


















Preview text:
lOMoARcPSD|364 906 32 Lecture
CHEMISTRY FOR ENGINEERS Dr. NGUYEN Ngoc Vinh 1 lOMoARcPSD|364 906 32 Chapter 7 Intermolecular forces Molecular forces 2 lOMoARcPSD|364 906 32 3 lOMoARcPSD|364 906 32
Intramolecular forces - Review 4 lOMoARcPSD|364 906 32 5 lOMoARcPSD|364 906 32 Intermolecular forces 6 lOMoARcPSD|364 906 32
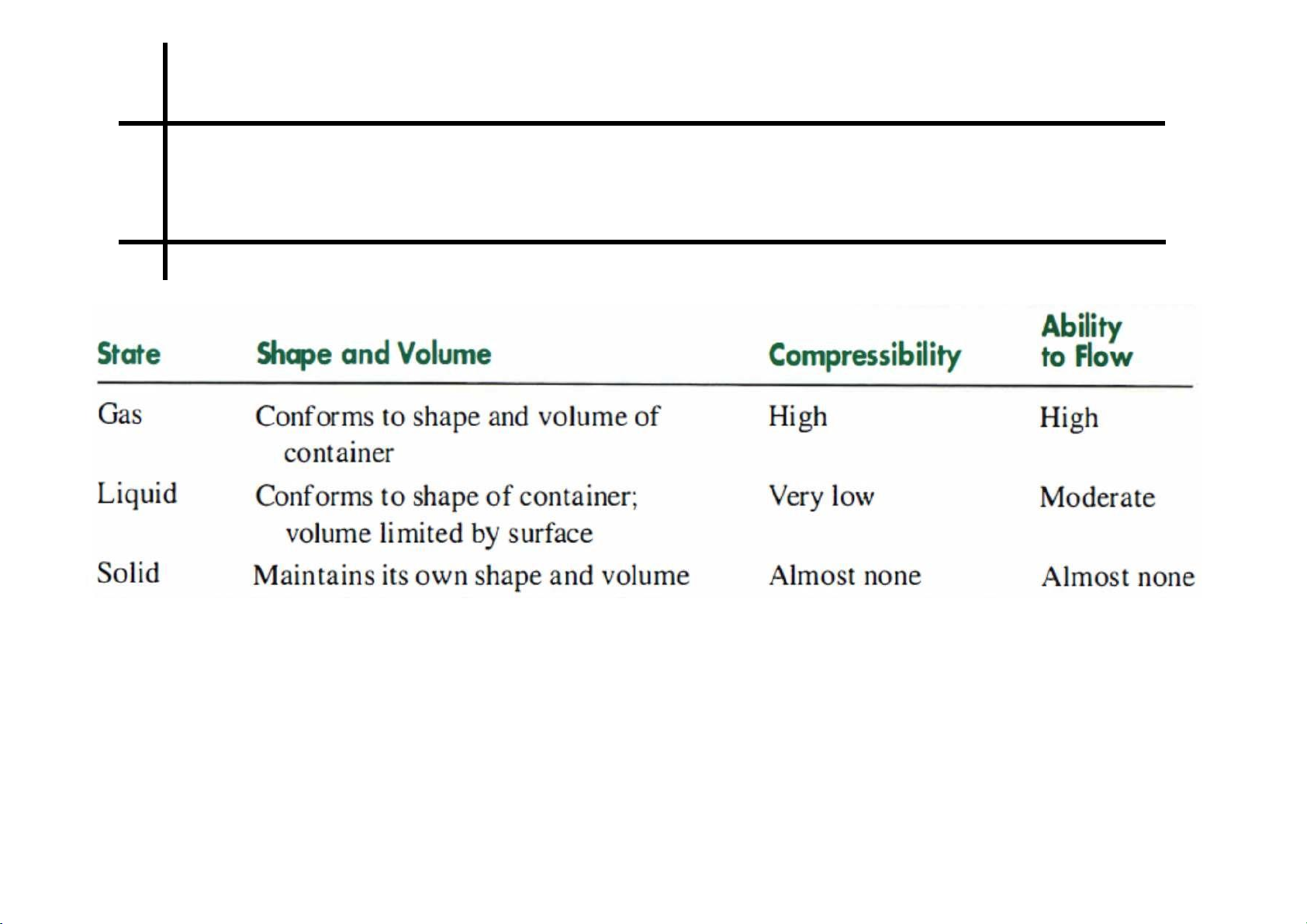
Review on physical states Phase changes 7 lOMoARcPSD|364 906 32 8 lOMoARcPSD|364 906 32
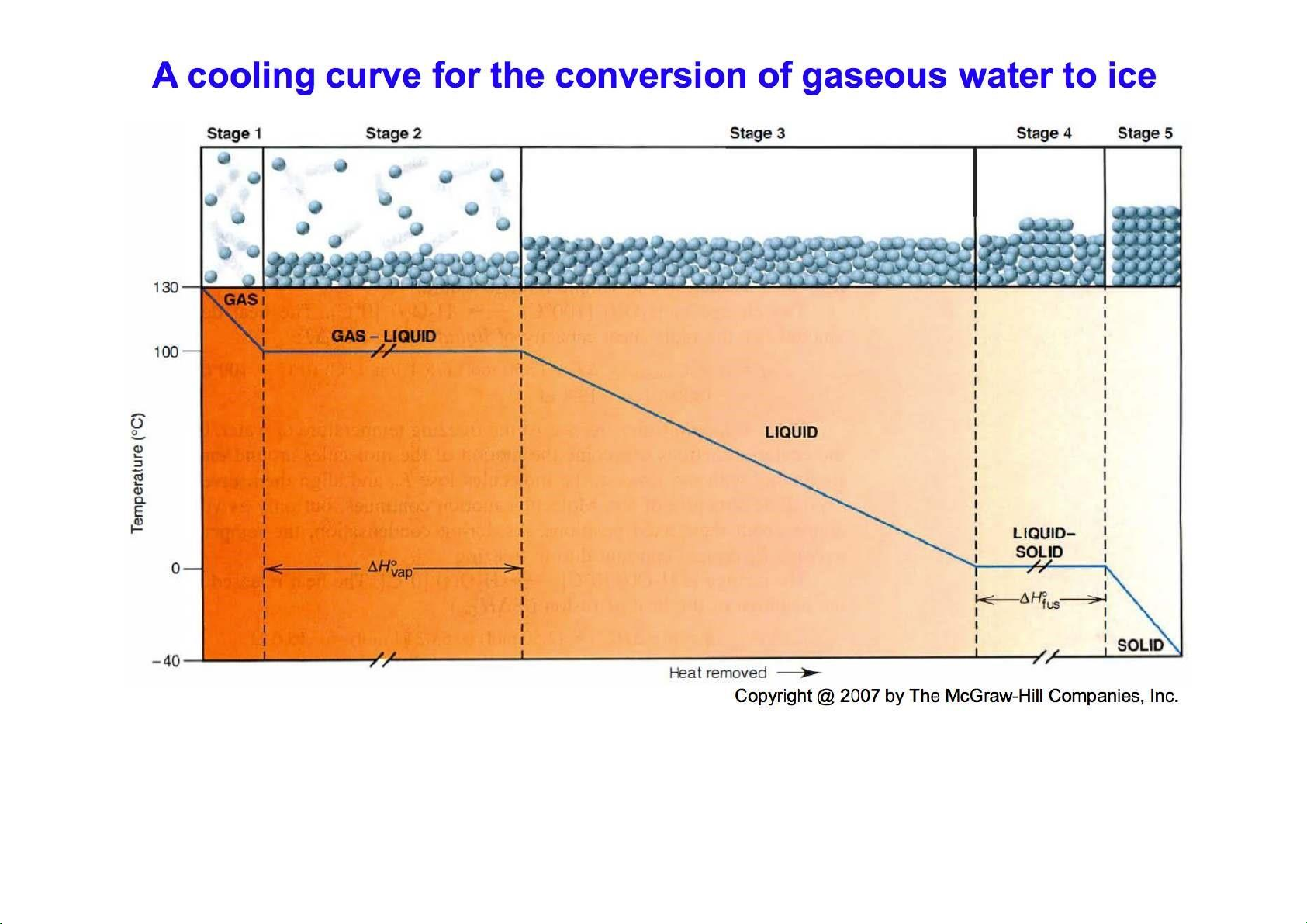
Heating – cooling curve 9 lOMoARcPSD|364 906 32 10 lOMoARcPSD|364 906 32
