















Preview text:
lOMoARcPSD| 59629529
LIÊN HIỆP CÁC HỘI KH&KT VIỆT NAM HỘI HÓA HỌC VIỆT
NAM TRƯỜNG ĐẠI HỌC CÔNG NGHIỆP HÀ NỘI BÀI THI
OLYMPIC HOÁ HỌC SINH VIÊN CÁC TRƯỜNG ĐẠI HỌC VÀ
CAO ĐẲNG TOÀN QUỐC LẦN THỨ IX (4/2016)
Bài thi lý thuyết Bảng: A
Họ và tên thí sinh: .............................................................. Số phách:
Ngày tháng năm sinh:..........................................................
Số báo danh:........................................................................
Đơn vị:................................................................................ lOMoARcPSD| 59629529 Số phách
Bài làm lý thuyết của thí sinh Bảng: A
KẾT QUẢ CHẤM BÀI THI LÝ THUYẾT Tổng Tổng điểm Câu 1 2 3 4 5 6 7 8 điểm bằng chữ bằng số Điểm
Hà Nội, ngày 18 tháng 04 năm 2016 TRƯỞNG TIỂU BAN
CÁN BỘ CHẤM THI 1
CÁN BỘ CHẤM THI 2 CHẤM THI BẢNG A
Hướng dẫn thí sinh khi làm bài thi
1. Thí sinh phải viết họ, tên, ngày tháng năm sinh và số báo danh vào tờ bìa của bài thi.
(Vì đây là phách của bài thi).
2. Các trang bên trong của bài thi không được viết bất cứ thông tin cá nhân nào, mà chỉ
làm bài bằng bút xanh hoặc đen, không được dùng bút đỏ. Tất cả các kết quả trả lời mỗi
câu hỏi phải được viết trong khung quy định của bài thi. Làm khác quy định sẽ không được chấm điểm.
3. Bài thi gồm 08 câu, 18 trang. Khi làm bài xong thí sinh phải nộp toàn bộ bài thi và ký
xác nhận trước khi ra về. 4. Thí sinh có 180 phút để làm bài thi.
5. Thí sinh không được sử dụng tài liệu tham khảo, chỉ được sử dụng máy tính không có thẻ nhớ. lOMoARcPSD| 59629529 Số phách
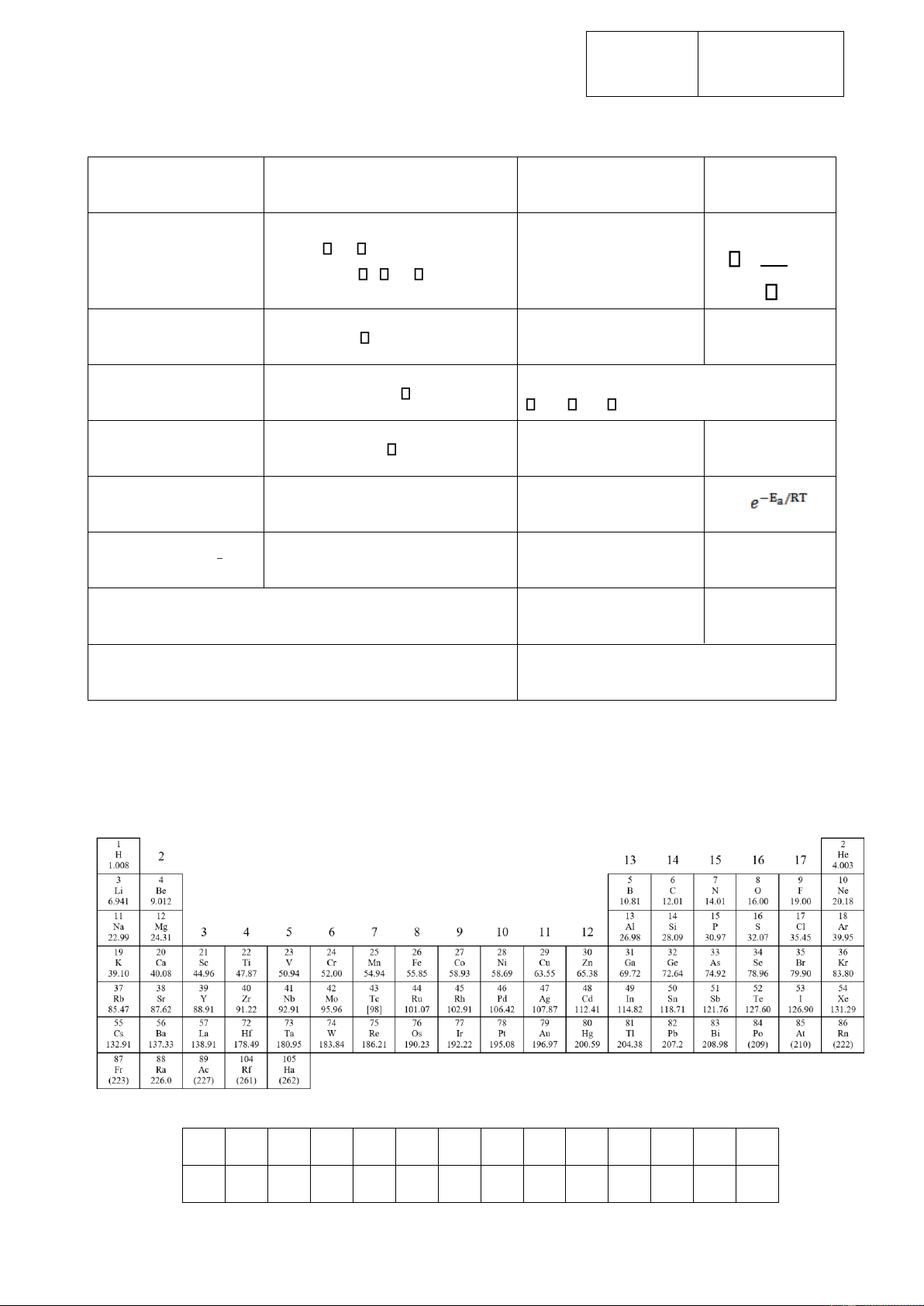
Các hằng số và công thức cần thiết Phương trình khí lý Số Avogadro:
NA = 6.0221×1023 mol–1 tưởng: PV = nRT hc 8.314 J K–1 mol–1 Hằng số khí: R =
Năng lượng của photon: E 0.08205 atm L K–1 mol–1 Năng lượng tự do Gibbs: Hằng số Faraday:
F = 96485 C mol–1
G = H – TS Hằng số Planck:
h = 6.6261×10–34 J s
H = E + nRT Vận tốc ánh sáng : c = 3.000×108 m s–1 Phương trình Faraday: Q = it Không độ C: 273.15 K
