











Preview text:
VIETNAM NATIONAL UNIVERSITY – HO CHI MINH CITY
INTERNATIONAL UNIVERSITY
SCHOOL OF BIOMEDICAL ENGINEERING
Chemistry for BME Laboratory BM098IU REPORT [Lab No.7] [CHROMATOGRAPHY] Submitted by
Thai Nguyen Hao – BEBEIU21145 Date Submitted: 12/12/2023 Date Performed: 28/11/2023 Lab Section: Tuesday Morning Course Instructor: Truong Phuoc Long, PhD. TAs: Vo Cam Duyen Bui Chi Bao Tran Duong An Hoa
GRADING GUIDELINE FOR LAB REPORT Number Content Score Comment Format (max 10%) - Font type Yes No - Font size Yes No - Lab title Yes No - Page number Yes No 1 - Table of contents Yes No - Header/Footer Yes No
- List of figures (if exists) Yes No
- List of tables (if exists) Yes No - Lab report structure Yes No Yes No - References
English Grammar and Spelling (max 5%)
(Max 0% if references = 0%) 2 - Grammar Yes No - Spelling Yes No Introduction (20pts):
Data and Result Analysis (max 85%) 3 04 parts Experimental procedure (20 pts): Results & Discussion (30 pts): Total Score Conclusion (15 pts): Overall Signature Date: …/…/2023 ___________ ________________________ i BM098IU International University
School of Biomedical Engineering Table of Contents
List of Figures ......................................................................................................................................... iii
List of Tables ............................................................................................................................................ iv
I. Introduction .......................................................................................................................................... 1
1. Background of Rutin ................................................................................................................... 1
2. Background of chromatography ............................................................................................... 1
a. Thin-layer chromatography ...................................................................................................... 2
b. High-performance liquid chromatography (HPLC) ........................................................... 3
II. Experimental procedures .................................................................................................................. 5
1. Materials and equipment ........................................................................................................... 5
a. Materials ........................................................................................................................................ 5
b. Equipment ..................................................................................................................................... 6
2. Experimental procedures ........................................................................................................... 7
a. Preparation for the materials of laboratory experiments ................................................... 7
a.1. Rutin preparation .................................................................................................................... 7
a.2. Green tea fresh extract preparation ...................................................................................... 7
b. Mobile phase preparation .......................................................................................................... 7
c. Determination of rutin and tannin by TLC ........................................................................... 8
III. Results and discussion...................................................................................................................... 9
1. Results ............................................................................................................................................. 9
2. Discussion .................................................................................................................................... 11
a. Rutin result ................................................................................................................................. 11
b. Green tea extract examination ............................................................................................... 12
IV. Conclusion ........................................................................................................................................ 12
References ............................................................................................................................................... 12 ii BM098IU List of Figures
Figure 1. The structure of Rutin - C27H30O16
[2] ...............................................................................................1 Figure 2. Equation to calculate Rf
[4] ....................................................................................................................3
Figure 3. Procedures of TLC
[1] ..............................................................................................................................3
Figure 4. Instrumentation in HPLC progress
[1] ...............................................................................................4 Figure 5.
Methanol ........................................................................................................................................................5 International University
School of Biomedical Engineering Figure 6. Ethyl
acetat ...................................................................................................................................................5 Figure 7.
Acetic acid.....................................................................................................................................................5 Figure 8.
Chloroform....................................................................................................................................................5 Figure 9. Rutin
pills ......................................................................................................................................................5 Figure 10. Rutin
95% ...................................................................................................................................................5 Figure 11. Analytical
balance ...................................................................................................................................6 Figure 12. Filter
paper .................................................................................................................................................6 Figure 13. Eppendorf
tubes ........................................................................................................................................6 Figure 14.
Eppendorf tubes ........................................................................................................................................6 Figure 15. Mortar and
pestle .....................................................................................................................................6 Figure 16. TLC
aluminum sheets ............................................................................................................................6
Figure 17. TLC plates dimensions
[1] ...................................................................................................................8 iii BM098IU
Figure 18. Green tea extract
result ..........................................................................................................................9 Figure 19. Rutin
result .................................................................................................................................................9 List of Tables Table 1.
