of 0.7 µM. The modified electrode demonstrated excel ent stability, retaining approximately 93.4%
of its initial current response after 30 days, highlighting its practical applicability in real-life sample analysis. Analysis was
performed to determine known concentrations of H2O2 in cow milk with a recovery rate between 96.76 and 102.42%
Cyclic voltammetry and amperometric detection were used to study the electrochemical reduction of hydrogen per-
oxide on an electrode coated with hollow carbon spheres and electrodeposited PtNPs [148]. The authors attribute the
high electrocatalytic sensitivity (42.8 µA µM−1 cm−2) and low detection limit (6.25 μM) to the combined effect of these
modifiers. The peroxide electrode has been used successfully to determine the concentration of H2O2 in samples of a
solution in which seafood has been immersed.
The detection of hydrogen peroxide released from living cells requires the development of electrocatalysts that are
highly sensitive and selective. In this regard, new atomic-thick PtNi nanowires have been synthesized and attached to
reduced carbon oxide through an ultrasonic method [149]. The GCE modified with the nanocomposite material shows
good performance in H2O2 quantification in a wide range of concentrations from 1 nM to 5.3 mM (detecting a minimum
of 0.3 nM) at an applied potential of -0.6 V (vs. Ag|AgCl). Furthermore, the atomic-thick PtNi nanowires provide high-
density active sites and facilitate efficient electron transfer, enhancing the selective detection of H2O2 while minimizing
interference from substances such as fructose, glucose, ascorbic acid, and uric acid, commonly present in biological
medium. The selectivity of the amperometric electrode is an indication of its practical applicability for the detection of
hydrogen peroxide released by cancer cells.
Many diseases are linked to the generation of reactive oxygen species (ROS) such as superoxide radicals, hydroxyl
radicals, and hydrogen peroxide. This imbalance between the ratio of oxidants and antioxidants (in favour of oxidants)
in living cells leads to oxidative stress. Neurotransmitters can also serve as an indicator of the amount of oxidative stress
in human metabolism. Therefore, monitoring these compounds has become increasingly important in recent years in
medical and environmental applications [150]. Conventional methods of measuring these compounds, including spectro-
photometry and chromatography, are time-consuming and expensive. An alternative possibility for quick, real-time, and
highly accurate quantification of catecholamines is to use electrochemical techniques and to develop electrochemical
sensing devices that include a biological element (such as an immobilized enzyme) and a signal transducer.
A major problem with an electrochemical enzyme sensor is an efficient electron transfer rate between the active
centre of an enzyme and the electrode surface. The development of enzyme electrodes by combining nanomaterials
and bioelements has several advantages: excellent electrical conductivity, with electron transfer rates increased by up
to 100-fold compared to traditional materials, a large electrochemically active surface area (e.g. up to 150 m2 g−1), and
reduced electron transfer resistance (often deceased by up to 50%) [151]. A new enzyme electrode based on a (AuNP,
MoS2, Nafion, or laccase)-modified GCE was developed for the determination of catechol [152]. The electrocatalytic
current was found to increase proportionally with increasing analyte concentration over a wide linear range (from 2 to
2000 µM) and a relatively low detection limit (2 µM). The authors hypothesize that the low detection limit is a result of the
excellent conductivity and electrocatalytic properties of the AuNPs, which enhance electron transfer and facilitate the
enzymatic reaction, thereby improving the sensitivity of the biosensor. Two different strategies for the quantification of
serotonin and dopamine were recently reported [153], where poly(2,6-bis(3,4-ethylenedioxythiophene)-4-methyl-4-octyl-
dithienosilol) and laccase-modified Pt electrode was developed to detect serotonin, and a poly(2,6-bis(selenophen-
2-yl)-4-methyl-4-octyl-dithienosilol) and horseradish peroxidase-modified Au electrode for detecting dopamine. These
silole derivative and oxidoreductase-based enzyme electrodes show high selectivity owing to enzyme specificity and
electroconducting polymer structure, and high reproducibility (± 3.9–0.88% relative standard deviation). According to Vol:.(1234567890)
Discover Electrochemistry (2024) 1:12
| https://doi.org/10.1007/s44373-024-00012-8 Review
the authors, the characteristics of both systems allow the development of a disposable biosensor for in vitro use or in situ applications.
Rubio-Govea et al. [154] developed an amperometric dopamine biosensor based on 2D layered MoS2 and LacII-
modified carbon paper electrodes (where LacII denotes a type of laccase). By applying the LacII-modified electrodes to
the determination of dopamine in a synthetic urine sample, a detection limit of 0.67 µM was estimated, which is below
the average dopamine concentration (~ 2 µM) typically found in real-life urine samples. However, further research is
required to facilitate the application of the biosensor to real-life sample analysis.
Coelho et al. [155] developed a laccase-loaded botryospherane film multiwalled carbon nanotube-immobilised GCE,
which showed excellent selectivity towards dopamine and spironolactone owing to he high sensitivity (linear range
of 2.99–38.5 μmol L−1 and detection limit of 0.127 μmol L−1 for dopamine) and selectivity of the biosensor, even in the
presence of interfering species including ascorbic acid, uric acid and phenolic compounds. The enzyme electrode is
characterized by excellent selectivity towards dopamine and spironolactone, with limit of detention of 0.94 µmol L−1
for spironolactone, despite the influence of side analytes. The developed system demonstrates sensitivity, stability, and
good intraday reproducibility, as well as the possibility of long-term storage. It has been successfully applied to quantify
dopamine in pharmaceutical and biological samples.
The electrocatalytic oxidation of dopamine on an electrode modified with CQDs and immobilized laccase was studied
by differential pulse voltammetry [156]. The large specific surface area of CQDs provides many catalytic centers on the
electrode surface, which facilitates electron exchange between the enzyme and the electrode. The electrocatalytic activity
of the laccase electrode showed a low detection limit of 0.08 µM and a wide linear concentration range of 0.25–76.81 µM.
The obtained analytical recovery of 101.9–103.5% with a standard deviation of 0.53% to 1.01% shows that the method
is acceptable for the determination of catechol in a complex environment of human serum.
By electropolymerization of pyrrole on a Pt electrode with subsequent immobilization of laccase, an amperometric
electrode for the quantification of epinephrine was developed [157]. Quantitative analysis of catecholamine was per-
formed by electrochemical reduction of enzymatically produced (generated) epinephrine-quinone at a constant poten-
tial of −0.220 V (vs. Ag|AgCl). The developed enzyme electrode demonstrated good analytical parameters, including a
low detection limit of 0.01 µM, high linearity over a wide concentration range (R2 = 0.998 for 0.1–1.0 µM, R2 = 0.995 for
1.0–10.0 µM), good reproducibility with 5.08% RSD after 31 measurements, and a stable response over 10 days, retain-
ing 80.9% of its initial response. The sensor was used for the determination of epinephrine in an antibiotic drug. A main
drawback is a 200-s duration for the sensor to reach a steady-state signal.
