














Preview text:
VIETNAM NATIONAL UNIVERSITY, HCMC INTERNATIONAL UNIVERSITY FOOD ANALYSIS LAB MANUAL
Instructor: Dr. Nguyen Hong Long
DEPARTMENT OF FOOD TECHNOLOGY HO CHI MINH CITY, 2023 CONTENTS
Determination of Moisture content .................................................................................................... 3
I. Objective ................................................................................................................................ 3
II. Materials ................................................................................................................................. 3
III. Method ................................................................................................................................... 3
IV. Data and Calculations............................................................................................................. 4
VI. References .............................................................................................................................. 4
Determination of Ash content ......................................................................................................... 5
I. Objective ................................................................................................................................ 5
II. Materials ................................................................................................................................. 5
III. Method ................................................................................................................................... 5
IV. Data and Calculations............................................................................................................. 6
VI. References .............................................................................................................................. 6
Determination of Lipid Content...................................................................................................... 7
I. Objective ................................................................................................................................ 7
II. Materials ................................................................................................................................. 7
III. Method ................................................................................................................................... 7 IV. Data and
calculations ............................................................................................................. 9
V. Questions ................................................................................................................................ 9
Determination of Total Carbohydrate in foods ........................................................................... 10
I. Objective .............................................................................................................................. 10
II. Materials ............................................................................................................................... 10
III. Method ................................................................................................................................. 10
IV. Questions .............................................................................................................................. 11
V. References ............................................................................................................................ 11
Determination of Total Flavonoid Content (TFC) ...................................................................... 13
I. Objective .............................................................................................................................. 13
II. Materials ............................................................................................................................... 13
III. Method ................................................................................................................................. 13
IV. Questions .............................................................................................................................. 13
V. References ............................................................................................................................ 13
Determination of Vitamin C in foods ........................................................................................... 15
I. Objective .............................................................................................................................. 15
II. Materials ............................................................................................................................... 15
III. Method ................................................................................................................................. 15
IV. Questions .............................................................................................................................. 17 V.
References ............................................................................................................................ 17
Determination of Moisture content I. Objective
Determine the moisture content of rice flour and ketchup (using moisture balance analyzer and forced draft oven). II. Materials • Rice flour, 50 g • Ketchup, 50 g • Plastic gloves • Tong • Spoon • Aluminum pan • Tray • Desiccators • Analytical balance • Moisture balance analyzer • Forced draft oven III. Method
Moisture content is determined in triplicate for both methods.
1. Use moisture balance analyzer
Note: test rice flour and liquid milk separately
Follow instruction manual from manufacturer Sample
• Weigh 0.5g samples into the aluminum pan
• Turn on the power switch and waiting for instrument calibration
• Specify settings: automatic operation mode for both rice flour and ketchup
• Place the pan in the instrument
• Carefully close the lid and wait until the weight of aluminum is constant (the sign O appears on the screen) • Tare instrument
• Place testing sample on the pan • Start the measurement • Obtain results
2. Using Forced Draft Oven Ketchup:
• Label and weigh aluminum pans
• Weigh 5 g into aluminum pans and weigh sample
• Evaporate majority of water on a hot plate; do not dry the sample completely (Apply to samples for ash determination as well).
• Place the aluminum pans containing sample in a forced draft oven at 130oC for 3 hours (weigh the aluminum
pans with samples after every 30 mins until no change in weight observed).
• Store in a desiccator until samples are weighed. Rice flour:
• Label and weigh aluminum pans
• Weigh 2g of sample into aluminum pans
• Place the aluminum pans containing sample in a forced draft oven at 130oC for 3 hours (weigh the aluminum
pans with samples after every 30 mins until no change in weight observed).