Materials ..........................................................................................................................................................5 Table 2.
Equipments .....................................................................................................................................................6
Table 3. Length values of rutin and green tea
extract ......................................................................................9 International University
School of Biomedical Engineering iv BM098IU International University
BM098IU School of Biomedical Engineering . Introduction
The topic of laboratory session 7 is Chromatography, which is used to separate a mixture
by putting it through a medium in which its components move at different speeds while in
suspension or solution. In the laboratory 7, the knowledge, new terminology and
procedures to apply chromatography of rutin and green tea extract are mentioned. The
laboratory no.07 is about theory information, practices and 3 main experiments: preparation
for the materials of laboratory experiments, mobile phase preparation, determination of rutin and tannin by TLC. 1. Background of Rutin
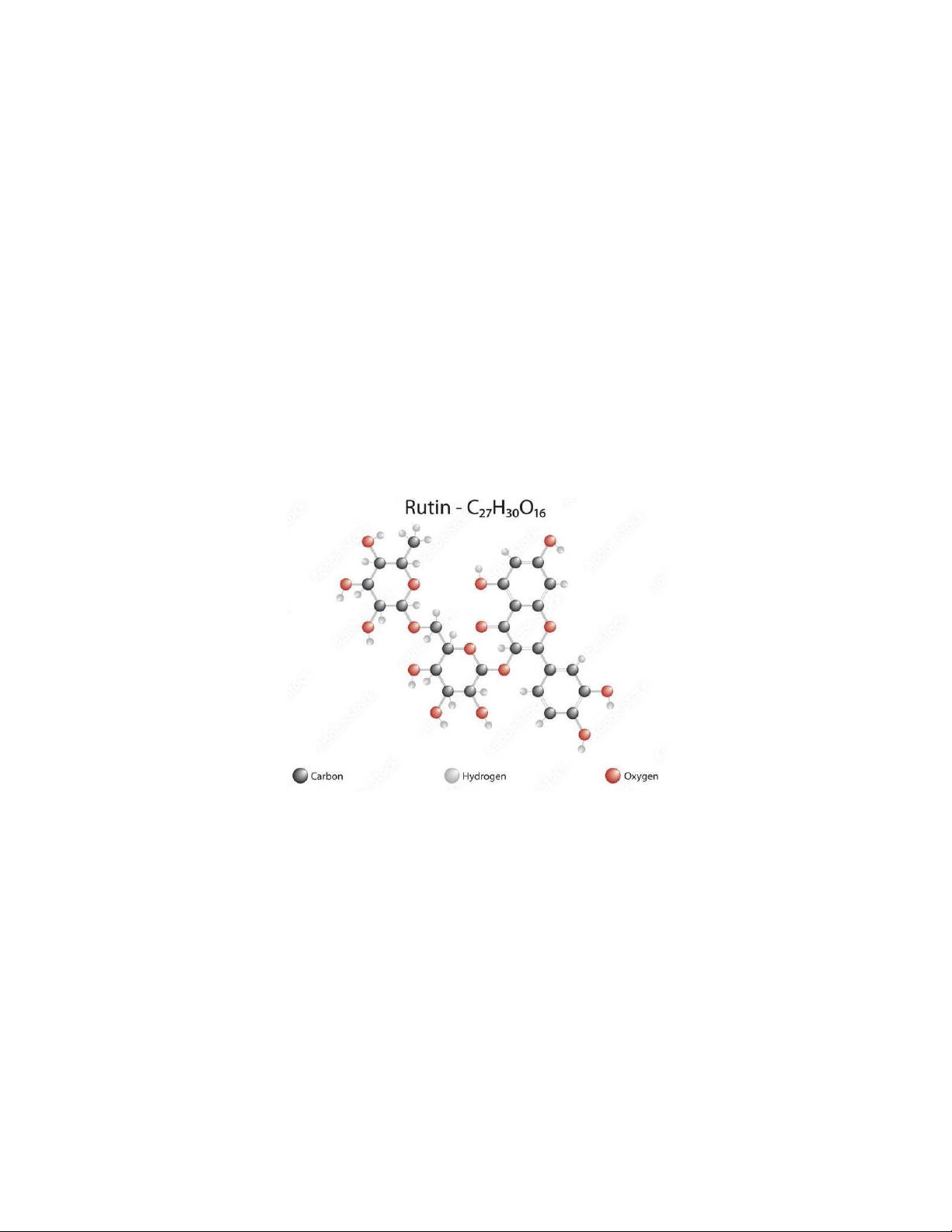
Rutin is is a flavonoid glycoside that can be found in many different plants, for instance,
flowers, tea, apples,…, citrus included. Rutin is also called as rutoside, quercetin-3-
Orutinoside or sophorin. It is the glycoside that combines the disaccharide rutinose (α-
Lrhamnopyranosyl-(1→6)-β-D-glucopyranose) with the flavonol quercetin. Numerous
pharmacological effects, including those of an antioxidant, cytoprotective, vasoprotective,
anticarcinogenic, neuroprotective, and cardioprotective agent, have been shown to be
present. Its appearance is solid, melting point is 242 °C (468 °F; 515 K).