Dopamine and epinephrine are structurally similar catecholamines that play the role of neurotransmitters for clinical
diagnosis, whose elevated or decreased levels in blood plasma or urine often indicate pathological conditions such as
tumors or serious neurological disorders [158]. Both catecholamines, dopamine, and L-epinephrine, are organic sub-
stances of pharmaceutical importance, as both compounds are marketed in the form of injectable solutions. Therefore,
the development of rapid and sensitive methods for their quantification is of important practical importance for the
pharmaceutical industry. In addition, both catecholamines are prone to rapid degradation even when exposed to air for
a short time. Despite the importance of NTs for clinical diagnosis, existing quantification methods still need improve-
ments in terms of selectivity [159], sensitivity, flexibility, and simplification [160]. In an attempt to minimise the limita-
tions inherent in routinely used diagnostic methods for the determination of catecholamines, such as chromatography
[161, 162], chemiluminescence [163], electrophoresis [164], or electroanalysis with chemically modified electrodes have been proposed [165–168].
Conventional analytical techniques lack the advantages of electrochemical techniques, which include device minia-
turization, fast response, high sensitivity, and good selectivity. Electrochemical techniques are also suitable for detecting
neurotransmitters in the presence of mixtures of other molecules with high temporal resolution. Electrochemical sen-
sors can be incorporated into portable devices for targeted applications in diagnostic and clinical fields as biomarkers
of some diseases, e.g. Alzheimer’s and Parkinson’s [169, 170].
Alvarez et al. [171] reported a specific, selective, and sensitive RNA-based dopamine aptasensor showing a submicro-
molar detection limit. Recently, various enzymatic catecholamine biosensors using monoamine oxidase [172], cellobiose
dehydrogenase [173], and pyrroloquinoline quinone-dependent glucose dehydrogenase [174] have also been reported.
Josypčuk et al. studied the conditions of four biosensors in a flow-injection system with mini reactors filled with dif-
ferent mesoporous powdered SiO2 covalently linked with the enzymes laccase and tyrosinase (polyphenol oxidases)
for the determination of catechols [175]. Utilizing an injector, a sample of the corresponding catecholamine was trans-
ported to the enzyme reactor, where the substrate was oxidized to a quinone by the immobilized polyphenol oxidase.
At a constant potential, the quinone was electrochemical reduction to form of current response, which enhanced the Vol.:(0123456789) Review
Discover Electrochemistry (2024) 1:12
| https://doi.org/10.1007/s44373-024-00012-8
detection signal. The reduction peak current was found to be directly proportional to the catecholamine concentration of the injected sample.
1.10 Challenges and perspectives
The development of new materials and modification methods for electrodes remains central to modern electroanalysis.
Nanomaterials like graphene, gold, and silver nanoparticles show great promise in improving the sensitivity and selec-
tivity of electrochemical sensors, but several key challenges must be addressed to advance the field. One major chal-
lenge is miniaturization and portability. The growing demand for smaller, wearable devices, such as implantable medical
sensors and smartwatches, calls for innovations that enable continuous monitoring. However, ensuring the necessary
accuracy and stability in these miniaturized systems is technically difficult, particularly with issues like environmental
interferences and power efficiency. Green synthesis is another priority, as traditional methods often use toxic chemicals
and energy-intensive processes. Future research will focus on sustainable, eco-friendly production methods, including
the use of biocompatible reducing agents and renewable resources, ensuring that new materials are both effective and
safe for the environment and human health. The durability and stability of modified electrodes are also critical. Many
nanomaterial-based electrodes face degradation when exposed to real-world conditions with strong interferences.
Developing more robust materials that maintain performance in harsh environments—such as extreme pH or salinity—is
essential. Conductive polymers hold particular promise here, offering improved stability, conductivity, and mechanical
strength as protective coatings or matrices for nanomaterials. In biosensors, achieving efficient electron transfer between
biomolecules and electrodes remains a challenge, limiting overall performance. Polymer-nanoparticle hybrids and con-
ductive polymer films are being explored to improve electron transfer and enzyme stabilization, which are essential for
longer sensor lifetimes and better response times. Reproducibility and scalability also present significant challenges, as
sensors that perform well in the lab often exhibit variability when scaled for industrial production. Standardizing fabrica-
tion methods and maintaining quality control will be crucial for commercial success.
Finally, cost-effectiveness remains a key issue. While noble metals like gold and silver enhance sensor performance,
their high cost limits large-scale manufacturing. To mitigate this, future efforts will focus on combining cheaper alterna-
tives, such as graphene and carbon nanotubes, with small amounts of noble metals and polymers, creating sensors that
balance high performance with affordability and scalability. 2 Conclusions
The future of electroanalysis lies in the continuous advancement of materials and sensor technologies to overcome the
current challenges in sensitivity, selectivity, and durability. Researchers are working to develop cost-effective and sustain-
able methods for synthesizing nanomaterials and improving the design of electrochemical sensors.
Key focus areas include the miniaturization of devices for wearable applications, enhancing electrode stability in com-
plex environments, and increasing the reproducibility of sensors for commercial use. The development of more robust,
portable, and eco-friendly sensors will significantly impact various fields, from medical diagnostics to environmental monitoring.
Overal , interdisciplinary col aboration between materials science, chemistry, and engineering wil be crucial for unlock-
ing the full potential of electrochemical sensing technologies in the future.
Acknowledgements The author gratefully acknowledges the support from the Bulgarian Ministry of Education and Science under the National
Program “Young Scientists and Postdoctoral Students – 2”.
Author contributions M.P. conducted the entire process of writing the review article, including conceptualization, literature research, analysis,
and synthesis of the collected information, as well as drafting the text. M.P. also prepared all tables and figures and reviewed and approved the final version.
Data availability No datasets were generated or analysed during the current study. Declarations
Competing interests The authors declare no competing interests. Vol:.(1234567890)
Discover Electrochemistry (2024) 1:12
| https://doi.org/10.1007/s44373-024-00012-8 Review
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adapta-
tion, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source,
provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article
are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in
the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will
need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. References
1. Apak R, Çekiç SD, Üzer A, Çelik SE, Bener M, Bekdeşer B, Can Z, Sağlam Ş, Önem AN, Erçağ E. Novel spectroscopic and electrochemical
sensors and nanoprobes for the characterization of food and biological antioxidants. Sensors. 2018. https:// doi. org/ 10. 3390/ s1801 0186.
2. Will-Cole AR, Hassanien AE, Calisgan SD, et al. Tutorial: Piezoelectric and magnetoelectric N/MEMS - materials, devices, and applications.
J Appl Physi. 2022. https:// doi. org/ 10. 1063/5. 00943 64.
3. Scozzari A. Electrochemical sensing methods: a brief review andrea scozzari*. Methods. 2008; 335–351
4. Moshirian-Farahi SS, Zamani HA, Abedi M. Nano-molar level determination of isoprenaline in pharmaceutical and clinical samples; a
nanostructure electroanalytical strategy. Eurasian Chem Commun. 2020;2:702–11.
5. Apak R, Üzer A, Sağlam Ş, Arman A. Selective electrochemical detection of explosives with nanomaterial based electrodes. Electroanalysis.