• Store in a desiccator until samples are weighed. IV. Data and Calculations 1. Recorded Data
Sample Repetition Pan (g) Pan + Wet Pan + % Moisture sample (g) Dried sample (g) Rice flour 1 𝑋̅ = 2 SD = 3 Ketchup 1 𝑋̅ = 2 SD = 3 2. Calculation V. Questions
1. What is the difference between moisture content and water activity measurement? (20%)
2. Why the ketchup sample was partially evaporated on a hot plate before being dried in the hot oven? (20%)
3. Compare moisture content results of different groups, compare results between ketchup and rice flour,
compare results between moisture balance analyzer and forced draft oven method. Explain any difference. (40%)
4. What are advantages and disadvantages of each method? (10%)
5. Lab-work behavior and participation (10%) VI. References
1. AACC International (2010) Approved methods of analysis, 11th edn. (On-line) AACC International, St. Paul, MN
2. AOAC International (2007) Official methods of analysis, 18th edn, 2005; Current through revision 2, 2007
(Online).AOAC International, Gaithersburg, MD
3. Bradley RL Jr (2010) Moisture and total solids analysis, Ch.6.In: Nielsen SS (ed) Food Analysis, 4th edn. Springer, New Your
4. Wehr HM, Frank JF (eds) (2004) Standard methods for the examination of dairy products, 16th edn.
American Public Health Association, Washington,
5. Pham, H. V. (n.d.). Food analysis lab manual (Vol. 2). Ho Chi Minh, International University. Vietnam National University HCMC 4
Determination of Ash content I. Objective
Determine ash content of rice flour and ketchup using muffle furnace. II. Materials • Ketchup, 50 g • Rice flour, 50g • Crucibles
• Desiccators (with dried desiccant) • Volumetric pipettes, 5 ml • Aluminum pan • Gloves • Tong • Spoon • Tray • Analytical balance • Muffle furnace III. Method • Ketchup:
1. Label and weigh the crucible
2. Weigh 2g sample into a crucible and weigh sample
3. Evaporate majority of water on a hot stirring plate
4. Place the crucible containing samples in a forced draft oven at 130oC
5. Weigh the crucible after each 30 min until no change in weight observed
• Rice flour:
6. Label and weigh the crucible
7. Weigh 2g of sample in the crucibles
8. Place crucibles containing samples in a forced draft oven at 110oC
9. Weigh the crucible after each 30 min until no change in weight observed Before drying in the oven After drying in the oven Before drying in the oven After drying in the oven
• Store samples in a desiccator until samples are weighed
• Weigh crucible containing dried sample
• Primary burning the content of materials by flame in a fume hood
• Place all the crucibles in muffle furnace at 550oC for 3h until only white matters can be seen
• Cool crucibles in a desiccator and weigh crucibles with ashed samples IV. Data and Calculations 1. Recorded Data Sample
Repetition Crucible (g)
Crucible + Wet sample (g)
Crucible + Ashed sample (g) % Ash Rice flour 1 𝑋̅ = 2 SD = 3 Milk 1 𝑋̅ = 2 SD = 3 2. Calculation: V. Questions
1. What is ash? What does ash consist of? Give examples to illustrate your answer (15%)
2. How many types of ashing procedures? What are they? (20%
3. Compare ash content results of different groups, compare results between milk and rice flour, compare your
result and ash content reported on the nutrition label or in literature. (40%)
4. Why do the samples need to be placed in desiccators, but not outside, before weighed? (15%)
5. Lab-work behavior and participation (10%) VI. References
1. AACC International (2010) Approved methods of analysis, 11th edn. (On-line) AACC International, St. Paul, MN
2. AOAC International (2007) Official methods of analysis, 18th edn, 2005; Current through revision 2, 2007
(On-line).AOAC International, Gaithersburg, MD
3. Bradley RL Jr (2010) Moisture and total solids analysis, Ch.6.In: Nielsen SS (ed) Food Analysis, 4th edn. Springer, New Your
4. Wehr HM, Frank JF (eds) (2004) Standard methods for the examination of dairy products, 16th edn. American
Public Health Association, Washington,
5. Pham, H. V. (n.d.). Food analysis lab manual (Vol. 2). Ho Chi Minh, International University. Vietnam National University HCMC 6
Determination of Lipid Content I. Objective
Determine the lipid content of snack
foods and rice bran by the Soxhlet method. II. Materials • Butter, 30g • Snack food, 30 g • Hexane
• Aluminum weighing pan, pre-dried in 70oC oven over night
• Filter paper, pre-dried in 70oC oven over night • Plastic gloves • Spatula • Tape • Tongs • Beaker, 250 ml
• Graduated cylinder, 500 ml • Desiccators • Mortar and pestle • Pumice • Analytical balance
• Soxhlet extractor, with glassware • Forced draft oven III. Method
• Record the fat content and serving size of products as shown on the package label Name of Product Label g fat / serving Label serving size (g) Label g fat / 100 g product
• Snack: Grind approx. 10 g sample with mortar and pestle. • Correct moisture content:
- Using the remainder of the ground sample and dried, labeled, and weighed aluminum sample pans, prepare
triplicate 2–3 g samples for moisture analysis.