Phương trình Arrhenius: k = A 1 N = 1 kg.m.-1s -2 1 eV = 1.602×10-19 J Kw = = 1.0×10-14
1 atm = 760 torr = 1.01325×105 Pa 1m = 109m = 1010Å
1 ppm là một phần triệu 1ppb là một phần tỷ
BẢNG TUẦN HOÀN CÁC NGUYÊN TỐ HÓA HỌC 1 18 58 59 Pr 61 62 63 64 65 66 67 68 69 70 71 Ce 140.91 60 Nd Pm Sm Eu Gd Tb Dy Ho Er Tm Yb Lu 140.12 144.24 (145)
150.36 151.96 157.25 158.93 162.50 164.93 167.26 168.93 173.05 174.97 90 91 92 U 94 95 96 97 98 99 100 101 102 103 Th Pa 238.03 93 Np Pu Am Cm Bk Cf Es Fm Md No Lr 232.04 231.04 237.05 (244) (243) (247) (247) (251) (254) (257) (256) (254) (257) lOMoARcPSD| 59629529 Số phách
(Cán bộ trông thi không giải thích gì thêm) Câu I (2,5 điểm) a b c Tổng điểm 0,75 1,0 0,75 2,5 Cán bộ 1 Cán bộ 2
a) Xây dựng giản đồ MO của CO và CN-. Viết cấu hình electron, xác định độ bội liên
kết, từ tính của chúng.
.............................................................................................................................................................
.............................................................................................................................................................
.............................................................................................................................................................
.............................................................................................................................................................
.............................................................................................................................................................
.............................................................................................................................................................
.............................................................................................................................................................
.............................................................................................................................................................