2023. https:// doi. org/ 10. 1002/ elan. 20220 0175.
6. Wang J. Analytical electrochemistry. Hoboken: Wiley; 2006. https:// doi. org/ 10. 1002/ 04717 90303.
7. Rana A, Baig N, Saleh TA. Electrochemically pretreated carbon electrodes and their electroanalytical applications – a review. J Electroanal Chem. 2019;833:313–32.
8. Fagiolari L, Sampò M, Lamberti A, Amici J, Francia C, Bodoardo S, Bella F. Integrated energy conversion and storage devices: interfacing
solar cells, batteries and supercapacitors. Energy Storage Mate. 2022;51:400–34.
9. Pollok D, Waldvogel SR. Electro-organic synthesis-a 21stcentury technique. Chem Sci. 2020;11:12386–400.
10. McCreery RL. The merger of electrochemistry and molecular electronics. Chem Rec. 2012;12:149–63.
11. Kraft A. Electrochromism: a fascinating branch of electrochemistry. ChemTexts. 2019;5:1–18.
12. Bi Li, Zhao R, Cao G, Xue,. Robust super-hydrophobic coating prepared by electrochemical surface engineering for corrosion protection. Coatings. 2019;9:452.
13. Díaz-Cruz JM, Serrano N, Pérez-Ràfols C, Ariño C, Esteban M. Electroanalysis from the past to the twenty-first century: challenges and
perspectives. J Solid State Electrochem. 2020;24:2653–61.
14. Mallakpour S, Behranvand V. Recent progress and perspectives on biofunctionalized CNT hybrid polymer nanocomposites. In: Thakur
VK, Thakur K, Pappu A, editors. Hybrid polymer composite materials: properties and characterisation. Amsterdam: Elsevier; 2017.
15. Bhagabati P, Rahaman M, Khastgir D. Morphology and spectroscopy of polymer-carbon. Composites. 2019. https:// doi. org/ 10. 1007/ 978- 981- 13- 2688-2_9.
16. Singh N, Sanyal U, Fulton JL, Gutiérrez OY, Lercher JA, Campbell CT. quantifying adsorption of organic molecules on platinum in aqueous
phase by hydrogen site blocking and in situ X-ray absorption spectroscopy. ACS Catal. 2019;9:6869–81.
17. Baffert C, Sybirna K, Ezanno P, Lautier T, Hajj V, Meynial-Salles I, Soucaille P, Bottin H, Léger C. Covalent attachment of FeFe hydrogenases
to carbon electrodes for direct electron transfer. Anal Chem. 2012;84:7999–8005.
18. Chen X, Cheng X, Gooding JJ. Detection of trace nitroaromatic isomers using indium tin oxide electrodes modified using β-cyclodextrin
and silver nanoparticles. Anal Chem. 2012;84:8557–63.
19. Bard AJ. Chemical modification of electrodes. J Chem Educ. 1983;60:302–4.
20. Mitzi DB, Kosbar LL, Murray CE, Copel M, Afzali A. High-mobility ultrathin semiconducting films prepared by spin coating. Nature. 2004;428:299–303.
21. Babar NUA, Asghar MN, Hussain F, Joya KS. Spray-assembled nanoscale cobalt-oxide as highly efficient and durable bifunctional elec-
trocatalyst for overall water splitting. Mater Today Energy. 2020;17:100434.
22. Dar RA, Khare NG, Cole DP, Karna SP, Srivastava AK. Green synthesis of a silver nanoparticle–graphene oxide composite and its applica-
tion for As( iii ) detection. RSC Adv. 2014;4:14432–40.
23. Suherman AL, Ngamchuea K, Tanner EEL, Sokolov SV, Holter J, Young NP, Compton RG. Electrochemical detection of ultratrace (picomolar)
levels of Hg2+ using a silver nanoparticle-modified glassy carbon electrode. Anal Chem. 2017;89:7166–73.
24. Qin X, Wang H, Miao Z, Wang X, Fang Y, Chen Q, Shao X. Synthesis of silver nanowires and their applications in the electrochemical
detection of halide. Talanta. 2011;84:673–8.
25. Zuo C, Scully AD, Gao M. Drop-casting method to screen ruddlesden-popper perovskite formulations for use in solar cells. ACS Appl
Mater Interfaces. 2021;13:56217–25.
26. Kaliyaraj Selva Kumar A, Zhang Y, Li D, Compton RG. A mini-review: how reliable is the drop casting technique? Electrochem Commun. 2020;121:106867.
27. Neuróhr K, Pogány L, Tóth BG, Révész Á, Bakonyi I, Péter L. Electrodeposition of Ni from various non-aqueous media: the case of alcoholic
solutions. J Electrochem Soc. 2015;162:D256–64.
28. Khudaish EA. Reversible potentiodynamic deposition of VO2/V2O5 network onto strongly oxidative glassy carbon electrode for quan-
tification of tamoxifen drug. Microchem J. 2020;152: 104327. Vol.:(0123456789) Review
Discover Electrochemistry (2024) 1:12
| https://doi.org/10.1007/s44373-024-00012-8
29. Hezard T, Fajerwerg K, Evrard D, Collière V, Behra P, Gros P. Influence of the gold nanoparticles electrodeposition method on Hg(II) trace
electrochemical detection. Electrochim Acta. 2012;73:15–22.
30. Tonelli D, Scavetta E, Gualandi I. Electrochemical deposition of nanomaterials for electrochemical sensing. Sensors. 2019. https:// doi. org/ 10. 3390/ s1905 1186.
31. Malyshev VV, Shakhnin DB. Titanium coating on carbon steel: direct-current and impulsive electrodeposition. Physicomech Chem Prop Mater Sci. 2014;50:80–91.
32. Ziyatdinova G, Guss E, Yakupova E. Electrochemical sensors based on the electropolymerized natural phenolic antioxidants and their
analytical application. Sensors. 2021. https:// doi. org/ 10. 3390/ s2124 8385.
33. Martins JI, Bazzaoui M, Reis TC, Costa SC, Nunes MC, Martins L, Bazzaoui EA. The effect of pH on the pyrrole electropolymerization on
iron in malate aqueous solutions. Prog Org Coat. 2009;65:62–70.
34. Abdel-Hamid R, Newair EF. Voltammetric determination of polyphenolic content in pomegranate juice using a poly(gallic acid)/multi-
walled carbon nanotube modified electrode. Beilstein J Nanotechnol. 2016;7:1104–12.
35. Fomo G, Waryo T, Feleni U, Baker P, Iwuoha E. Electrochemical polymerization BT - functional polymers. Cham: Springer International Publishing; 2019. p. 105–31.
36. Lee SA, Yang JW, Choi S, Jang HW. Nanoscale electrodeposition: dimension control and 3D conformality. Exploration. 2021. https:// doi. org/ 10. 1002/ EXP. 20210 012.
37. Nagaiah TC, Schäfer D, Schuhmann W, Dimcheva N. Electrochemically deposited Pd-Pt and Pd-Au codeposits on graphite electrodes for
electrocatalytic H2O2 reduction. Anal Chem. 2013;85:7897–903.