- Dry sample at 70°C, for 24 h in a drying oven.
- Reweigh after drying and calculate moisture content of the sample.
• Weigh filter paper and label on the filter paper
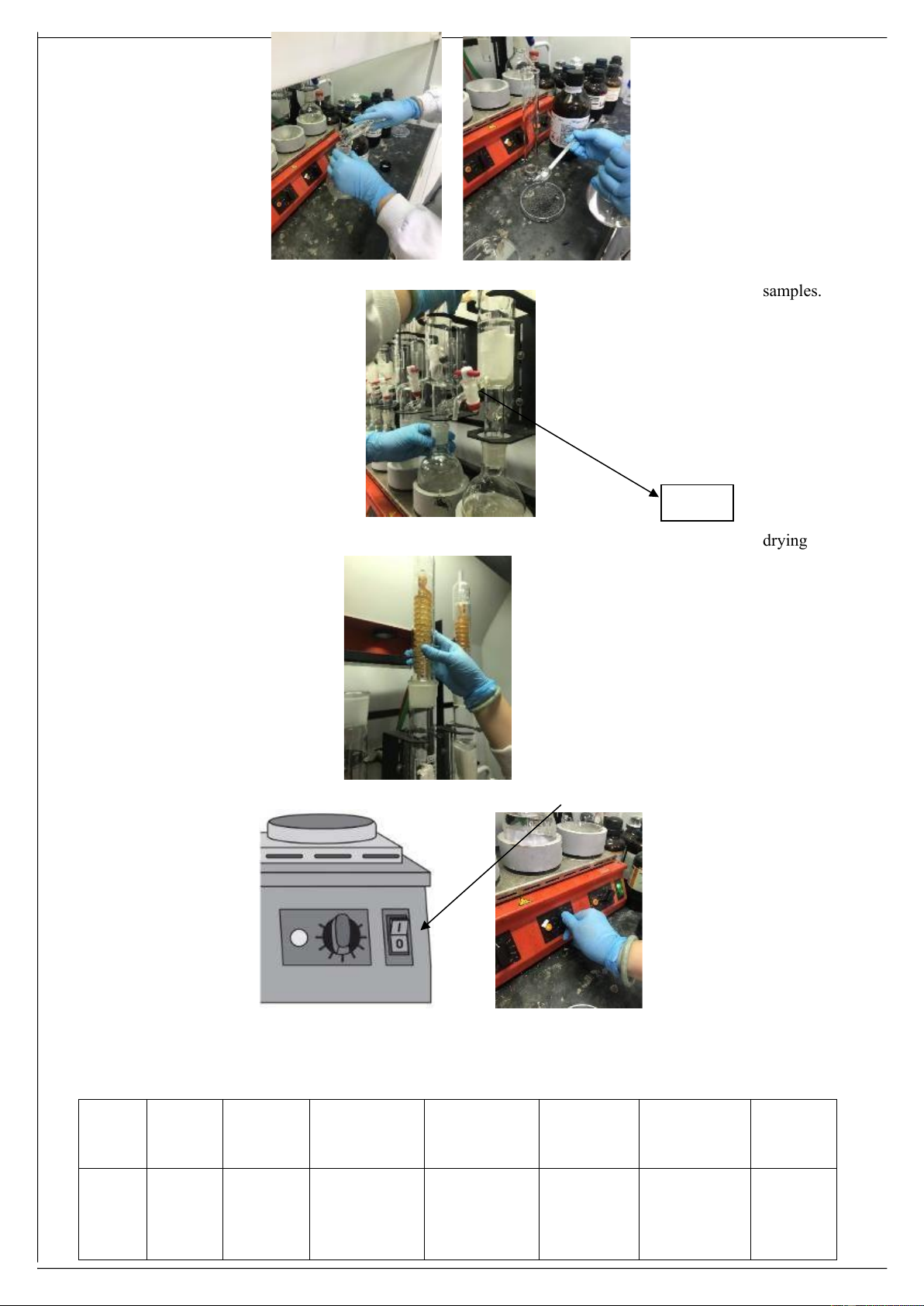
• Prepare samples: place 5 g of snack or rice bran in the pre-weighed filter paper.