.............................................................................................................................................................
.............................................................................................................................................................
.............................................................................................................................................................
.............................................................................................................................................................
.............................................................................................................................................................
.............................................................................................................................................................
.............................................................................................................................................................
.............................................................................................................................................................
.............................................................................................................................................................
.............................................................................................................................................................
.............................................................................................................................................................
.............................................................................................................................................................
.............................................................................................................................................................
............................................................................................................................................................. lOMoARcPSD| 59629529 Số phách
.............................................................................................................................................................
.............................................................................................................................................................
.............................................................................................................................................................
............................................................................................................................................................. lOMoARcPSD| 59629529 Số phách
.............................................................................................................................................................
.............................................................................................................................................................
.............................................................................................................................................................
.............................................................................................................................................................
.............................................................................................................................................................
.............................................................................................................................................................
b) Dựa vào thuyết liên kết hóa trị (VB), hãy cho biết dạng hình học và từ tính của các
phức chất [Ni(CO)4] và [Ni(CN)4]2-.
.............................................................................................................................................................
.............................................................................................................................................................
.............................................................................................................................................................
.............................................................................................................................................................
.............................................................................................................................................................
.............................................................................................................................................................
.............................................................................................................................................................
.............................................................................................................................................................
.............................................................................................................................................................
.............................................................................................................................................................
.............................................................................................................................................................
.............................................................................................................................................................
.............................................................................................................................................................
.............................................................................................................................................................
.............................................................................................................................................................
.............................................................................................................................................................
.............................................................................................................................................................
.............................................................................................................................................................
.............................................................................................................................................................
.............................................................................................................................................................
.............................................................................................................................................................
.............................................................................................................................................................
.............................................................................................................................................................
............................................................................................................................................................. lOMoARcPSD| 59629529 Số phách
c) Dùng thuyết liên kết hóa trị (VB) hãy cho biết cấu tạo, dạng hình học của CO2, NO2 và O3.
.............................................................................................................................................................
.............................................................................................................................................................
.............................................................................................................................................................
.............................................................................................................................................................
.............................................................................................................................................................
.............................................................................................................................................................
.............................................................................................................................................................
.............................................................................................................................................................
.............................................................................................................................................................
.............................................................................................................................................................
.............................................................................................................................................................
.............................................................................................................................................................
.............................................................................................................................................................
.............................................................................................................................................................
.............................................................................................................................................................
.............................................................................................................................................................
.............................................................................................................................................................
.............................................................................................................................................................
.............................................................................................................................................................
.............................................................................................................................................................
.............................................................................................................................................................
............................................................................................................................................................. Câu II (3,0 điểm) a b c Tổng điểm 0,5 2,0 0,5 3,0 Cán bộ 1 Cán bộ 2
Người ta tiến hành khai thác vàng bằng phương pháp xyanua như sau: đầu tiên quặng
vàng được nghiền vụn, rồi trộn với dung dịch NaCN trong môi trường kiềm và liên tục sục lOMoARcPSD| 59629529 Số phách
oxi không khí vào hỗn hợp phản ứng. Khi đó oxi sẽ oxi hóa vàng thành [Au (CN)2]-. Sau đó
người ta cho kẽm bột tác dụng với dung dịch [Au (CN)2]- để thu hồi vàng kim loại.
Cho biết: E0(Au+/Au) =1,70V; E0(O2/H2O) =1,23V; E0(OCl-/Cl-) =1,49V;
E0(CNO-/CN-) = -0,14V; pKHCN = 9,2; Hằng số phân ly tổng cộng của phức chất [Au (CN)2]- bằng 7,04 10-40.
a) Hãy viết các phương trình phản ứng hóa học xảy ra trong quá trình trên.
...........................................................................................................................................................
...........................................................................................................................................................
...........................................................................................................................................................
...........................................................................................................................................................
...........................................................................................................................................................
...........................................................................................................................................................
........................................................................................................................................................... b) Tính E0(Au(CN) -
2 /Au) và E0(O2 kk/OH-) (coi áp suất của oxi trong không khí bằng 0,2 atm).
Chứng minh rằng khi có mặt ion CN- trong môi trường kiềm thì oxi không khí có thể
oxi hóa Au thành [Au (CN)2]-. pH tối thiểu của dung dịch CN- phải bằng bao nhiêu? Tại sao?