38. Sadabadi KK, Ramesh P, Guezennec Y, Rizzoni G. Development of an electrochemical model for a Lithium Titanate oxide nickel manganese
cobalt battery module. J Energy Storage. 2022;50:104046.
39. Amin HMA, Königshoven P, Hegemann M, Baltruschat H. Role of lattice oxygen in the oxygen evolution reaction on Co3O4: isotope
exchange determined using a small-volume differential electrochemical mass spectrometry cell design. Anal Chem. 2019;91:12653–60.
40. Garcia-Segura S, Qu X, Alvarez PJJ, et al. Opportunities for nanotechnology to enhance electrochemical treatment of pollutants in potable
water and industrial wastewater-a perspective. Environ Sci Nano. 2020;7:2178–94.
41. Daraee H, Eatemadi A, Abbasi E, Aval SF, Kouhi M, Akbarzadeh A. Application of gold nanoparticles in biomedical and drug delivery.
Artif Cells Nanomed Biotechnol. 2016;44:410–22.
42. Paulchamy B, Arthi G, Lignesh BD. A simple approach to stepwise synthesis of graphene oxide nanomaterial. J Nanomed Nanotechnol. 2015;06:1–4.
43. Boles MA, Ling D, Hyeon T, Talapin DV. Erratum: the surface science of nanocrystals. Nat Mater. 2016;15:364–364.
44. Duan H, Wang D, Li Y. Green chemistry for nanoparticle synthesis. Chem Soc Rev. 2015;44:5778–92.
45. Tajik S, Taher MA, Beitollahi H. First report for electrochemical determination of levodopa and cabergoline: application for determination
of levodopa and cabergoline in human serum, urine and pharmaceutical formulations. Electroanalysis. 2014;26:796–806.
46. Venu M, Venkateswarlu S, Reddy YVM, Seshadri Reddy A, Gupta VK, Yoon M, Madhavi G. Highly sensitive electrochemical sensor for
anticancer drug by a zirconia nanoparticle-decorated reduced graphene oxide nanocomposite. ACS Omega. 2018;3:14597–605.
47. Soltani H, Beitollahi H, Hatefi-Mehrjardi AH, Tajik S, Torkzadeh-Mahani M. Voltammetric determination of glutathione using a modified
single walled carbon nanotubes paste electrode. Anal Bioanal Electrochem. 2014;6:67–79.
48. Shankar SS, Shereema RM, Ramachandran V, Sruthi TV, Kumar VBS, Rakhi RB. Carbon quantum dot-modified carbon paste electrode-
based sensor for selective and sensitive determination of adrenaline. ACS Omega. 2019;4:7903–10.
49. Chauhan N, Chawla S, Pundir CS, Jain U. An electrochemical sensor for detection of neurotransmitter-acetylcholine using metal nano-
particles, 2D material and conducting polymer modified electrode. Biosens Bioelectron. 2017;89:377–83.
50. Tajik S, Taher MA, Beitollahi H, Torkzadeh-Mahani M. Electrochemical determination of the anticancer drug taxol at a ds-DNA modified
pencil-graphite electrode and its application as a label-free electrochemical biosensor. Talanta. 2015;134:60–4.
51. Yaqoob AA, Ahmad H, Parveen T, Ahmad A, Oves M, Ismail IMI, Qari HA, Umar K, Mohamad Ibrahim MN. Recent advances in metal deco-
rated nanomaterials and their various biological applications: a review. Front Chem. 2020;8:1–23.
52. Katz E, Willner I. Integrated nanoparticle-biomolecule hybrid systems: synthesis, properties, and applications. Angew Chem Int Ed. 2004;43:6042–108.
53. Srivastava S, Kumar V, Arora K, Singh C, Ali MA, Puri NK, Malhotra BD. Antibody conjugated metal nanoparticle decorated graphene
sheets for a mycotoxin sensor. RSC Adv. 2016;6:56518–26.
54. Srivastava S, Abraham S, Singh C, Ali MA, Srivastava A, Sumana G, Malhotra BD. Protein conjugated carboxylated gold@reduced graphene
oxide for aflatoxin B1 detection. RSC Adv. 2015;5:5406–14.
55. Li M, Wang P, Li F, Chu Q, Li Y, Dong Y. An ultrasensitive sandwich-type electrochemical immunosensor based on the signal amplification
strategy of mesoporous core–shell Pd@Pt nanoparticles/amino group functionalized graphene nanocomposite. Biosens Bioelectron. 2017;87:752–9.
56. Li YY, Kang P, Wang SQ, Liu ZG, Li YX, Guo Z. Ag nanoparticles anchored onto porous CuO nanobelts for the ultrasensitive electrochemical
detection of dopamine in human serum. Sens Actuators, B Chem. 2021;327:128878.
57. Grogan SP, Dorthé EW, Glembotski NE, Gaul F, D’Lima DD. Cartilage tissue engineering combining microspheroid building blocks and
microneedle arrays. Connect Tissue Res. 2020;61:229–43.
58. Qu L, Yang L, Ren Y, Ren X, Fan D, Xu K, Wang H, Li Y, Ju H, Wei Q. A signal-off electrochemical sensing platform based on Fe3S4-Pd and
pineal mesoporous bioactive glass for procalcitonin detection. Sens Actuators, B Chem. 2020;320:128324.
59. Ozcelikay G, Kurbanoglu S, Yarman A, Scheller FW, Ozkan SA. Au-Pt nanoparticles based molecularly imprinted nanosensor for electro-
chemical detection of the lipopeptide antibiotic drug Daptomycin. Sens Actuators, B Chem. 2020;320:128285.
60. Elseman AM, Abdelbasir SM. Nanosilver-based electrocatalytic materials. In: Shukla SK, Hussain CM, Patra S, Choudhary M, editors.
Electrocatalytic materials for renewable energy. Hoboken: Wiley; 2024. p. 71–110.
61. Fekry AM. A new simple electrochemical Moxifloxacin Hydrochloride sensor built on carbon paste modified with silver nanoparticles.
Biosens Bioelectron. 2017;87:1065–70. Vol:.(1234567890)
Discover Electrochemistry (2024) 1:12
| https://doi.org/10.1007/s44373-024-00012-8 Review
62. Faisal M, Alam MM, Asiri AM, Alsaiari M, Saad Alruwais R, Jalalah M, Madkhali O, Rahman MM, Harraz FA. Detection of hydrogen peroxide
with low-dimensional silver nanoparticle-decorated PPy-C/TiO2 nanocomposites by electrochemical approach. J Electroanal Chem. 2023;928:117030.
63. Sheng Q, Shen Y, Zhang J, Zheng J. Ni doped Ag@C core-shell nanomaterials and their application in electrochemical H2O2 sensing. Anal Methods. 2017;9:163–9.
64. Jahanbakhshi M, Habibi B. A novel and facile synthesis of carbon quantum dots via salep hydrothermal treatment as the silver nanopar-
ticles support: application to electroanalytical determination of H 2 O 2 in fetal bovine serum. Biosens Bioelectron. 2016;81:143–50.
