
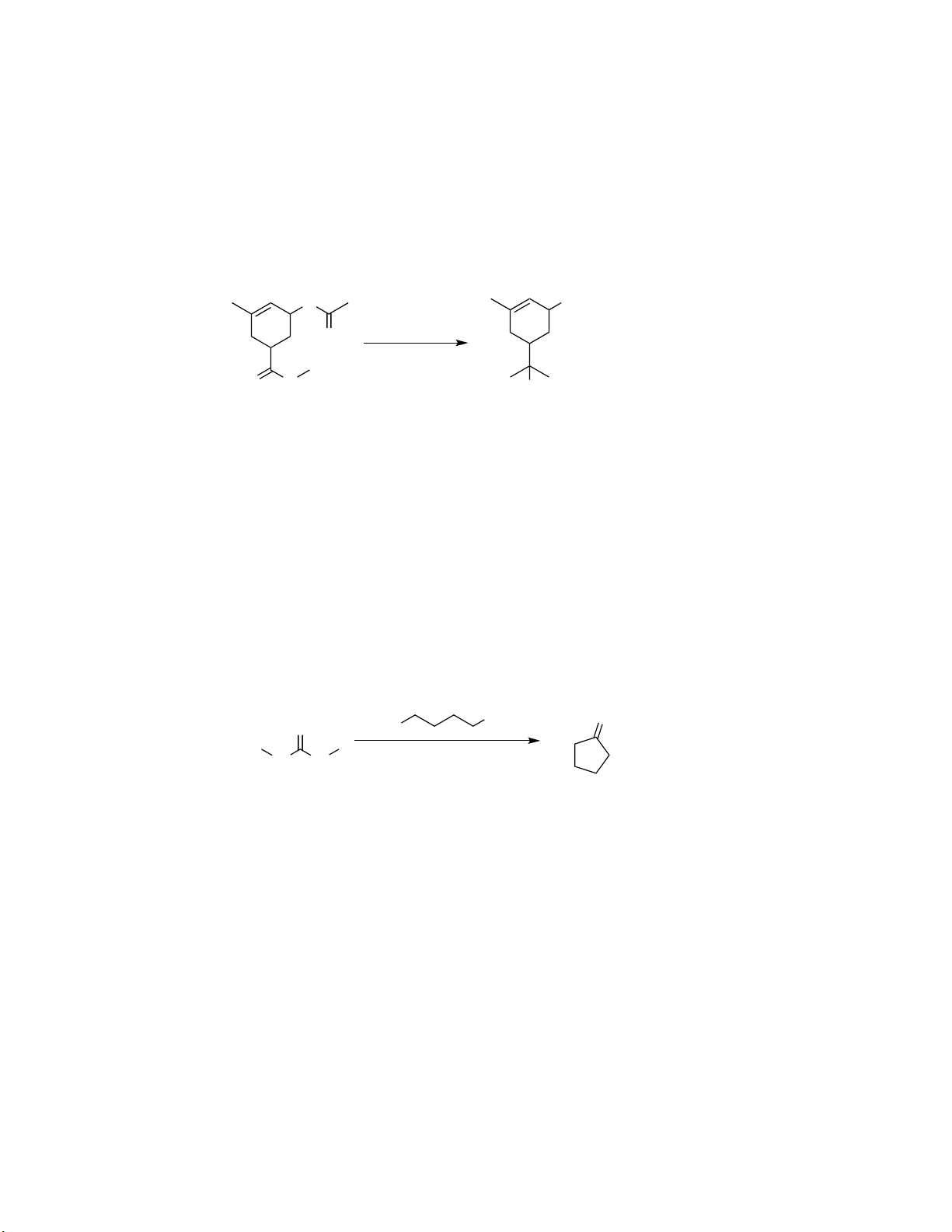
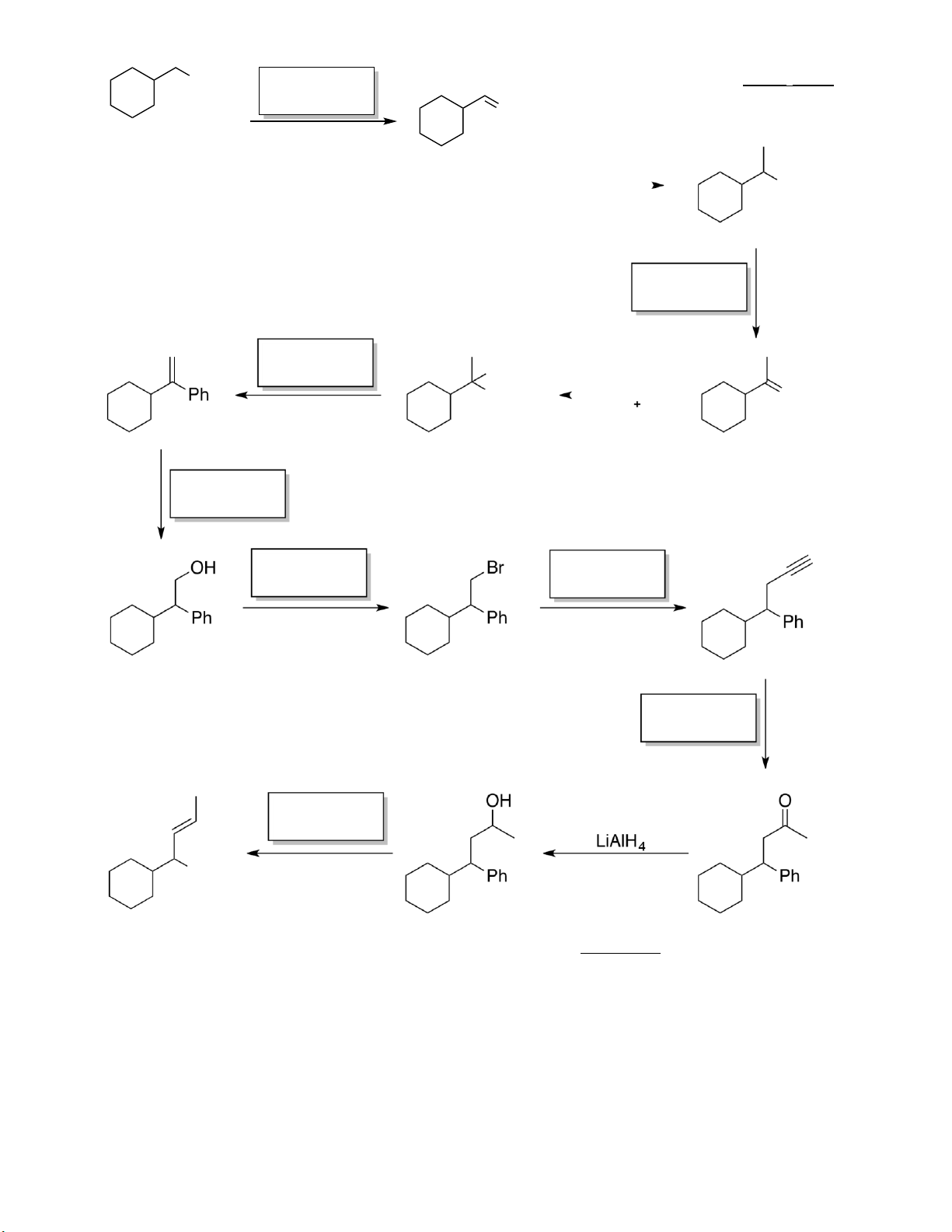
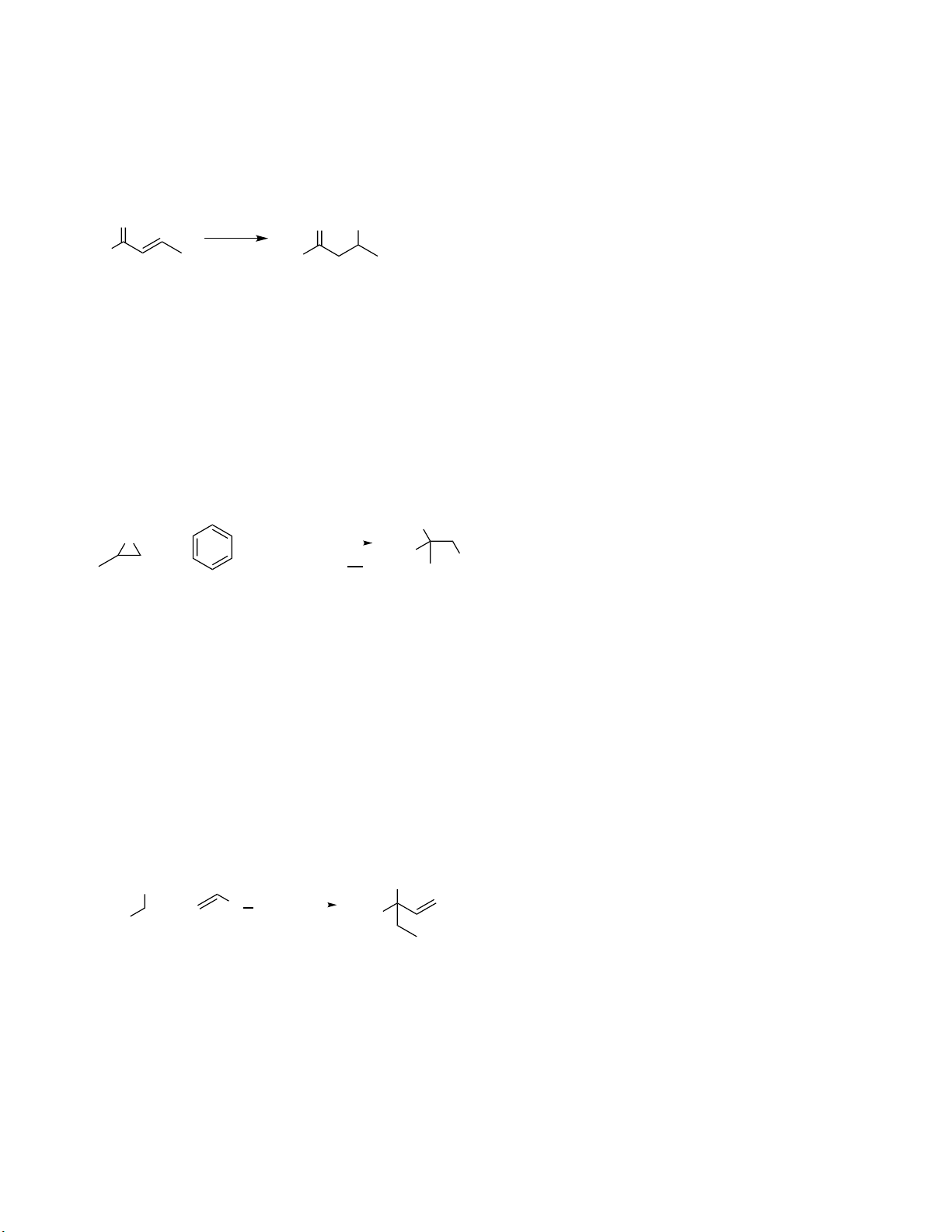

Preview text:
lOMoAR cPSD| 22014077 lOMoAR cPSD| 22014077 Chemistry 234
Synthetic Transformations of Carbonyl Compounds – Problem Set Reduction
Reactions 1) Predict the product for each reaction shown below. O NaBH 4 O 1. LiAlH4 CH 3 OH 3 + 2. H O NH 2 1 . LiAlH O . H 2 3 O H NaBH4 4 O CH3OH + O 1. L iAlH O 1 . LiAlH 4 OH . H 2 3 O + 2 . H O + 4 Cl 3 O NaBH OH CH 3 OH O 1. LiAlH4 4 O + O 2. H3O 1. L iAlH 4 O O 2 . H 3 O + 1. DIBAL-H -78 ¡C O 1 . LiAlH Cl 2. H 4 2O NH 2 . H 3 O + 1. DIBAL-H O -78 ¡C O 2. 2 H O 2) Predict the product for each reduction reaction below. Pay close attention to chemoselectivity. O O NaBH4 OCH3 CH3OH lOMoAR cPSD| 22014077 O 1. DIBAL-H OH -78 ¡C Cl2. H2O O O NaBH4 N CH3OH O Oxidation Reactions 