Figure 1. The structure of Rutin - C27H30O16 [2]
2. Background of chromatography
Chromatography is a method used in laboratories to separate mixture components
according to their relative quantities. The mixture is passed through a medium in
suspension or solution, with the constituents moving through it at varying speeds. The
medium, also known as the mobile phase, can be either a liquid or a gas. The mobile phase
is adjacent to the stationary phase, which can be either a liquid or a solid. Chromatography
finds extensive application in diverse fields such as environmental science, biochemistry,
and chemistry, to mention a few. It is employed to determine, separate, and measure the
constituents of a mixture. [3] International University
BM098IU School of Biomedical Engineering
Referred to as the "stationary" phase, the stationary phase comes before the mobile phase.
The mixture's various components are split between the stationary and moving phases as
the mixture is transported through the stationary phase by the moving mobile phase.
Because different components interact with the stationary and mobile phases differently,
this method allows the components of a mixture to be separated.
a. Thin-layer chromatography
Thin-layer chromatography (TLC) is a chromatographic method that uses an inert substrate
to support a thin stationary phase to separate the constituents of a mixture. It can be done
on an analytical scale to track the development of a reaction or on a preparative scale to
purify minute amounts of a substance.
Thin layer chromatography (TLC) is a potent analytical method for separating and
identifying various substances in a mixture. Following are a few typical uses for TLC: drug
analysis (TLC is widely used for both drug analysis and quality control and can be used to
find impurities, degradation products, and active ingredients in drug formulations.),
analysis of natural products (TLC is used to examine natural products like essential oils
and plant extracts. It is useful for determining the various compounds present in these
products and their quantities.), chemical analysis: TLC is a widely used in separating and
identifying various compounds, and can be applied to identify unknown compounds, as
well as to ascertain the composition of mixtures and sample purity.)
The concept of separation is the foundation of thin layer chromatography. The degree to
which compounds are drawn to each phase determines how the compounds are separated.
The mobile phase's compounds traverse the stationary phase's surface. The compounds that
adhere more to the stationary phase are slowed down by the movement, whereas the other
compounds move more quickly. Thus, the mixture is divided. Following the separation
process, the various components of the mixture appear as spots on the plates at varying
depths. With the appropriate techniques, one can discover their nature and personality.
