

Preview text:
lOMoARcPSD|364 906 32
International University - VNUHCM
School of Chemical and Environmental Engineering ----------------- Final Examination
Date: August 09, 2023; Duration: 120 minutes Closed
book; Offline; Laptops/Cell-phone are not allowed.
SUBJECT: Chemistry for Engineers (ID:CH011IU)
Approval by the School of Chemical and Lecturer: Environmental Engineering Signature Signature Full name: Full name: Proctor 1 Proctor 2 Signature Signature Full name: Full name: STUDENT INFO Student name: Student ID:
INSTRUCTIONS: the total point is 100 (equivalent to 40% of the course) 1. Purpose:
• Test your knowledge in (1) intermolecular forces, (2) properties of gases, liquids, and solids, (3)
chemical reactions, chemical kinetics, and chemical equilibrium, (4) chemistry of acids and bases,
(5) thermal chemistry, and (6) chemical thermodynamics (CLOi) 2. Requirement:
• Read carefully each question and answer it following the requirements of each question including
multiple-choice questions and free-response questions.
• Write the answers and draw models CLEAN and TIDY directly in this exam paper PART I
Multiple-choice __________________/80 pts
PART II Free response __________________/20 pts
Total __________________/100 pts QUESTIONS lOMoARcPSD|364 906 32 HCMC National University
Student Name:………...………………………. International
University Student ID:……………………………. ------------
PART I: MULTIPLE CHOICE (40 questions – 2 points/question)
PART II: FREE-RESPONSE QUESTIONS (20 points) Q41. (5 pts) Q42. (5 pts) Q43. (10 pts) /5 2 lOMoARcPSD|364 906 32 HCMC National University