3) Predict the product for each reaction below. Assume an excess of oxidizing agent is present. O OH PCCPCC HO O OH Na2Cr2O7Na2Cr2O7 H HO HO2SO4H H2SO4 OH Na2Cr2O7Na2Cr2O7 H2SO4 OH H2SO4 Addition of Organometallic Reagents
(Grignard, Lithiates, and Acetylide ions) 4) Predict the product for each reaction shown below. Assume an excess of organometallic reagent. O 1. H 3 CMgBr 2 . H 3 O + lOMoAR cPSD| 22014077 O 1. Ph MgBr 2 . H O + H 3 Cl 3C Li 1. H 2. H3O+ O O 1. H3C MgBr O 2. H3O+ 1. Ph Na O 2. H3O+ OH 1. H3C Li O 2. H3O+ 1. MgBr O OCH3 2. H3O+ 1. Mg Br 2. O 3. H3O+ Addition of Organometallic Reagents
(Organocuprates) 5) Predict the product for each reaction shown below. 1 . Ph 2 CuLi 2 . H 2 O O 1 . Ph 2 CuLi 2 . H O lOMoAR cPSD| 22014077 O 2 1 . (CH 3 ) 2 CuLi H 2 . H 2 O O O 1 . (CH 3 ) 2 CuLi 2 . H 2 O Cl O 1 . (CH 3 ) 2 CuLi O 2. H2O 6) What