.............................................................................................................................................................
.............................................................................................................................................................
.............................................................................................................................................................
.............................................................................................................................................................
.............................................................................................................................................................
.............................................................................................................................................................
.............................................................................................................................................................
.............................................................................................................................................................
.............................................................................................................................................................
.............................................................................................................................................................
.............................................................................................................................................................
.............................................................................................................................................................
.............................................................................................................................................................
............................................................................................................................................................. lOMoARcPSD| 59629529 Số phách
.............................................................................................................................................................
............................................................................................................................................................. lOMoARcPSD| 59629529 Số phách
.............................................................................................................................................................
.............................................................................................................................................................
.............................................................................................................................................................
.............................................................................................................................................................
.............................................................................................................................................................
.............................................................................................................................................................
.............................................................................................................................................................
.............................................................................................................................................................
.............................................................................................................................................................
.............................................................................................................................................................
.............................................................................................................................................................
.............................................................................................................................................................
.............................................................................................................................................................
.............................................................................................................................................................
.............................................................................................................................................................
.............................................................................................................................................................
.............................................................................................................................................................
.............................................................................................................................................................
.............................................................................................................................................................
.............................................................................................................................................................
.............................................................................................................................................................
.............................................................................................................................................................
.............................................................................................................................................................
.............................................................................................................................................................
.............................................................................................................................................................
.............................................................................................................................................................
.............................................................................................................................................................
.............................................................................................................................................................
.............................................................................................................................................................
............................................................................................................................................................. lOMoARcPSD| 59629529 Số phách
c) Để xử lý CN- có trong nước thải của quá trình khai thác vàng bằng phương pháp xyanua,
người ta thường dùng NaOCl để oxi hóa CN- thành CNO- theo phản ứng: CN- + OCl- CNO- + Cl-
Nếu cho 5ml dung dịch NaOCl 0,2M vào 1 lit nước thải có nồng độ CN- là 10-3M
(coi thể tích dung dịch không đổi ) thì có thể oxi hóa hoàn toàn CN- thành CNO- được không?.
Tính nồng độ CN- còn lại trong dung dich sau khi xử lý?
.............................................................................................................................................................
.............................................................................................................................................................
.............................................................................................................................................................
.............................................................................................................................................................
.............................................................................................................................................................
.............................................................................................................................................................
.............................................................................................................................................................
.............................................................................................................................................................
.............................................................................................................................................................
.............................................................................................................................................................
.............................................................................................................................................................
.............................................................................................................................................................
.............................................................................................................................................................
.............................................................................................................................................................
.............................................................................................................................................................
.............................................................................................................................................................
.............................................................................................................................................................
.............................................................................................................................................................
.............................................................................................................................................................
.............................................................................................................................................................
.............................................................................................................................................................
.............................................................................................................................................................
.............................................................................................................................................................
............................................................................................................................................................. lOMoARcPSD| 59629529 Số phách
Câu III (3,0 điểm) a b c Tổng điểm 1,0 1,0 1,0 3,0 Cán bộ 1 Cán bộ 2
Amoniac được tổng hợp theo phản ứng sau: N2(k) + 3H2(k) 2NH3(k)
a) Chứng minh rằng ở nhiệt độ và áp suất nhất định, phần mol của NH3 là cực đại nếu
xuất phát từ hỗn hợp N2 và H2 có tỷ lệ N2 : H2 = 1: 3 theo số mol.
.............................................................................................................................................................
.............................................................................................................................................................
.............................................................................................................................................................
.............................................................................................................................................................
.............................................................................................................................................................
.............................................................................................................................................................
.............................................................................................................................................................
.............................................................................................................................................................
.............................................................................................................................................................
.............................................................................................................................................................
.............................................................................................................................................................
.............................................................................................................................................................
.............................................................................................................................................................
.............................................................................................................................................................
.............................................................................................................................................................
.............................................................................................................................................................
.............................................................................................................................................................
............................................................................................................................................................. lOMoARcPSD| 59629529 Số phách
............................................................................................................................................................