65. Sangili A, Annalakshmi M, Chen SM, Balasubramanian P, Sundrarajan M. Synthesis of silver nanoparticles decorated on core-shell struc-
tured tannic acid-coated iron oxide nanospheres for excellent electrochemical detection and efficient catalytic reduction of hazardous
4-nitrophenol. Compos B Eng. 2019;162:33–42.
66. Kozlova M, Butrim S, Solovyev M, Pushkarev A, Pushkareva I, Kalinichenko V, Akelkina S, Grigoriev S. Structural and electrochemical
characteristics of platinum nanoparticles supported on various carbon carriers. C. 2022;8:14.
67. Chen A, Ostrom C. Palladium-based nanomaterials: synthesis and electrochemical applications. Chem Rev. 2015;115:11999–2044.
68. Shabana N, Arjun AM, Nubla K, Ankitha M, Rasheed PA. Platinum nanoparticles decorated Nb2CTx MXene as an efficient dual functional
catalyst for hydrogen evolution and oxygen reduction reaction. Int J Hydrogen Energy. 2023;48:7698–707.
69. Guerrini L, Alvarez-Puebla RA, Pazos-Perez N. Surface modifications of nanoparticles for stability in biological fluids. Materials. 2018;11:1–28.
70. Shahid MM, Rameshkumar P, Pandikumar A, Lim HN, Ng YH, Huang NM. An electrochemical sensing platform based on a reduced gra-
phene oxide–cobalt oxide nanocube@platinum nanocomposite for nitric oxide detection. J Mater Chem A. 2015;3:14458–68.
71. Ali Sandhu Z, Farwa U, Danish M, Asam Raza M, Ashraf H, Hamayun M, Elahi M, Manzoor A, Toor S, Al-Sehemi AG. Gold nanoparticles as
a promising catalyst for efficient oxygen reduction in fuel cells: perils and prospects. Inorg Chem Commun. 2024;160:111961.
72. Li J, Wang Y, Sun Y, Ding C, Lin Y, Sun W, Luo C. A novel ionic liquid functionalized graphene oxide supported gold nanoparticle composite
film for sensitive electrochemical detection of dopamine. RSC Adv. 2017;7:2315–22.
73. Han T, Zhang Y, Xu J, Dong J, Liu CC. Monodisperse AuM (M = Pd, Rh, Pt) bimetallic nanocrystals for enhanced electrochemical detection
of H2O2. Sens Actuators, B Chem. 2015;207:404–12.
74. Wei H, Pan D, Hu X, Liu M, Han H, Shen D. Voltammetric determination of copper in seawater at a glassy carbon disk electrode modified
with Au@MnO2 core-shell microspheres. Microchim Acta. 2018. https:// doi. org/ 10. 1007/ s00604- 018- 2799-1.
75. Alonso-Lomillo MA, Domínguez-Renedo O, Saldaña-Botín A, Arcos-Martínez MJ. Determination of ascorbic acid in serum samples by
screen-printed carbon electrodes modified with gold nanoparticles. Talanta. 2017;174:733–7.
76. Won YH, Huh K, Stanciu LA. Au nanospheres and nanorods for enzyme-free electrochemical biosensor applications. Biosens Bioelectron. 2011;26:4514–9.
77. Zhang Y, Sun Y, Liu Z, Xu F, Cui K, Shi Y, Wen Z, Li Z. Au nanocages for highly sensitive and selective detection of H 2O2. J Electroanal Chem. 2011;656:23–8.
78. Jayabal S, Ramaraj R. Synthesis of core/shell Au/Ag nanorods embedded in functionalized silicate sol–gel matrix and their applications
in electrochemical sensors. Electrochim Acta. 2013;88:51–8.
79. Dimcheva N. Nanostructures of noble metals as functional materials in biosensors. Curr Opin Electrochem. 2020;19:35–41.
80. Costa D, Pradier C-M, Tielens F, Savio L. Adsorption and self-assembly of bio-organic molecules at model surfaces: a route towards
increased complexity. Surf Sci Rep. 2015;70:449–553.
81. Bollella P, Gorton L, Ludwig R, Antiochia R. A third generation glucose biosensor based on cellobiose dehydrogenase immobilized on a
glassy carbon electrode decorated with electrodeposited gold nanoparticles: characterization and application in human saliva. Sensors.
2017. https:// doi. org/ 10. 3390/ s1708 1912.
82. Novoselov KS, Geim AK, Morozov SV, Jiang D, Zhang Y, Dubonos SV, Grigorieva IV, Firsov AA. Electric field effect in atomically thin carbon
films. Science. 2004;306:666–9.
83. Tan C, Cao X, Wu XJ, et al. Recent advances in ultrathin two-dimensional nanomaterials. Chem Rev. 2017;117:6225–331.
84. Mamba G, Mishra AK. Graphitic carbon nitride (g-C3N4) nanocomposites: a new and exciting generation of visible light driven photo-
catalysts for environmental pollution remediation. Appl Catal B. 2016;198:347–77.
85. Hu T, Mei X, Wang Y, Weng X, Liang R, Wei M. Two-dimensional nanomaterials: fascinating materials in biomedical field. Sci Bull. 2019;64:1707–27.
86. Moraes LPR, Mei J, Fonseca FC, Sun Z. Two-dimensional metal oxide nanomaterials for sustainable energy applications. In: Zafeiratos S,
editor. 2D nanomaterials for energy applications: graphene and beyond. Amsterdam: Elsevier; 2019.
87. Gowthaman NSK, Amalraj M, Kesavan S, et al. Zero-, one- and two-dimensional carbon nanomaterials as low-cost catalysts in optical
and electrochemical sensing of biomolecules and environmental pollutants. Microchem J. 2023;194:109291.
88. Joshi N, Hayasaka T, Liu Y, Liu H, Oliveira ON, Lin L. A review on chemiresistive room temperature gas sensors based on metal oxide
nanostructures, graphene and 2D transition metal dichalcogenides. Microchim Acta. 2018;185:213.
89. Tiwari SK, Kumar V, Huczko A, Oraon R, De AA, Nayak GC. Magical allotropes of carbon: prospects and applications. Crit Rev Solid State Mater Sci. 2016;41:257–317.
90. Shao Y, Wang J, Wu H, Liu J, Aksay IA, Lin Y. Graphene based electrochemical sensors and biosensors: a review. Electroanalysis. 2010;22:1027–36.
91. Khan R, Radoi A, Rashid S, Hayat A, Vasilescu A, Andreescu S. Two-dimensional nanostructures for electrochemical biosensor. Sensors. 2021;21:3369.
92. Han L, Shen H, Zhu J-X, Li Y-T. Mini review: electrochemical electrode based on graphene and its derivatives for heavy metal ions detec-
tion. Talanta Open. 2022;6:100153.
93. Al-Gahouari T, Sayyad P, Bodkhe G, Ingle N, Mahadik M, Shirsat S, Shirsat M. Controlling reduction degree of graphene oxide-based
electrode for improving the sensing performance toward heavy metal ions. Appl Phys A. 2021;127:170.