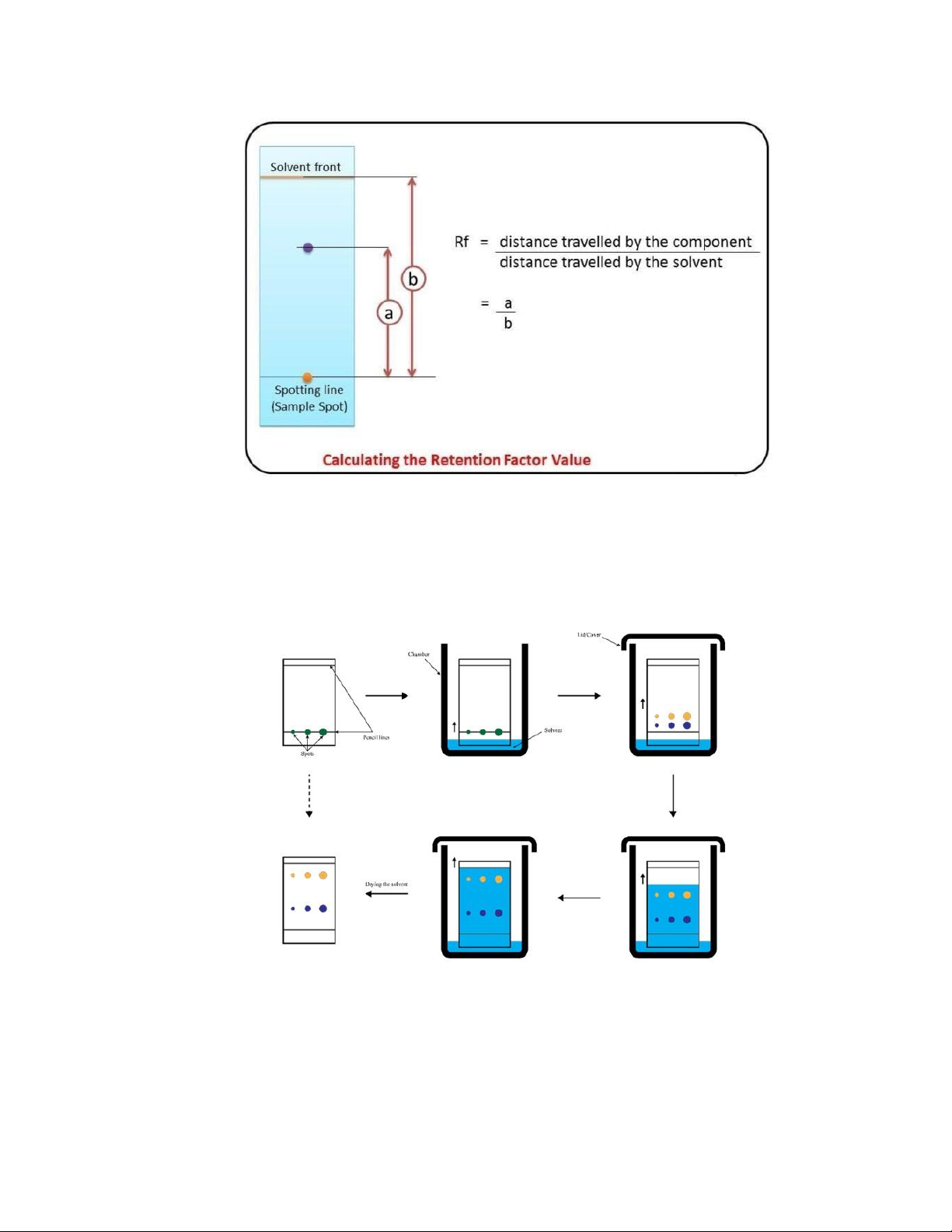
A specific point's distance traveled is used to calculate the position of the moveable phase.
The retention factor, or Rf number, is the ratio of these distances. Among other things, the
Rf value is used to calculate relative masses, relative solubilities, and polarity for both
stationary and mobile phases. This is how it can be calculated: 2 International University
BM098IU School of Biomedical Engineering
Figure 2. Equation to calculate Rf [4]
Sample components are located using a range of techniques following separation. The term
visualization refers to the method of locating analytes on a thin-layer plate. The steps of TLC are illustrated below:
Figure 3. Procedures of TLC [1]
b. High-performance liquid chromatography (HPLC)
High-performance liquid chromatography, which is essentially an improved form of column
chromatography, can be understood as having its roots in column chromatography. High pressures International University
BM098IU School of Biomedical Engineering
of up to 400 atmospheres are used to force a solvent through a column against the natural tendency
of the solvent to slowly trickle through due to gravity. As a result, it proceeds rather rapidly.