reagent is needed to convert propanoyl chloride to each of the following? HPh Ph O O OH OH 7) Draw the electron pushing mechanism for the addition of methyl cuprate to 3-buten-2-one. O 1. Me2CuLi 2. H O 2 lOMoAR cPSD| 22014077
Protecting Groups in Organic Synthesis 8) Propose how you could carry out the following synthetic sequence. Hint: PBr3 converts alcohols to bromides. O? Br OHOH 9) A student tried to carry out the reaction sequence below, but none of the diol (A) was formed. Explain what went wrong with the plan and design a successful synthesis of A. 1. O OH Mg 2 . H 3 O + OH OH BrBrMgOH A General Mechanisms 10) Draw the electron pushing mechanism for each reaction shown below. O 1. H 3 CMgBr OH O 2 . H 3 O + OH lOMoAR cPSD| 22014077 OH O 1. H 3 CMgBr O ( excess ) + CH3OH + (CH3)3COH 2 . H 3 O + OH O O MgBr O O BrMg + 2 CH3OH followed by dilute H + O O Synthesis 11) Consider the following reaction sequence. Identify the missing reagents. lOMoAR cPSD| 22014077 OHO 1. CH3MgBr+ OH 2. H3O OH 1. PhMgBr Ph O 2. H3O lOMoAR cPSD| 22014077 Ph 12) Provide a reasonable synthetic route to carry out the synthetic transformation shown below. O ? O Ph H Ph 13) Design a reasonable synthesis for each of the following using the provided starting materials. Unless otherwise specified, you may use any other organic or inorganic reagents as necessary. HO O ? Ph OCH3 OH OH ? Br Ph Ph (these are your only allowed sources of carbon) lOMoAR cPSD| 22014077 O O CH3 Br ? H BrH3C (these are your only allowed sources of carbon) 14) Challenge Question: Show how 2-hexanone could be synthesized using only ethanol and any other inorganic reagents. Hint: you might need to use a couple of reactions from organic 1.