Tightly wrap it up and reweigh • Prepare 1 control (blank)
• Fill 250 ml hexane in the round bottom flask. Add some 5 – 8 pumices.
• Place the wrapped filter paper in a Soxhlet extractor 7 Group 1 2 3 4 5 Extractor sample Blank Rice bran Rice bran Snack Snack lOMoARcPSD|47206417 Group 1 2 3 4 5 Extractor sample Blank Rice bran Rice bran Snack Snack • Adjust the power levels at 60% first, then to 70 – 80% so that the solvent boils uniformly in all positions and condenses in sufficient amounts to uniformly extract all of the
• Place the flask on the hotplate flask adapter and insert the extractor into the mouth of the flask. samples. • Remove wrapped filter paper from the Soxhlet extractor using tongs, air dry overnight in a hood, and then open filter paper to expose the closed content; dry in a
• Attach the reflux condenser to the extractor and start the flow of cooling water drying oven at 70oC for 24 h. • Cool dried samples in a desiccator then reweigh. 8
• Turn the power switch on the front of the inline heating unit to the “I” position
IV. Data and calculations Filter Wet Wet sample + Dried sample %Fat + % Sample paper sample Filter paper + Filter paper % moisture Moisture % fat (g) (g) (g) (g) 1 1 1 1 2 2 2 2 Snack 𝑿 = 𝑿 = 𝑿 = 𝑿 = SD = SD = SD = SD = lOMoARcPSD|47206417 1 1 1 1 Rice 2 2 2 2 bran 𝑿 = 𝑿 = 𝑿 = 𝑿 = SD = SD = SD = SD = 1. Recorded data 2. Calculation - Calculation of % moisture: - Calculation of % fat:
[(𝐼𝑛𝑖𝑡𝑖𝑎𝑙 𝑤𝑡 𝑜𝑓 𝑠𝑎𝑚𝑝𝑙𝑒 + 𝐹𝑖𝑙𝑡𝑒𝑟 𝑝𝑎𝑝𝑒𝑟) − (𝐹𝑖𝑛𝑎𝑙 𝑤𝑡 𝑜𝑓 𝑠𝑎𝑚𝑝𝑙𝑒 + 𝐹𝑖𝑙𝑡𝑒𝑟 𝑝𝑎𝑝𝑒𝑟)]𝑋̅100
%(𝑓𝑎𝑡 + 𝑚𝑜𝑖𝑠𝑡𝑢𝑟𝑒) =
[(𝑊𝑡 𝑜𝑓 𝑤𝑒𝑡 𝑠𝑎𝑚𝑝𝑙𝑒 + 𝐹𝑖𝑙𝑡𝑒𝑟 𝑝𝑎𝑝𝑒𝑟) − (𝑊𝑡 𝑜𝑓𝐹𝑖𝑙𝑡𝑒𝑟)]
%𝐹𝑎𝑡(𝑤𝑡/𝑤𝑡) = (%𝐹𝑎𝑡 + %𝑀𝑜𝑖𝑠𝑡𝑢𝑟𝑒) − (%𝑀𝑜𝑖𝑠𝑡𝑢𝑟𝑒) V. Questions
1. Calculate % moisture and % fat. (30%)
2. Compare your findings to fat content reported on the nutrition label. Explain any similarities and/or differences between the two. (30%)
3. What were the advantages of using the Soxhlet extraction method? Compare Soxhlet extraction method with another
lipid extraction method you know to illustrate your answer. (30%)