The equilibrium nature of phase changes (reading) Intermolecular forces 11 lOMoARcPSD|364 906 32
Dipole – dipole forces 12 lOMoARcPSD|364 906 32
Hydrogen bonding forces 13 lOMoARcPSD|364 906 32 14 lOMoARcPSD|364 906 32 Problem 15 lOMoARcPSD|364 906 32
Polarity and boiling point 16 lOMoARcPSD|364 906 32 17 lOMoARcPSD|364 906 32
The significance of hydrogen bonding 18 lOMoARcPSD|364 906 32
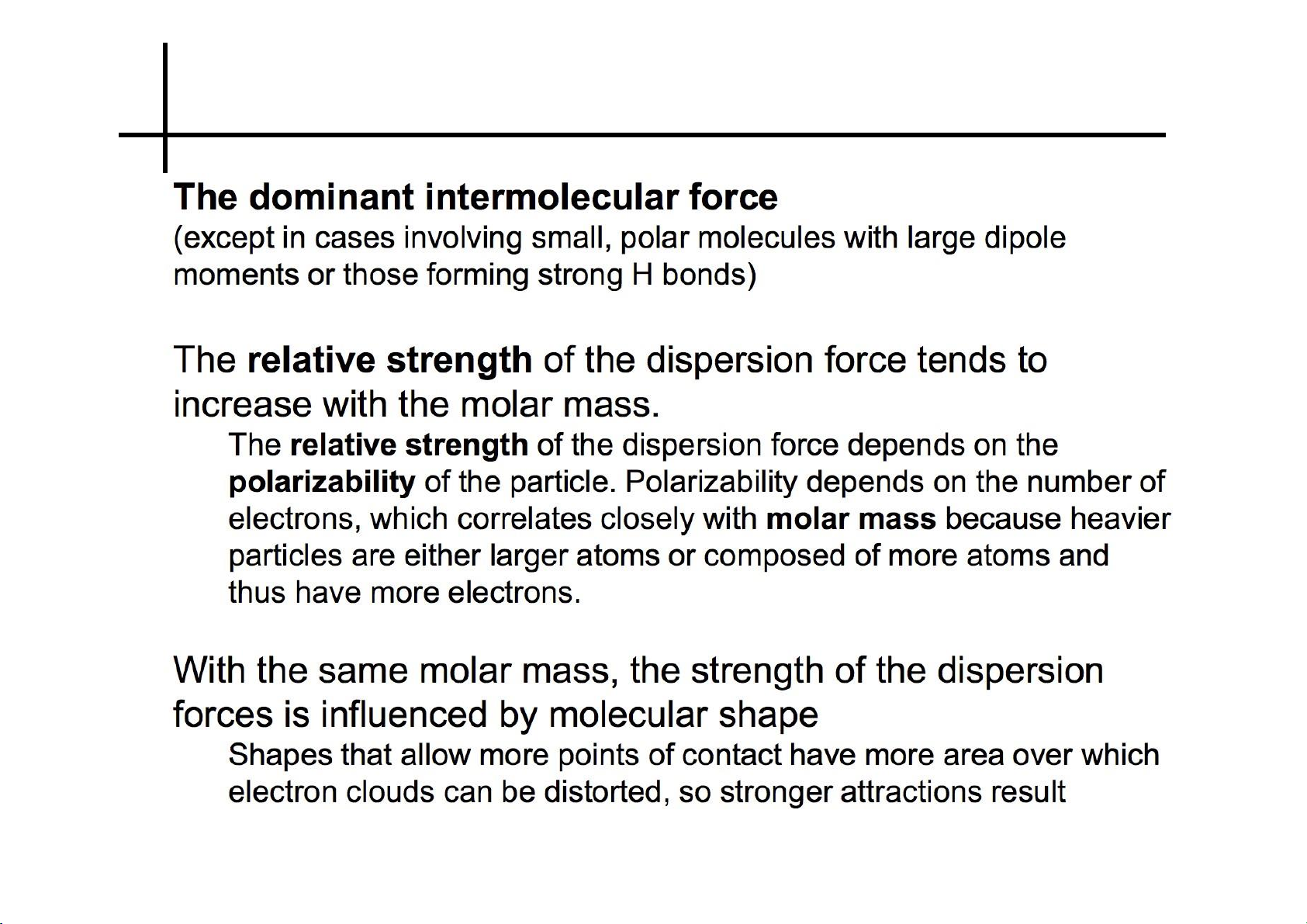
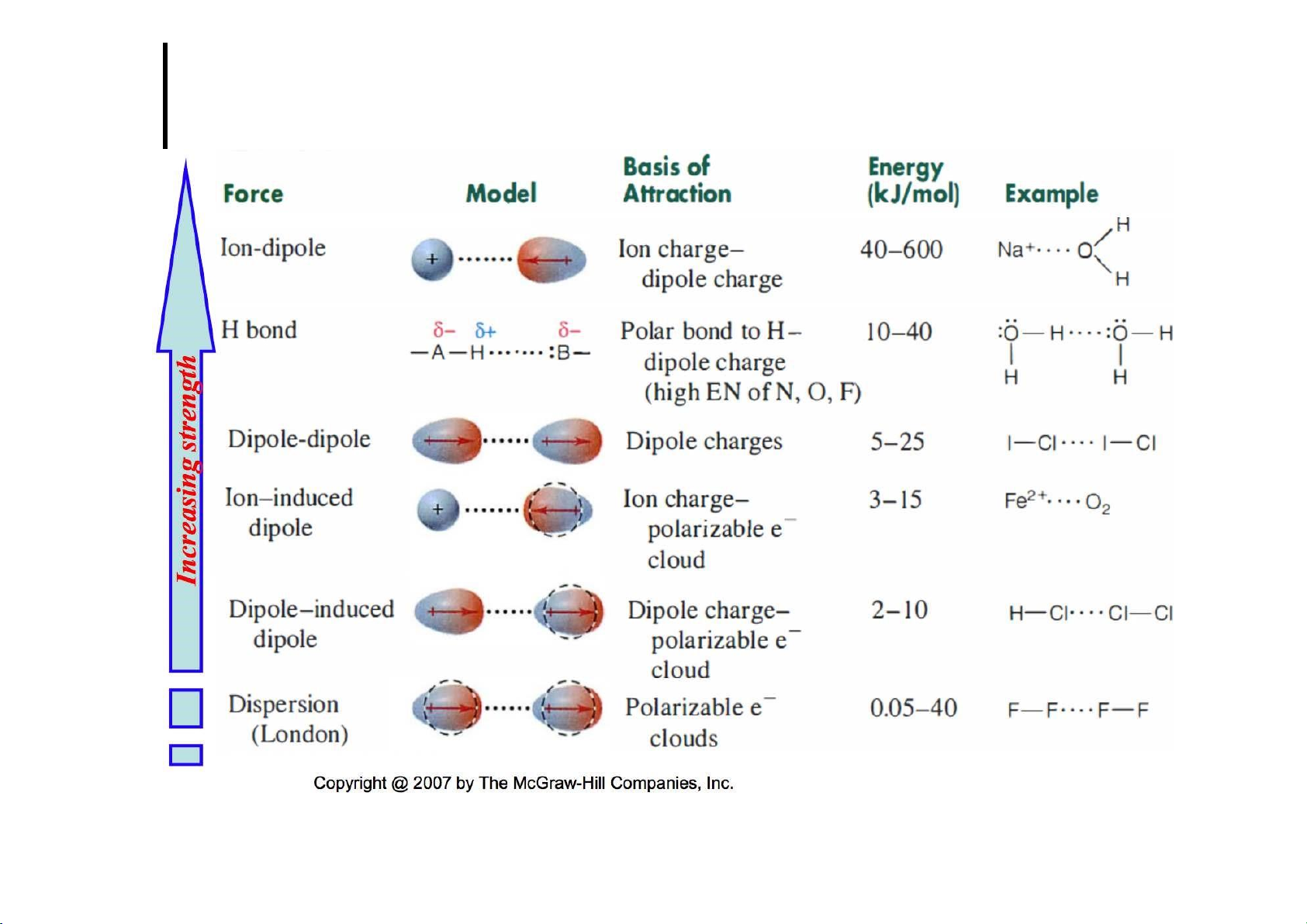
Ion – dipole interactions 19 lOMoARcPSD|364 906 32 Induced dipole forces 20 lOMoARcPSD|364 906 32 21 lOMoARcPSD|364 906 32 Dispersion