94. Cui L, Wu J, Ju H. Synthesis of bismuth-nanoparticle-enriched nanoporous carbon on graphene for efficient electrochemical analysis of
heavy-metal ions. Chem Eur J. 2015;21:11525–30. Vol.:(0123456789) Review
Discover Electrochemistry (2024) 1:12
| https://doi.org/10.1007/s44373-024-00012-8
95. Wang M, Wu Z, Dai L. Graphitic carbon nitrides supported by nitrogen-doped graphene as efficient metal-free electrocatalysts for oxygen
reduction. J Electroanal Chem. 2015;753:16–20.
96. Jiang S, Zhang Z, Yang N, Li L, Wei Z. Probing the interaction between nitrogen dopants and edge structures of doped graphene catalysts
for the highly efficient oxygen reduction reaction. J Phys Chem C. 2022;126:19113–21.
97. Hao Q, Jia G, Wei W, Vinu A, Wang Y, Arandiyan H, Ni B-J. Graphitic carbon nitride with different dimensionalities for energy and environ-
mental applications. Nano Res. 2020;13:18–37.
98. Ong W-J, Tan L-L, Ng YH, Yong S-T, Chai S-P. Graphitic carbon Nitride (g-C 3 N 4)-based photocatalysts for artificial photosynthesis and
environmental remediation: are we a step closer to achieving sustainability? Chem Rev. 2016;116:7159–329.
99. Baranowska D, Kędzierski T, Aleksandrzak M, Mijowska E, Zielińska B. Influence of hydrogenation on morphology, chemical structure
and photocatalytic efficiency of graphitic carbon nitride. Int J Mol Sci. 2021. https:// doi. org/ 10. 3390/ ijms2 22313 096.
100. Wang X, Maeda K, Thomas A, Takanabe K, Xin G, Carlsson JM, Domen K, Antonietti M. A metal-free polymeric photocatalyst for hydrogen
production from water under visible light. Nat Mater. 2009;8:76–80.
101. Yew YT, Lim CS, Eng AYS, Oh J, Park S, Pumera M. Electrochemistry of layered graphitic carbon nitride synthesised from various precur-
sors: searching for catalytic effects. ChemPhysChem. 2016;17:481–8.
102. Li J, Fang J, Ye P, Wu D, Wang M, Li X, Xu A. Peroxymonosulfate activation by iron oxide modified g-C3N4 under visible light for pollutants
degradation. J Photochem Photobiol A. 2017;342:85–93.
103. Agnihotri AS, Varghese A, Nidhin A. Transition metal oxides in electrochemical and bio sensing: a state-of-art review. Appl Surf Sci Adv.
2021. https:// doi. org/ 10. 1016/j. apsadv. 2021. 100072.
104. Trujillo RM, Barraza DE, Zamora ML, Cattani-Scholz A, Madrid RE. Nanostructures in hydrogen peroxide sensing. Sensors. 2021;21:2204.
105. Jung YC, Yu YJ, Kim YK, Lee JH, Seo JH, Choi JY. Asymmetric tmo–metal–tmo structure for enhanced efficiency and long-term stability
of si-based heterojunction solar cells. Materials. 2023;16:5550.
106. Kokulnathan T, Joseph Anthuvan A, Chen SM, Chinnuswamy V, Kadirvelu K. Trace level electrochemical determination of the neurotrans-
mitter dopamine in biological samples based on iron oxide nanoparticle decorated graphene sheets. Inorg Chem Front. 2018;5:705–18.
107. Chandra S, Lang H, Bahadur D. Polyaniline-iron oxide nanohybrid film as multi-functional label-free electrochemical and biomagnetic
sensor for catechol. Anal Chim Acta. 2013;795:8–14.
108. George JM, Antony A, Mathew B. Metal oxide nanoparticles in electrochemical sensing and biosensing: a review. Microchim Acta. 2018;185:358.
109. Luo SX, Wu YH, Gou H, Liu Y. A novel electrochemical sensor for the analysis of salbutamol in pork samples by using NiFe2O4 nanopar-
ticles modified glassy carbon electrode. Adv Mater Res. 2014;850–851:1279–82.
110. Cui X, Sun YQ, Ma R, Song XC. A hydrogen peroxide electrochemical sensor based on co-doped Fe3O4 nanoparticles. Adv Mater Res. 2014;941–944:377–80.
111. Bonyani M, Mirzaei A, Leonardi SG, Bonavita A, Neri G. Electrochemical properties of Ag@iron Oxide nanocomposite for application as
nitrate sensor. Electroanalysis. 2015;27:2654–62.
112. Lee S, Oh J, Kim D, Piao Y. A sensitive electrochemical sensor using an iron oxide/graphene composite for the simultaneous detection
of heavy metal ions. Talanta. 2016;160:528–36.
113. Xie H, Liu J, Yones HA, Niu Y, Zhao Y, Xi Y, Li X, Li G, Sun W, Wang X. Fe3O4 decorated reduced graphene oxide modified electrode for
electrochemistry of hemoglobin and its application as trichloroacetic acid and nitrite sensor. Int J Electrochem Sci. 2019;14:9141–9.
114. Madrakian T, Maleki S, Heidari M, Afkhami A. An electrochemical sensor for rizatriptan benzoate determination using Fe3O4 nanoparticle/
multiwall carbon nanotube-modified glassy carbon electrode in real samples. Mater Sci Eng C. 2016;63:637–43.
115. Shaikh SF, Mane RS, Min BK, Hwang YJ, Joo O. D-sorbitol-induced phase control of TiO2 nanoparticles and its application for dye-sensitized
solar cells. Sci Rep. 2016;6:20103.
116. Ramanavicius S, Ramanavicius A. Insights in the application of stoichiometric and non-stoichiometric titanium oxides for the design of
sensors for the determination of gases and vocs (Tio2−x and tino2n−1 vs. tio2). Sensors. 2020;20:1–18.
117. Tian WC, Ho YH, Chen CH, Kuo CY. Sensing performance of precisely ordered TiO2 nanowire gas sensors fabricated by electron-beam
lithography. Sensors. 2013;13:865–74.
118. Bumajdad A, Madkour M, Abdel-Moneam Y, El-Kemary M. Nanostructured mesoporous Au/TiO2 for photocatalytic degradation of a
textile dye: The effect of size similarity of the deposited Au with that of TiO2 pores. J Mater Sci. 2014;49:1743–54.
119. Toledo WD, Couto AB, Almeida DAL, Ferreira NG. Facile synthesis of TiO2/rGO neatly electrodeposited on carbon fiber applied as ternary
electrode for supercapacitor. Mater Res Express. 2019;6:65040.
120. Paradowska E, Arkusz K, Pijanowska DG. Comparison of gold nanoparticles deposition methods and their influence on electrochemical
and adsorption properties of titanium dioxide nanotubes. Materials. 2020;13:1–20.