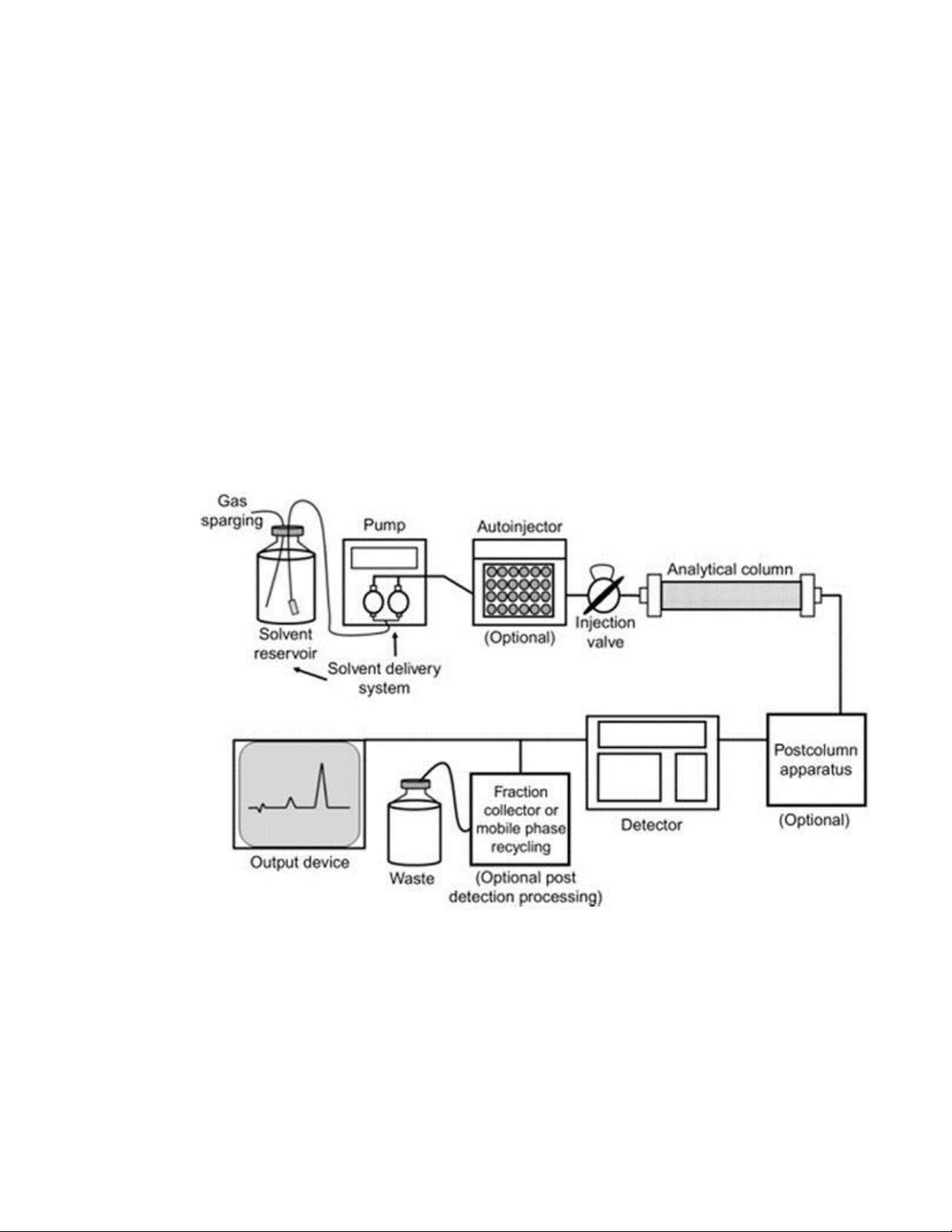
In the process known as high-performance liquid chromatography (HPLC) (stationary
phase), a sample combination or analyte is passed down a column containing
chromatographic packing material in a solvent while being subjected to high pressure,
which is referred to as the mobile phase. Helium or nitrogen carrier gas flows in a stream
to move the sample through the system. Almost any substance that can dissolve in a liquid
can have its molecules separated and identified using the high-performance liquid
chromatography (HPLC) technique, even in incredibly small quantities (as low as parts per trillion).
In many circumstances, HPLC is a widely used method of separation and detection. Gas
chromatography (GC) is most effective with substances that do not evaporate because it
requires the samples to be in their gas phase. Non-volatile substances include vitamins,
metabolites, medications, and carbohydrates. Additionally, because it is non-destructive,
every component can be preserved for later research (like mass spectrometry).