forces 22 lOMoARcPSD|364 906 32 Dispersion forces 23 lOMoARcPSD|364 906 32 Dispersion forces 24 lOMoARcPSD|364 906 32 Dispersion forces 25 lOMoARcPSD|364 906 32 Strength of forces 26 lOMoARcPSD|364 906 32 27 lOMoARcPSD|364 906 32
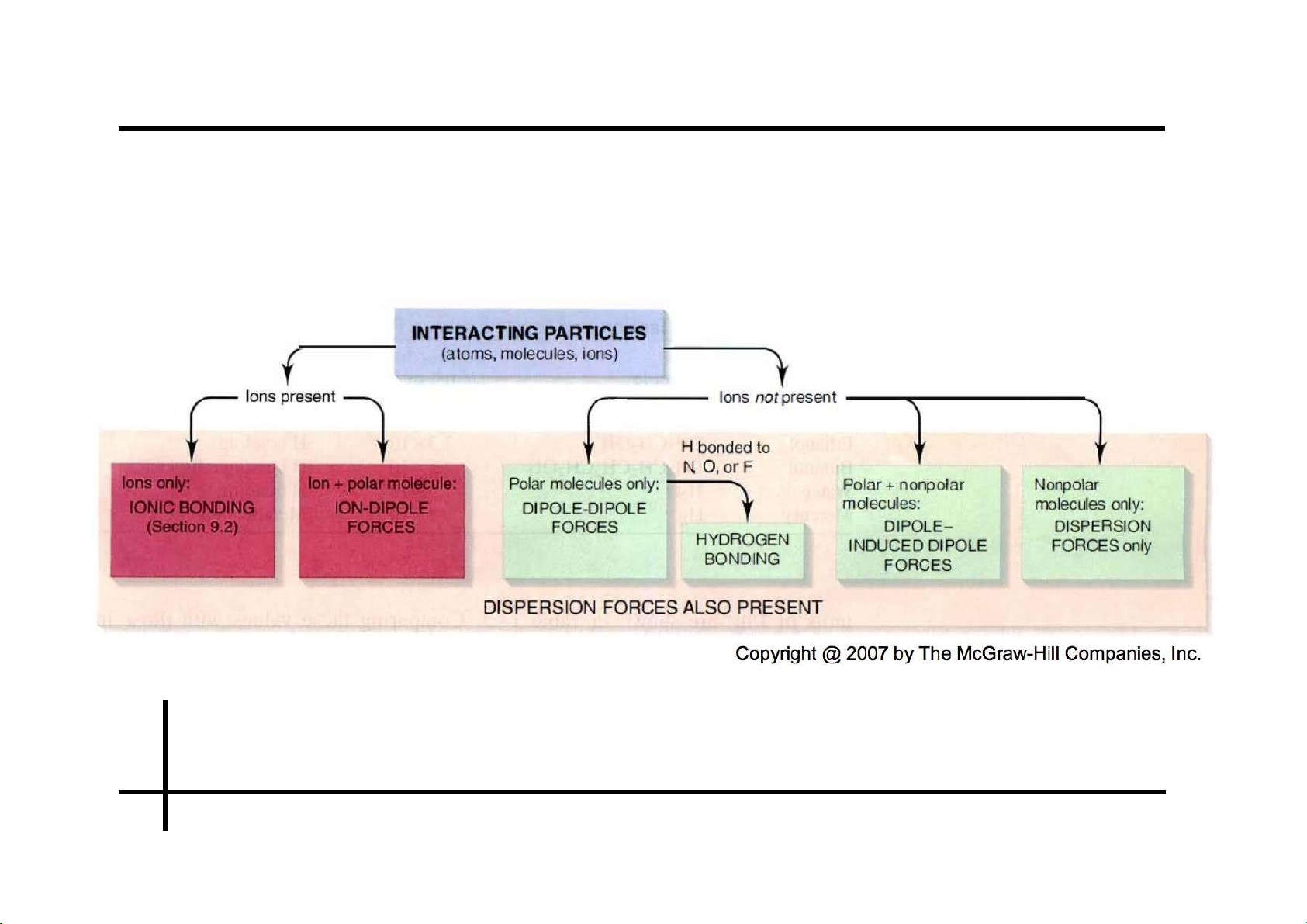
Summary on molecular forces