121. Maity D, Sahoo SR, Saha S. Synthesis and characterization of nanomaterials for electrochemical sensors. ACS Symp Ser. 2023;1437:193–222.
122. Luo M, Zhang Y, Zhao S. Non-enzymatic hydrogen peroxide sensor based on Fe 3 O 4 @Polydopamine-Ag nanocomposite modified
magnetic glassy carbon electrode. J Electrochem Soc. 2021;168:067511.
123. Arciga-Duran E, Meas Y, Pérez-Bueno JJ, Ballesteros JC, Trejo G. Electrochemical synthesis of Co 3 O 4–x films for their application as
oxygen evolution reaction electrocatalysts: role of oxygen vacancies. J Electrochem Soc. 2018;165:H3178–86.
124. Chekin F, Vahdat SM, Asadi MJ. Green synthesis and characterization of cobalt oxide nanoparticles and its electrocatalytic behavior. Russ J Appl Chem. 2016;89:816–22.
125. Saghatforoush LA, Sanati S, Hasanzadeh M. Synthesis, characterization and electrochemical properties of Co3O4 nanostructures by
using cobalt hydroxide as a precursor. Res Chem Intermed. 2015;41:4361–72.
126. Li S-J, Du J-M, Zhang J-P, Zhang M-J, Chen J. A glassy carbon electrode modified with a film composed of cobalt oxide nanoparticles and
graphene for electrochemical sensing of H2O2. Microchim Acta. 2014;181:631–8.
127. Ivanova-Kolcheva V, Sygellou L, Stoyanova M. Enhanced catalytic degradation of acid orange 7 dye by peroxymonosulfate on Co3O4
promoted by Bi2O3. Acta Chim Slov. 2020;67:609–21.
128. Ivanova-Kolcheva VV, Stoyanova MK. Catalytic activation of PMS by Co3O4 modified g-C3N4 for oxidative degradation of the azo dye
Acid Orange 7 in aqueous solutions. Bul Chem Commun. 2019;51:158–64. Vol:.(1234567890)
Discover Electrochemistry (2024) 1:12
| https://doi.org/10.1007/s44373-024-00012-8 Review
129. Ramanavicius S, Ramanavicius A. Conducting polymers in the design of biosensors and biofuel cells. Polymers. 2021;13:1–19.
130. Ramanavicius S, Ramanavicius A. Development of molecularly imprinted polymer based phase boundaries for sensors design
(review). Adv Coll Interface Sci. 2022;305:102693.
131. Ramanavicius S, Ramanavicius A. Charge transfer and biocompatibility aspects in conducting polymer-based enzymatic biosensors
and biofuel cells. Nanomaterials. 2021;11:1–22.
132. Ramanavicius S, Jagminas A, Ramanavicius A. Advances in molecularly imprinted polymers based affinity sensors (review). Polymers.
2021. https:// doi. org/ 10. 3390/ polym 13060 974.
133. Baranwal J, Barse B, Gatto G, Broncova G, Kumar A. Electrochemical sensors and their applications: a review. Chemosensors. 2022;10:363.
134. Barhoum A, Hamimed S, Slimi H, Othmani A, Abdel-Haleem FM, Bechelany M. Modern designs of electrochemical sensor platforms for
environmental analyses: principles, nanofabrication opportunities, and challenges. Trends Environ Anal Chem. 2023;38:e00199.
135. Yunus G, Singh R, Raveendran S, Kuddus M. Electrochemical biosensors in healthcare services: bibliometric analysis and recent develop- ments. PeerJ. 2023;11:1–27.
136. Nath S. Advancements in food quality monitoring: integrating biosensors for precision detection. Sustain Food Technol. 2024;2:976–92.
137. Ghabach M, Davarpanah AH. Hydrogen peroxide poisoning. Lancet Gastroenterol Hepatol. 2020;5:418.
138. Giorgio M, Trinei M, Migliaccio E, Pelicci PG. Hydrogen peroxide: a metabolic by-product or a common mediator of ageing signals? Nat
Rev Mol Cell Biol. 2007;8:722–8.
139. Mulgund A, Doshi S, Agarwal A. The role of oxidative stress in endometriosis. In: Watson RR, editor. Handbook of fertility. Amsterdam: Elsevier; 2015. p. 273–81.
140. Iacobini C, Vitale M, Pesce C, Pugliese G, Menini S. Diabetic complications and oxidative stress: a 20-year voyage back in time and back
to the future. Antioxidants. 2021. https:// doi. org/ 10. 3390/ antio x1005 0727.
141. Pizzino G, Irrera N, Cucinotta M, Pallio G, Mannino F, Arcoraci V, Squadrito F, Altavilla D, Bitto A. Oxidative stress: harms and benefits for
human health. Oxid Med Cell Longev. 2017;2017:1–13.
142. Kong ASY, Lai KS, Hee CW, Loh JY, Lim SHE, Sathiya M. Oxidative stress parameters as biomarkers of cardiovascular disease towards the
development and progression. Antioxidants. 2022;11:1–13.
143. Qi Y-T, Jiang H, Wu W-T, Zhang F-L, Tian S-Y, Fan W-T, Liu Y-L, Amatore C, Huang W-H. Homeostasis inside single activated phagolysosomes:
quantitative and selective measurements of submillisecond dynamics of reactive oxygen and nitrogen species production with a nano-
electrochemical sensor. J Am Chem Soc. 2022;144:9723–33.
144. Ichimura Y, Habuchi T, Tsuchiya N, Wang L, Oyama C, Sato K, Nishiyama H, Ogawa O, Kato T. Increased risk of bladder cancer associated
with a glutathione peroxidase 1 codon 198 variant. J Urol. 2004;172:728–32.
145. Silva RA, Lima MSF, Viana JBM, Bezerra VS, Pimentel MCB, Porto ALF, Cavalcanti MTH, Lima Filho JL. Can artisanal “Coalho” cheese from
Northeastern Brazil be used as a functional food? Food Chem. 2012;135:1533–8.
146. Waifalkar PP, Chougale AD, Kollu P, Patil PS, Patil PB. Magnetic nanoparticle decorated graphene based electrochemical nanobiosensor
for H2O2 sensing using HRP. Colloids Surf B. 2018;167:425–31.
147. Guler M, Turkoglu V, Bulut A, Zahmakiran M. Electrochemical sensing of hydrogen peroxide using Pd@Ag bimetallic nanoparticles
decorated functionalized reduced graphene oxide. Electrochim Acta. 2018;263:118–26.
148. Zhang Y, Duan H, Yuan W, Li Y, Zhang Y, Yang X, Liao X. An amperometric hydrogen peroxide sensor based on hollow carbon spheres/Pt
NPs modified glassy carbon electrode. Int J Electrochem Sci. 2022;17:221294.
149. Sun Y, Luo M, Qin Y, Zhu S, Li Y, Xu N, Meng X, Ren Q, Wang L, Guo S. Atomic-thick PtNi nanowires assembled on graphene for high-
sensitivity extracellular hydrogen peroxide sensors. ACS Appl Mater Interfaces. 2017;9:34715–21.