Student Name:………...………………………. International
University Student ID:……………………………. ------------ – END – lOMoARcPSD|364 906 32 HCMC National University
Student Name:………...………………………. International
University Student ID:……………………………. ------------ /53
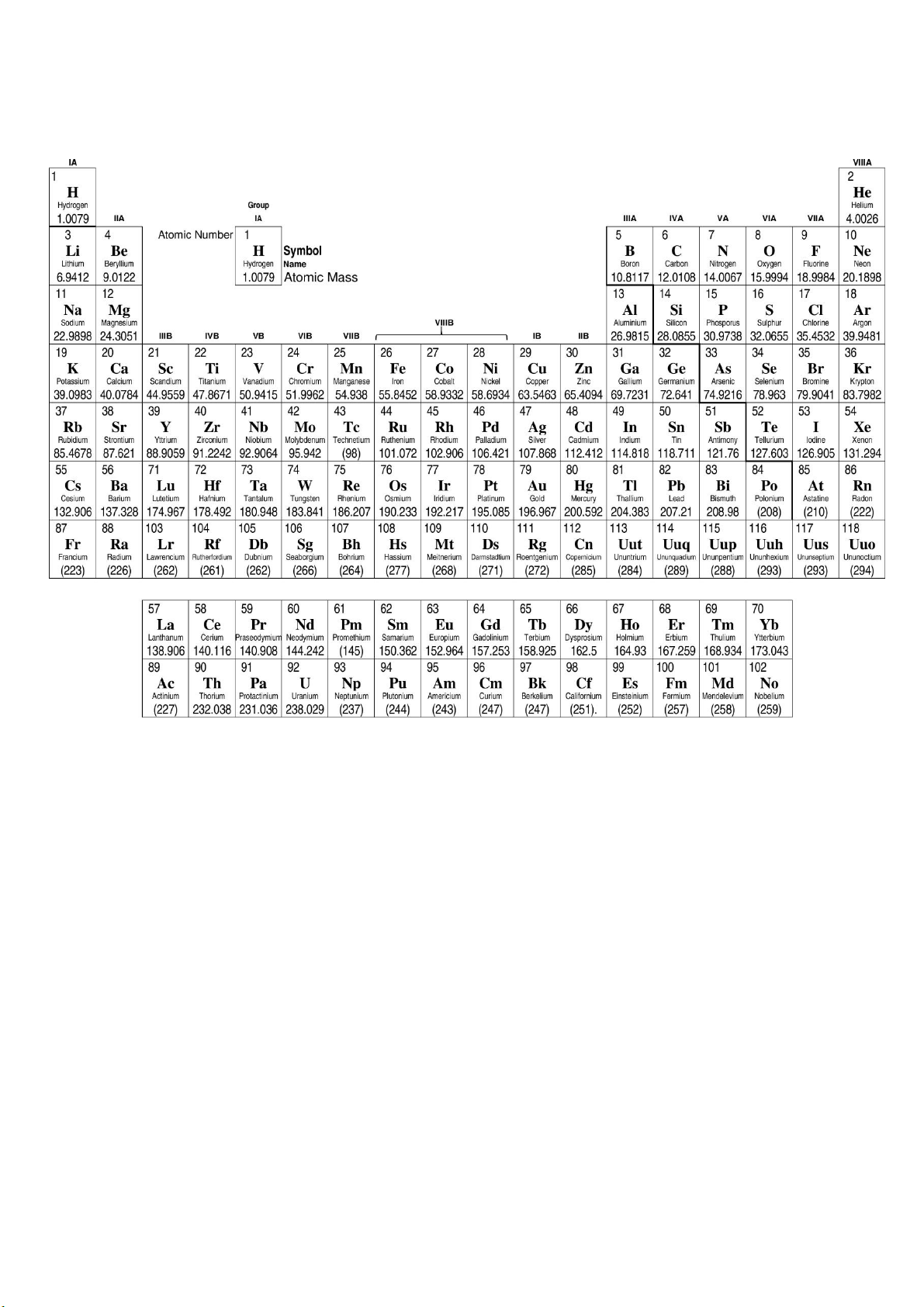
Appendix 1: Periodic Table with Relative Atomic Masses
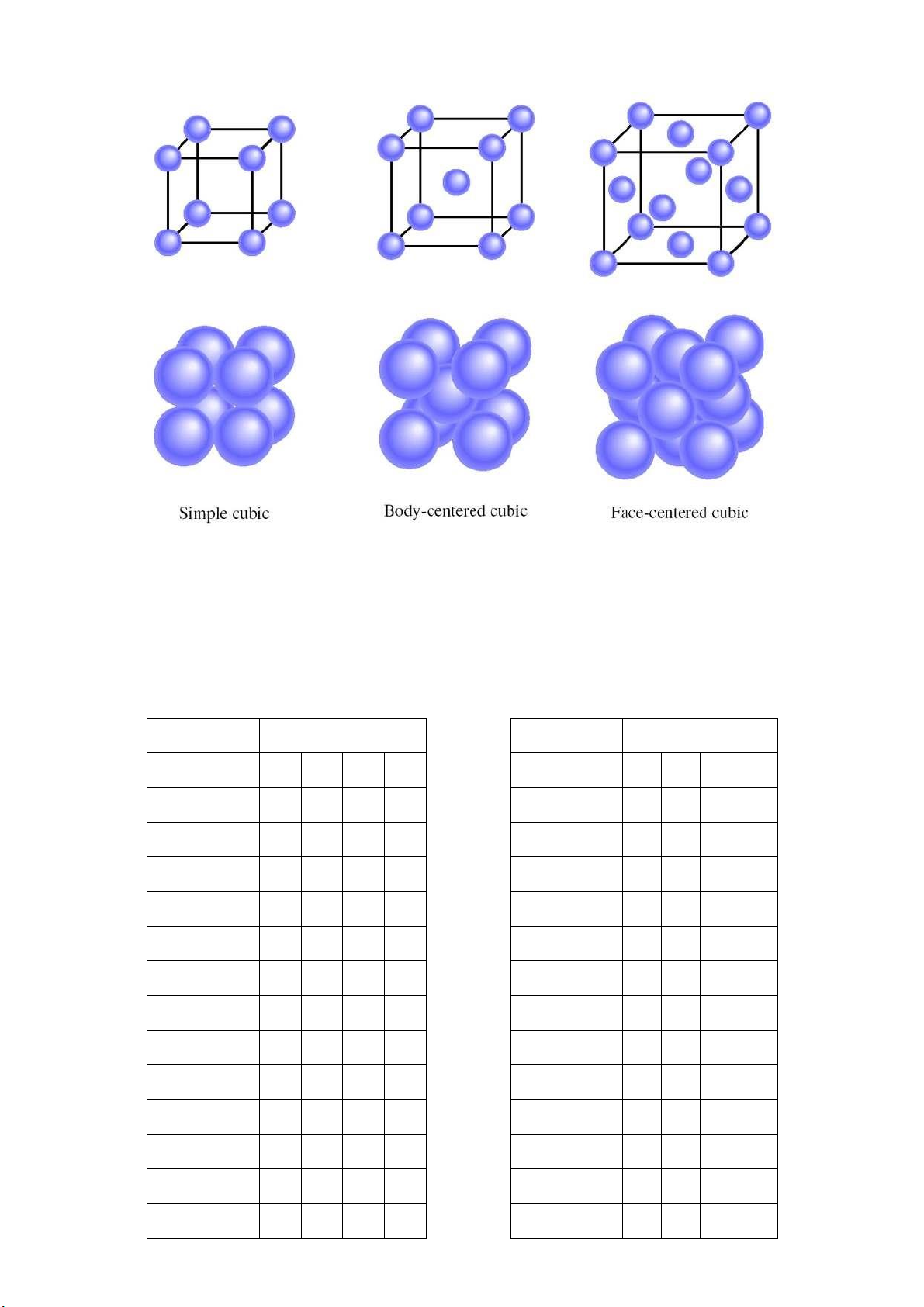
Appendix 2: Three types of cubic unit cells lOMoARcPSD|364 906 32 HCMC National University
Student Name:………...………………………. International
University Student ID:……………………………. ------------ /5 4 ANSWER SHEET
Student’s name: ………………………………………………………………………………………………………….
Student ID: ……………………………………………………………………………………………………………….. QUESTION ANSWER QUESTION ANSWER 1 A B C D 21 A B C D 2 A B C D 22 A B C D 3 A B C D 23 A B C D 4 A B C D 24 A B C D 5 A B C D 25 A B C D 6 A B C D 26 A B C D 7 A B C D 27 A B C D 8 A B C D 28 A B C D 9 A B C D 29 A B C D 10 A B C D 30 A B C D 11 A B C D 31 A B C D 12 A B C D 32 A B C D 13 A B C D 33 A B C D 14 A B C D 34 A B C D lOMoARcPSD|364 906 32 HCMC National University
Student Name:………...………………………. International
University Student ID:……………………………. ------------ 15 A B C D 35 A B C D 16 A B C D 36 A B C D 17 A B C D 37 A B C D 18 A B C D 38 A B C D 19 A B C D 39 A B C D 20 A B C D 40 A B C D INSTRUCTION: QUESTION ANSWER n A B C D Chose A n A B C D Chose C instead of A n A b C D Chose A again /55
Document Outline
- Final Examination
- Q43. (10 pts)
- ANSWER SHEET