. ......................................................................................................................................................... .... lOMoARcPSD| 59629529 Số phách
b) Để hiệu suất tổng hợp NH3 cao cần thực hiện phản ứng ở nhiệt độ và áp suất như thế nào? Giải thích? Cho biết: N2 H2 NH3 Ho298 (kJ/mol) - - - 46,19
So298 (J/mol.K) 191,49 130,59 192,51
...........................................................................................................................................................................
...........................................................................................................................................................................
...........................................................................................................................................................................
...........................................................................................................................................................................
...........................................................................................................................................................................
...........................................................................................................................................................................
...........................................................................................................................................................................
...........................................................................................................................................................................
...........................................................................................................................................................................
...........................................................................................................................................................................
...........................................................................................................................................................................
...........................................................................................................................................................................
...........................................................................................................................................................................
...........................................................................................................................................................................
...........................................................................................................................................................................
...........................................................................................................................................................................
...........................................................................................................................................................................
...........................................................................................................................................................................
...........................................................................................................................................................................
...........................................................................................................................................................................
...........................................................................................................................................................................
...........................................................................................................................................................................
...........................................................................................................................................................................
...........................................................................................................................................................................
...........................................................................................................................................................................
...........................................................................................................................................................................
........................................................................................................................................................................... lOMoARcPSD| 59629529 Số phách
c) Ở 4500C, nếu xuất phát từ hỗn hợp có tỷ lệ N2 : H2 = 1:3 theo số mol, thì cần thực
hiện phản ứng tổng hợp NH3 dưới áp suất bằng bao nhiêu để hiệu suất chuyển hóa bằng 90%?
...........................................................................................................................................................................
...........................................................................................................................................................................
...........................................................................................................................................................................
...........................................................................................................................................................................
...........................................................................................................................................................................
...........................................................................................................................................................................
...........................................................................................................................................................................
...........................................................................................................................................................................
...........................................................................................................................................................................
...........................................................................................................................................................................
...........................................................................................................................................................................
...........................................................................................................................................................................
...........................................................................................................................................................................
...........................................................................................................................................................................
...........................................................................................................................................................................
...........................................................................................................................................................................
........................................................................................................................................................................... .
.......................................................................................................................................................................... Câu IV (2,5 điểm) a b Tổng điểm 0,5 2,0 2,5 Cán bộ 1 Cán bộ 2
Dung dÞch chøa ion Fe (SCN)2+ cã mµu ®á khi nång ®é cña Fe(SCN)2+ lín h¬n 10-5M. H»ng
sè bÒn cña ion Fe (SCN)2+ Kb1 = 2.102.
a) Trong 500ml dung dÞch cã chøa 10-3 mol FeCl3 vµ 5.10-3 mol KSCN. TÝnh nång ®é
cña ion Fe (SCN)2+ ë tr¹ng th¸i c©n b»ng. Dung dÞch cã mµu ®á kh«ng?
...............................................................................................................................................
...............................................................................................................................................
............................................................................................................................................... lOMoARcPSD| 59629529 Số phách
............................................................................................................................................... .
..............................................................................................................................................
............................................................................................................................................... .
..............................................................................................................................................
...............................................................................................................................................
...............................................................................................................................................
...............................................................................................................................................
...............................................................................................................................................
...............................................................................................................................................
...............................................................................................................................................
...............................................................................................................................................
...............................................................................................................................................
...............................................................................................................................................
...............................................................................................................................................
...............................................................................................................................................
b) Hßa tan tinh thÓ NaF vµo dung dÞch trªn (thÓ tÝch dung dÞch kh«ng ®æi) sÏ t¹o thµnh
ion FeF2+ cã h»ng sè bÒn Kb2 = 1,6.105. Hái ph¶i thªm Ýt nhÊt bao nhiªu gam NaF th× mµu ®á míi biÕn mÊt?
...............................................................................................................................................
...............................................................................................................................................
...............................................................................................................................................
...............................................................................................................................................
...............................................................................................................................................
...............................................................................................................................................
...............................................................................................................................................
...............................................................................................................................................
...............................................................................................................................................
...............................................................................................................................................
...............................................................................................................................................
...............................................................................................................................................
...............................................................................................................................................
............................................................................................................................................... .
..............................................................................................................................................
...............................................................................................................................................
...............................................................................................................................................
...............................................................................................................................................
............................................................................................................................................... lOMoARcPSD| 59629529 Số phách
...............................................................................................................................................
