
Preview text:
HCMC Vietnam National University
Student Name:………...……………………….
International University Student ID:……………………………. ------------ Final Exam
Date: 21/02/2022 Duration: 120 minutes Open Book and Online
SUBJECT: PHYSICS 2 (FLUID MECHANICS AND THERMAL PHYSICS) (ID: PH014IU)
Approval by Deputy Chair of Department of Physics
Lecturer: Nguyễn Đức Diệu Signature Signature: Full name: Phan Hiền Vũ
Full name: Nguyễn Đức Diệu Proctor 1 Proctor 2 Signature Signature Full name: Full name: STUDENT INFO Student name: Student ID:
INSTRUCTIONS: the total of point is 100 (equivalent to 30% of the course) 1. Purpose:
Test your understanding of basic knowledge of The Kinetic Energy of Ideal Gas and the Second Law of Thermal Dynamics (CLO3).
Examine your skill in analysis and design a problem in science and engineering (CLO2).
Test your ability in applying knowledge of physics (CLO1).
Evaluate your English skills in writing communication manner (CLO4). 2. Requirement:
Read carefully each question and answer it following the requirements.
Write the answers and draw models CLEAN and TIDY directly in the exam paper.
HCMC Vietnam National University
Student Name:………...……………………….
International University Student ID:……………………………. ------------
Submit your exam including this cover page and the solutions of the following problems. Q1 (20 marks):
a. A stream of water of density 𝜌 cross-sectional area ,
A, and speed 𝑣 strikes a
wall that is perpendicular to the direction of the stream, as shown in the aside
Figure. The water then flows sideways across the wall. What is the force exerted by the stream on the wall?
b. The temperature of the Universe (i.e., the microwave radiation background or CMB) is about 3 K at the
present time. When the temperature was 12 K, what was the volume of the Universe compare to its
current volume? Explain your answer! (add figure 1)
Q2 (20 marks): A mole of ideal gas initially at temperature T0 and volume V0 undergoes a reversible isothermal
expansion to volume V1. 𝑉
a. Prove that the work done by the gas is 𝑊 = 𝑅𝑇 1 0ln(
). What is the change of the internal energy of the 𝑉0
gas during this expansion isotherm?
b. When a gas expands adiabatically, its volume is doubled (V1 = 2V0) while its absolute temperature is
decreased by a factor 1.32. Compute the number of degrees of freedom for the gas molecules?
Q3 (20 marks): The adiabatic expansion at an ideal gas is described by the equation 𝑃𝑉𝛾 = 𝐶, where 𝛾 and 𝐶 are constants.
a. Prove that the work done by the gas is expanding adiabatically from the state (Vi, Pi) to the state (Vf, Pf) 𝑃
is 𝑊 = 𝑓𝑉𝑓− 𝑃𝑖𝑉𝑖 . 1−𝛾 𝐶
b. Show that the above work formula can be rewritten as 𝑊 = 𝑉 (𝑃 𝐶 −𝐶
𝑓𝑉𝑓 − 𝑃𝑖𝑉𝑖). 𝑃 𝑉
Q4 (20 marks): A body of mass m = 1 k
g with specific heat C = 1,500 J/K at temperature 500 K is brought to
contact with an identical body at temperature 100 K, and the two are isolated from their surroundings. Find the
change in entropy of the system formed by these two objects? Q 5 (20 marks):
a. A sealed and thermally insulated container of total volume V is di
vided into two equal volumes by an
impermeable wall. The left half of the container is initially occupied by moles of an ideal gas at n
temperature T. Calculating the change in entropy of the system when the wall is suddenly removed and
the gas expands to fill the entire volume?
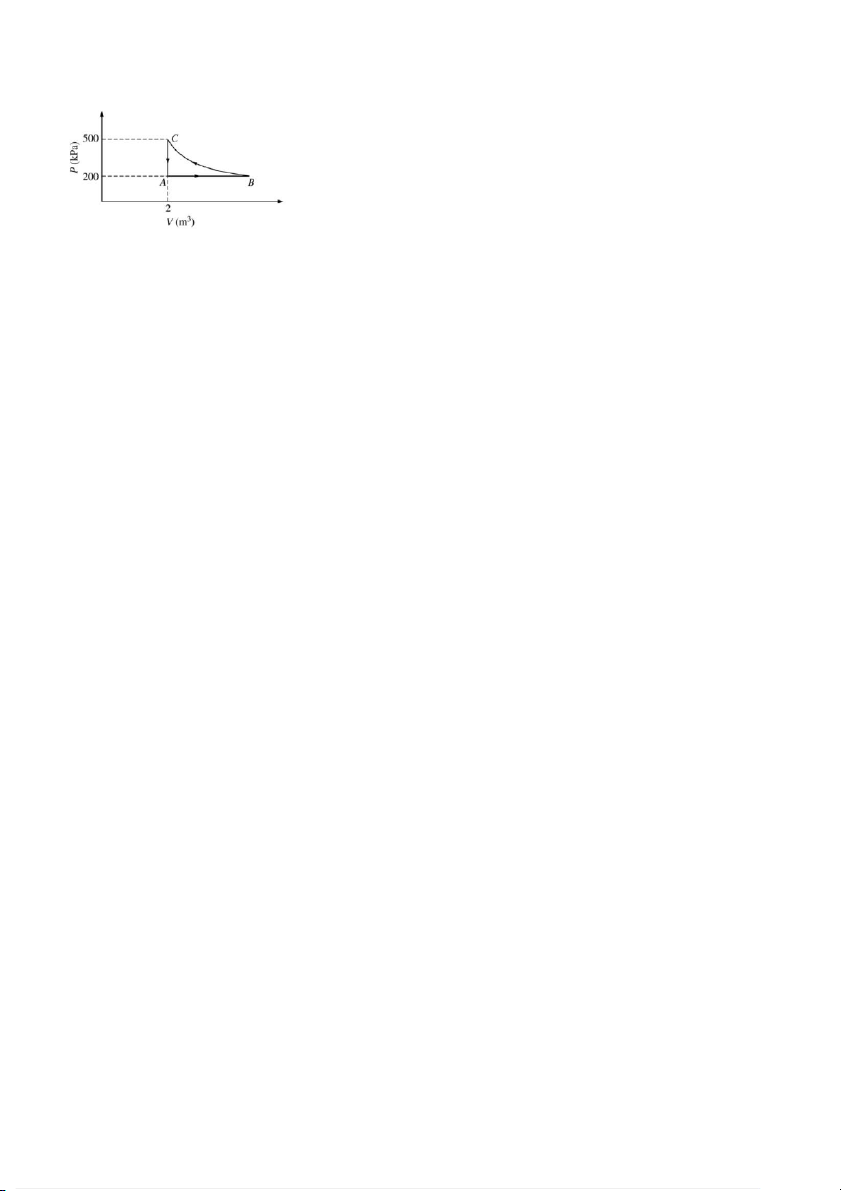
b. An ideal gas undergoes the cyclic process ABCA in the P-V diagram shown in the aside Figure. The path
BC is isothermal. Make an approximation of the work done by the gas during one complete cycle,
beginning and ending at A with the most nearly and rounded number. 2/3
HCMC Vietnam National University
Student Name:………...……………………….
International University Student ID:……………………………. ------------ (add figure 2) – END – 3/3