Figure 4. Instrumentation in HPLC progress [1] 4 International University
BM098IU School of Biomedical Engineering
II. Experimental procedures
1. Materials and equipment a. Materials
Table 1. Materials - FeCl3 2.5% - Rutin pills - Ethanol 70% - Methanol - Green tea powder - Distilled water - Rutin powder 97% - Chloroform - Standard tannin solution - Acetic acid Figure 7. Methanol Figure 5. Ethyl acetat Figure 6. Acetic acid International University
BM098IU School of Biomedical Engineering Figure 8. Chloroform Figure 10. Rutin pills Figure 9. Rutin 95% b. Equipment
Figure 14. Eppendorf tubes
Table 2. Equipments - Bécher - Paper - Filter paper - Ultrasonic bath - Weighing paper - Analytical balance - Medical gloves - Volumetric pipette - Pipette aid - Graduated pipette - Micropipette tip 20μL - Micropipette - Pencil
- TLC aluminum sheets - Parafilm. - Analytical balance - Mortar and pestle - Measuring spoon - Eppendorf tubes - Stirring rod
Figure 11. Analytical balance
Figure 13. Filter paper
Figure 12. Eppendorf tubes 6 International University
BM098IU School of Biomedical Engineering
Figure 16. Mortar and pestle
Figure 15. TLC aluminum sheets
2. Experimental procedures
a. Preparation for the materials of laboratory experiments a.1. Rutin preparation
Step 1: By analytical balance and weighing spoon, weigh 10mg of standard rutin
95% and weigh 10mg of rutin of unknown concentration from crushed rutin tablets into powder.
Step 2: Prepare 2 Eppendorf tubes, which are labeled as “Control” and “Test”
before pouring standard rutin 95% and unknown concentration rutin into the
“Control” tube and “Test” tube, respectively.
Step 3: Add 1mL of ethanol 70° into each tube by volumetric pipette and then shake
gently until the rutin in 2 tubes are completely dissolved. Finally, close the tubes’ cap.
a.2. Green tea fresh extract preparation
Step 1: Weigh 1g of green tea fresh and 1g of standard tannin solutions by analytical balance.
Step 2: After dissolving 1g green tea fresh with 20mL of methanol in the beaker
and using ultrasonicator to fully dissolve the solution, filtrate the dissolved solution by filter paper.
Step 2: Label 2 Eppendorf tubes as “Control” and “Test” before transferring the
green tea fresh solution and standard tannins solution into “Control” tube and “Test”
tube, respectively. Finally, close the tubes’ cap.
b. Mobile phase preparation
Step 1: Mobile phase 1, which for rutin determination, is made of ethyl acetate,
acetic acid, and water with ratio 10:2:2 (v/v). Next, the beaker is covered with paraffin film.
Step 2: Mobile phase 2 , which for analysis of green tea extract, is made of
chloroform, ethyl acetate, and methanol with ratio 5:3:2 (v/v). Next, the beaker is covered with paraffin film.
The 2 mobile phases are subjected to a 30-minute sonication. They are then left for twenty minutes on one side.