150. Zeng X, Xu C, Xu X, Huang Y, Wang Q, Huo X. Combined toxicity of air pollutants related to e-waste on inflammatory cytokines linked
with neurotransmitters and pediatric behavioral problems. Ecotoxicol Environ Saf. 2022;239:113657.
151. Tulli F, Gulotta FA, Martino DM, Zanini VIP, Borsarelli CD. Ultrasensitive Amperometric biosensing of polyphenols using horseradish
peroxidase immobilized in a laponite/Au/DNA-bioinspired polycation nanocomposite. J Electrochem Soc. 2018;165:B452–7.
152. Zhang Y, Li X, Li D, Wei Q. A laccase based biosensor on AuNPs-MoS2 modified glassy carbon electrode for catechol detection. Colloids Surf B. 2020;186:110683.
153. Baluta S, Zając D, Szyszka A, Malecha K, Cabaj J. Enzymatic platforms for sensitive neurotransmitter detection. Sensors. 2020;20:423.
154. Rubio-Govea R, Hickey DP, García-Morales R, Rodriguez-Delgado M, Domínguez-Rovira MA, Minteer SD, Ornelas-Soto N, García-García
A. MoS2 nanostructured materials for electrode modification in the development of a laccase based amperometric biosensor for non-
invasive dopamine detection. Microchem J. 2020;155:104792.
155. Coelho JH, Eisele APP, Valezi CF, Mattos GJ, Schirmann JG, Dekker RFH, Barbosa-Dekker AM, Sartori ER. Exploring the exocellular fungal
biopolymer botryosphaeran for laccase-biosensor architecture and application to determine dopamine and spironolactone. Talanta. 2019;204:475–83.
156. Wu R, Yu S, Chen S, Dang Y, Wen S-H, Tang J, Zhou Y, Zhu J-J. A carbon dots-enhanced laccase-based electrochemical sensor for highly
sensitive detection of dopamine in human serum. Anal Chim Acta. 2022;1229:340365.
157. Arslan F, Durmuş S, Çolak Ö, Arslan H. A new laccase-based biosensor for epinephrine determination. Gazi Univ J Sci. 2015;28:1–9.
158. Kurian MA, Gissen P, Smith M, Heales SJR, Clayton PT. The monoamine neurotransmitter disorders: an expanding range of neurological
syndromes. Lancet Neurol. 2011;10:721–33.
159. Hjemdahl P. Plasma catecholamines-analytical challenges and physiological limitations. Bailliere’s Clin Endocrinol Metab. 1993;7:307–53.
160. Ribeiro JA, Fernandes PMV, Pereira CM, Silva F. Electrochemical sensors and biosensors for determination of catecholamine neurotrans-
mitters: a review. Talanta. 2016;160:653–79.
161. Chung H, Tajiri S, Hyoguchi M, Koyanagi R, Shimura A, Takata F, Dohgu S, Matsui T. Analysis of catecholamine and their metabolites in
mice brain by liquid chromatography-mass spectrometry using sulfonated mixed-mode copolymer column. Anal Sci. 2019;35:433–9.
162. Ferreira FDP, Silva LIB, Freitas AC, Rocha-Santos TAP, Duarte AC. High performance liquid chromatography coupled to an optical fiber
detector coated with laccase for screening catecholamines in plasma and urine. J Chromatogr A. 2009;1216:7049–54. Vol.:(0123456789) Review
Discover Electrochemistry (2024) 1:12
| https://doi.org/10.1007/s44373-024-00012-8
163. Ye H, Xu H, Xu X, Zheng C, Li X, Wang L, Liu X, Chen G. An electrochemiluminescence sensor for adrenaline assay based on the tyrosinase/
SiC/chitosan modified electrode. Chem Commun. 2013;49:7070–2.
164. Gunawardhana SM, Bulgakova GA, Barybin AM, Thomas SR, Lunte SM. Progress toward the development of a microchip electrophoresis
separation-based sensor with electrochemical detection for on-line in vivo monitoring of catecholamines. Analyst. 2020;145:1768–76.
165. Lavanya N, Leonardi SG, Sekar C, Ficarra S, Galtieri A, Tellone E, Neri G. Detection of catecholamine neurotransmitters by nanostructured
SnO2-based electrochemical sensors: a review of recent progress. Mini-Rev Org Chem. 2018;15:382–8.
166. Ges IA, Currie KPM, Baudenbacher F. Electrochemical detection of catecholamine release using planar iridium oxide electrodes in nano-
liter microfluidic cell culture volumes. Biosens Bioelectron. 2012;34:30–6.
167. Hardi GW, Rahman SF. Amperometric detection of dopamine based on a graphene oxide/PEDOT:PSS composite electrode. Int J Technol. 2020;11:974–83.
168. Sanghavi BJ, Mobin SM, Mathur P, Lahiri GK, Srivastava AK. Biomimetic sensor for certain catecholamines employing copper(II) complex
and silver nanoparticle modified glassy carbon paste electrode. Biosens Bioelectron. 2013;39:124–32.
169. Dobryakova E, Genova HM, DeLuca J, Wylie GR. The dopamine imbalance hypothesis of fatigue in multiple sclerosis and other neurologi-
cal disorders. Front Neurol. 2015;6:1–8.
170. Kumar SJ, Kumar P. insight into the emerging role of striatal neurotransmitters in the pathophysiology of Parkinson’s disease and Hun-
tington’s disease: a review. Curr Neuropharmacol. 2019;17:165–75.
171. Álvarez-Martos I, Ferapontova EE. Electrochemical label-free aptasensor for specific analysis of dopamine in serum in the presence of
structurally related neurotransmitters. Anal Chem. 2016;88:3608–16.
172. Becerra-Hernández A, Galindo-de-la-Rosa J, Martínez-Pimentel Y, Ledesma-García J, Álvarez-Contreras L, Guerra-Balcázar M, Aguilar-
Elguezabal A, Álvarez A, Chávez-Ramírez AU, Vallejo-Becerra V. Novel biomaterial based on monoamine oxidase-A and multi-walled
carbon nanotubes for serotonin detection. Biochem Eng J. 2019;149:107240.
173. Jayakumar K, Reichhart TMB, Schulz C, Ludwig R, Felice AKG, Leech D. An oxygen insensitive amperometric glucose biosensor based
on an engineered cellobiose dehydrogenase: direct versus mediated electron transfer responses. ChemElectroChem. 2022. https:// doi.
org/ 10. 1002/ celc. 20220 0418.
174. Molinnus D, Hardt G, Käver L, Willenberg HS, Poghossian A, Keusgen M, Schöning MJ. Detection of Adrenaline Based on Bioelectrocata-
lytical System to Support Tumor Diagnostic Technology. In: Proceedings of Eurosensors. 2017; Paris, France, 3–6 September 2017. MDPI, Basel Switzerland. 506
175. Josypčuk O, Barek J, Josypčuk B. Amperometric determination of catecholamines by enzymatic biosensors in flow systems. Electroa- nalysis. 2018;30:1163–71.
Publisher’s Note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations. Vol:.(1234567890)