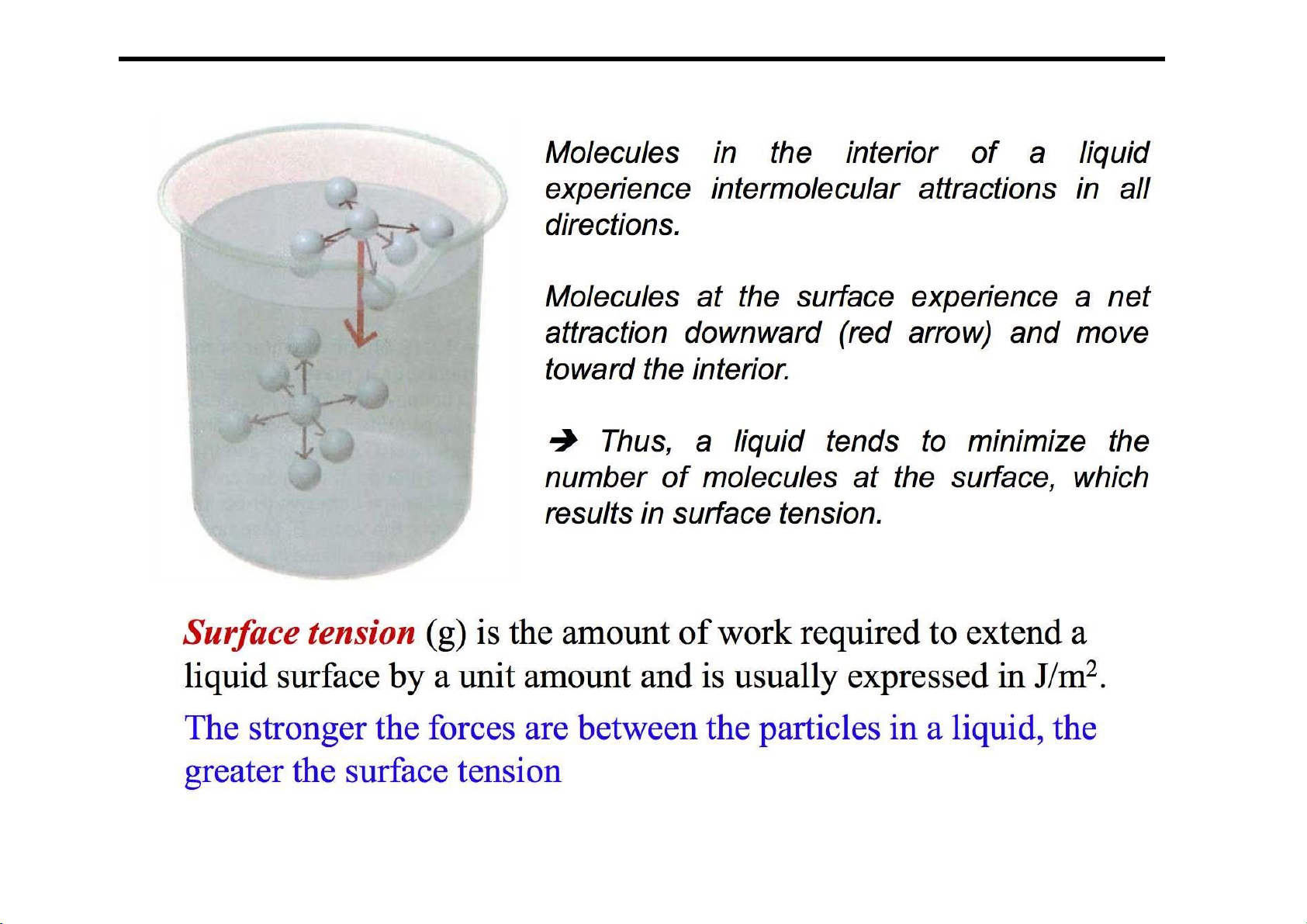
Liquids and intermolecular forces 28 lOMoARcPSD|364 906 32 Surface tension 29 lOMoARcPSD|364 906 32 30 lOMoARcPSD|364 906 32 Viscosity 31 lOMoARcPSD|364 906 32 End of Chapter 7 Downloaded by Hoa Minh (minhhoaanthea@gmail.com)
Document Outline
- End of Chapter 7