#NOTES: The acid substances are not allowed let outside and wear chemical
gloves before taking acid substances. International University
BM098IU School of Biomedical Engineering
c. Determination of rutin and tannin by TLC
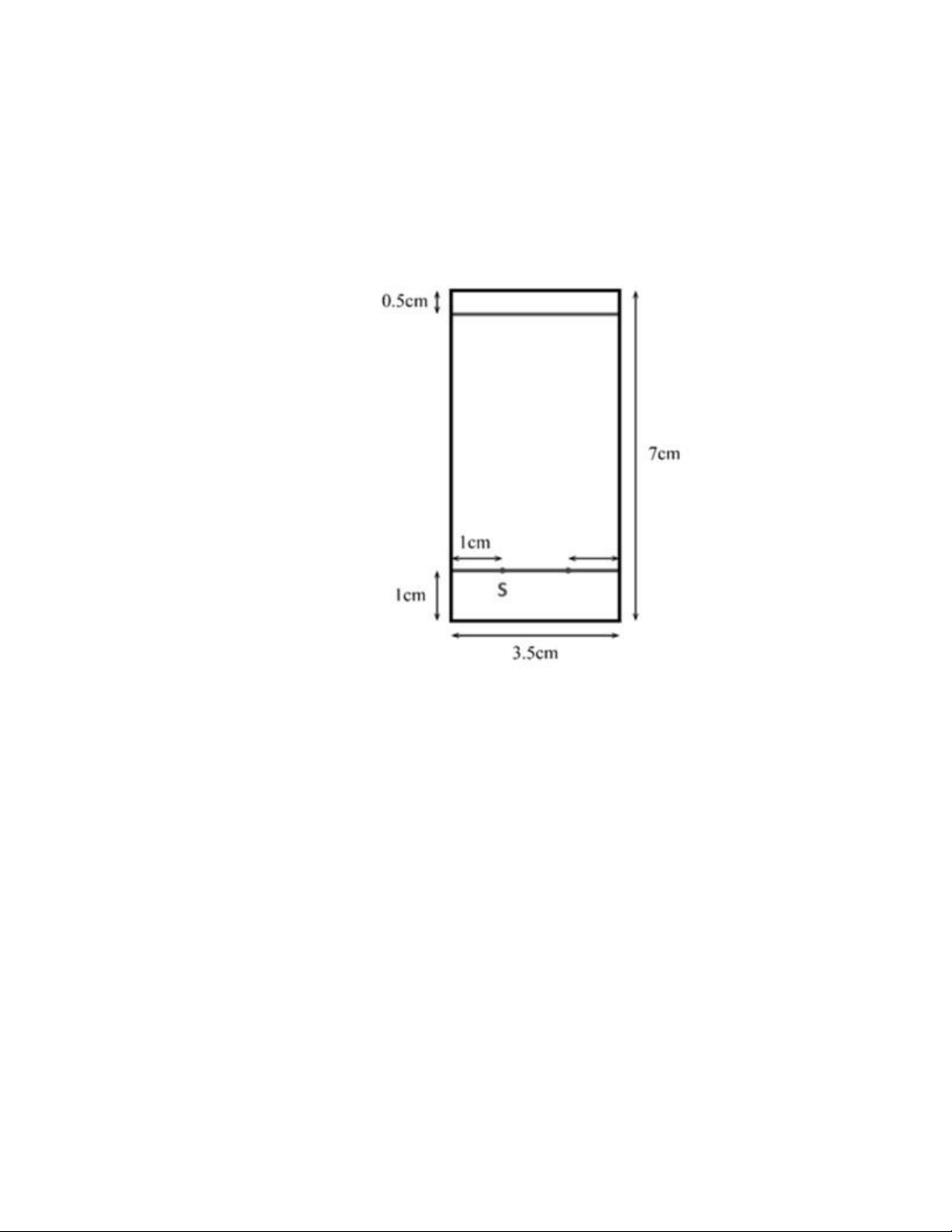
Step 1: Draw 2 lines by pencil on the TLC plates have size of around 7 x 3.5cm
with figure 17 is the correct drawing.
Figure 17. TLC plates dimensions [1]
Step 2: Insert a 20 uL micropipette tip into the “Control” tube and, working from
dot C on the TLC plates, dot each dot three to five times with a 95% rutin solution,
making sure that every dot is completely dry.
Step 3: Once again, follow step 2 and submerge a 20uL micropipette tip in the “Test”
tube. Next, proceed to add dots of the rutin test sample to the TLC plates in an equal
number to that of C, dot by dot, after making sure the prior dot solution is totally dry.
Step 4: Utilizing a different TLC plate, repeat steps 2 and 3 using the tannin control
substance in place of the rutin control substance and the tannin solution for the T-
dot test instead of the rutin sample.
Step 5: After dipping the TLC plates of rutin in the beaker mobile phase 1 and the
TLC plates of tannin in the beaker mobile phase 2, respectively, spray FeCl3 2.5%
on all of the rutin and tannin surfaces (allow technician can see the result clearly) 8 International University
BM098IU School of Biomedical Engineering
and allow them to dry. Lastly, clean the lab apparatus and testing areas and record
them on the results table.