...............................................................................................................................................
...............................................................................................................................................
............................................................................................................................................... Câu V: (2,0 đ) a (1,0 đ)
b (1,0 đ) Tổng điểm (2,0 đ) Cán bộ 1 Cán bộ 2
a) Viết công thức chiếu Fisơ các đồng phân quang học của hợp chất sec-butyl lactat.
...............................................................................................................................................
...............................................................................................................................................
...............................................................................................................................................
...............................................................................................................................................
...............................................................................................................................................
...............................................................................................................................................
...............................................................................................................................................
...............................................................................................................................................
...............................................................................................................................................
...............................................................................................................................................
...............................................................................................................................................
...............................................................................................................................................
...............................................................................................................................................
b) Từ benzen và các hóa chất vô cơ cần thiết khác, viết sơ đồ điều chế 2,4,5-trinitroanilin.
...............................................................................................................................................
...............................................................................................................................................
...............................................................................................................................................
...............................................................................................................................................
...............................................................................................................................................
...............................................................................................................................................
...............................................................................................................................................
...............................................................................................................................................
...............................................................................................................................................
...............................................................................................................................................
...............................................................................................................................................
............................................................................................................................................... lOMoARcPSD| 59629529 Số phách
...............................................................................................................................................
...............................................................................................................................................
...............................................................................................................................................
............................................................................................................................................... .
.............................................................................................................................................. lOMoARcPSD| 59629529 Số phách Câu VI: (2,0 đ)
Tổng điểm (2,0 đ) Cán bộ 1 Cán bộ 2
Từ xiclohexanon và etyl acrylat làm thế nào điều chế được đietyl 9-hidroxidecalin-
1,3đicacboxylat. Ở đây có sử dụng những loại phản ứng nào?
.................................................................................................................................
.................................................................................................................................
.................................................................................................................................
.................................................................................................................................
.................................................................................................................................
.................................................................................................................................
.................................................................................................................................
.................................................................................................................................
...............................................................................................................................................
...............................................................................................................................................
...............................................................................................................................................
...............................................................................................................................................
.................................................................................................................................
.................................................................................................................................
.................................................................................................................................
.................................................................................................................................
.................................................................................................................................
.................................................................................................................................
.................................................................................................................................
.................................................................................................................................
.................................................................................................................................
.................................................................................................................................
................................................................................................................................. Câu VII: (3,5 đ) 1 2 Tổng điểm (3,5 đ)
a (2,0 đ) b (1,0 đ) c (0,5 đ) a (2,0 đ) b (1,0 đ) c (0,5 đ) Cán bộ 1 Cán bộ 2 lOMoARcPSD| 59629529 Số phách
.................................................................................................................................
.................................................................................................................................
a) Hợp chất A (C3H6O) không cho phản ứng đặc trưng của anđehit và xeton. Cho A tác dụng
với etyl magie iodua, sau khi trung hòa môi trường phản ứng thì thu được hai chất B và C, có
cùng công thức C5H12O. Viết công thức cấu tạo và gọi tên A, B, C. Trong hai sản phẩm B và C, chất nào là sản phẩm
chính? ........................................................................................................................ .........
.................................................................................................................................
.................................................................................................................................
.................................................................................................................................
.................................................................................................................................
.................................................................................................................................
.................................................................................................................................
.................................................................................................................................
.................................................................................................................................
.................................................................................................................................
.................................................................................................................................
.................................................................................................................................
.................................................................................................................................
.................................................................................................................................
.................................................................................................................................
.................................................................................................................................
b) Oxi hóa chất chính nhận được ở trên bằng CrO3 thì được D, cho D phản ứng với brom
trong môi trường kiềm và sau khi trung hòa thì được E. Viết công thức cấu tạo của D và E.
.................................................................................................................................
.................................................................................................................................
.................................................................................................................................
.................................................................................................................................
.................................................................................................................................
.................................................................................................................................
.................................................................................................................................
.................................................................................................................................
.................................................................................................................................
.................................................................................................................................
.................................................................................................................................
.................................................................................................................................
.................................................................................................................................
.................................................................................................................................
.................................................................................................................................
c) Viết sơ đồ phản ứng điều chế E từ propilen và các hóa chất vô cơ cần thiết khác.