III. Results and discussion 1. Results
Figure 18. Green tea extract result
Figure 19. Rutin result
Table 3. Length values of rutin and green tea extract International University
BM098IU School of Biomedical Engineering Control Test 2 cm 2.4 cm Green tea extract 3.3 cm 3 cm 3.9 cm 3.35 cm 4.6 cm 4 cm Rutin 2.3 cm 2.2 cm
Green tea extract examination:
1) Measured length values: - Control sample (C): 2 cm - Test sample (T): 2.4 cm Calculate Rf: 2 - Control sample: Rf(C) = = 0.363 5.5 2.4 - Test sample: Rf(T) = = 0.436 5.5
2) Measured length values: - Control sample (C): 3.3 cm - Test sample (T): 3 cm Calculate Rf: 3.3 - Control sample: Rf(C) = = 0.6 5.5 3 - Test sample: Rf(T) = = 0.545 5.5
3) Measured length values: - Control sample (C): 3.9 cm - Test sample (T): 3.35 cm Calculate Rf: 10 International University
BM098IU School of Biomedical Engineering 3.9 Control sample: Rf(C) = = 0.709 5.5 3.35 - Test sample: Rf(T) = = 0.609 5.5
4) Measured length values: - Control sample (C): 4.6 cm - Test sample (T): 4 cm Calculate Rf: 4 - Control sample: Rf(C) = = 0.727 5.5 4.6 - Test sample: Rf(T) = = 0.836 5.5 Rutin examination:
Measured length values: - Control sample (C): 2.3 cm
- Test sample (T): 2.2 cm Calculate Rf: 2.3
- Control sample: Rf(C) = = 0.418 5.5 2.2 - Test sample: Rf(T) = = 0.4 5.5 2. Discussion a. Rutin result
There are two spots in the test sample and the control sample. It indicates that every sample
consists of a single component. When the Rf(C) > Rf(T) (0.418 > 0.4), the test sample is
considered to be more polar than the control sample. International University
BM098IU School of Biomedical Engineering
b. Green tea extract examination.
It is impossible to see the spot because of the technician's technique. In the C and T spots,
there are four striations each. The phenomena happens as a result of either an excessively
high sample concentration or an excessive number of sample droplets concentrated in one
location. The test sample 1; 4 is less polar than the control sample 1; 4 if Rf(C) < Rf(T)
(0.0360 < 0.436 and 0.727 < 0.836). The test sample 2; 3 is more polar than the control
sample 2; 3 if Rf(C) > Rf(T) (0.6 > 0.545 and 0.709 > 0.609). Furthermore, the test sample
and control sample's Rf values demonstrate the compounds' ability to separate.
The experimenters' technique, skill in placing the sample on the TLC plate and materials’
condition are what led to this outcome. The experimental result deviates from the
theoretical tolerance for the reasons included: Green tea extract contains many different
components and each of components can have a different affinity for the stationary phase
(the TLC plate) and the mobile phase (the solvent), leads to distinct spots on the TLC plate;
The solvent and plate temperatures fluctuate; the calculated value is inaccurate; low-quality
measuring device; size is incorrect technically; the solution must be taken from the tool
multiple times with a smaller volume than is possible due to the error number; there is still
water inside the beaker when it is weighed, which causes an inaccuracy in the mass value;
the technician executing the task is not accurate. IV. Conclusion
By the end of the laboratory 7, the knowlege about how chromatography, especially
thinlayer chromatography, works and what its characteristics are gained more. The speed
of the test sample and the speed of the control sample are determined based on the amount
of each substance that influences the chemical streak. If the technician did not want the
chemical to run as a line during the test rather than a spot, then the sample concentration
awareness could not be too high. References
1. Truong Phuoc Long, Ph.D. (2023). LABORATORY #7 CHROMATOGRAPHYIn Lab
Manual of Chemistry for BME. essay, Semester 1, Academic Year: 2023-2024.
2. National Library of Medicine. The Pharmacological Potential of Rutin, from:
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5355559/
3. Giddings, J. C., & Keller, R. A. (2023, December 8). Chromatography | Definition, Types, & Facts. Encyclopedia Britannica.
https://www.britannica.com/science/chromatography
4. Aryal, S. (2022, April 20). Paper Chromatography- definition, types, principle, steps,
uses. Microbe Notes. https://microbenotes.com/paper-chromatography/ 12