4. Lab-work behavior and participation (10%) VI. References
1. AOAD International (2007) Official Methods of Analysis, 18thedn, 2005 ; Current through Revision 2, 2007 (Online).
2. AOAC International, Gaithersburg, MDMin DB, Ellefson WC (2010) Fat analysis.Ch.8.In : Nielsen SS (ed) Food
analysis, 4thedn. Springer, New York
3. Nielsen SS (2010) Determination of fat content, Ch.4.In: Nielsen SS, Food analysis laboratory manual, 2ndedn. Springer, New York
4. Wehr HM, Frank JF (eds) (2004) Standard methods for the examination of dairy products. 17thedn. American Public
Health Administration, Washington, DC
5. Pham, H. V. (n.d.). Food analysis lab manual (Vol. 2). Ho Chi Minh, International University. Vietnam National University HCMC 9 lOMoARcPSD|47206417
Determination of Total Carbohydrate in foods I. Objective
Determine the total carbohydrate content of soft drinks and beers. II. Materials
• Beer, regular; opened and stored in 10oC fridge over night
• Soft drink, regular; opened and stored 10oC fridge over night
• Erlenmeyer flask, 100 ml, for distilled water
• Erlenmeyer flasks, 500 ml, for beverages • Bottle to collect waste
• Cuvettes for spectrophotometer • Gloves
• Mechanical pipettes, 5000 µm, 1000 µm and 100 µm (or 200 µm), with plastic tips
• 20 Test tubes, 16-20 mm internal diameter • Test tube rack
• Volumetric flasks, 1000 ml • Volumetric pipette, 5 ml
• Volumetric pipettes, 10 ml • Spectrophotometer • Vortex mixer
• Water bath, maintained at 25oC III. Method
Prepare standard curve tubes: • Using the
µg Glucose/ ml glucose standard solution (100 mg 0 20 40 60 80 100 glucose/L) and
glucose stock solution (100 µg/ml) 0 2 4 6 8 10 distilled water as indicated in distilled water 10 8 6 4 2 0 the table below.
• Record caloric content from labels of soft drinks and beers.
Prepare sample tubes:
• Dilute sample tubes as 1:2000 dilution by taking o 1ml sample + 9ml distilled water 10ml A (dilute 10 times) o 1
ml A + 9ml distilled water 10ml B (dilute 100 times) o 0.5ml B + 9.5ml distilled water 10ml C (dilute 2000 times)
• Then pipette 1.0 ml sample and 1.0 ml of distilled water into a test tube.
*Note: Phenol and H2SO4 additions are carried out in a hood with fan on
• Phenol addition: add 0.05 ml 80% phenol to each tube of Standard curve tubes and Sample tubes containing a total volume of 2 ml.
• H2SO4 addition: after adding phenol, add 5.0 ml H2SO4 to each tube. Mix on a Vortex test tube mixer. Let tubes stand
for 10 min to cool them to room temperature. Vortex. 10 lOMoARcPSD|47206417
• Reading absorbance: transfer samples from test tubes into cuvettes. Do not rinse cuvettes with water between samples.
• Zero the spectrophotometer with the blank.
• Read absorbance of all other samples at 490 nm. Triplicate IV. Questions
1. Construct a standard curve for your total carbohydrate determinations, expressed in terms of glucose
(A490 versus µg glucose/ 2ml). Determine the equation of the line for the standard curve. (20%) 2.
Calculate the concentration of glucose in your soft drink samples and beer samples in terms of g/ L. (20%)
3. Calculate the caloric content (based only on carbohydrate content) of your soft drink samples and
beer samples in term of Cal/ L and compare to the label. Explain any differences. (30%)
4. Compare and explain any differences between the calories that carbohydrates contributed to soft drinks and to beers. (20%)
5. Lab-work behavior and participation. (10%) V. References lOMoARcPSD|47206417
1. BeMiller JN (2010) Carbohydrate analysis. Ch. 10. In: Nielsen SS (ed) Food Analysis, 4thedn. Springer, New York
2. Dubois M, Gilles KA, Hamilton JK, Rebers PA, Smith F (1956) Colorimetric method for
determination of sugars and related substances. Anal Chem 28: 350 – 356
3. Nielsen SS (2010) Phenol-Sulphuric acid method for total carbohydrates, Ch.6.In: Nielsen SS, Food
analysis laboratory manual, 2ndedn. Springer, New York
4. Pham, H. V. (n.d.). Food analysis lab manual (Vol. 2). Ho Chi Minh, International University.
Vietnam National University HCMC 15 lOMoARcPSD|47206417
Determination of Total Flavonoid Content (TFC) I. Objective
Determine the total flavonoid content of tea using the aluminum chloride colorimetric method. II. Materials • Tea, 10g • AlCl3 10% • CH3COOK 1M • Ethanol 95% • Distilled water • Rutin stock 100µg/ml III. Method
1. Prepare tea samples
• Brew 1g tea with 100ml boiling water in a covered beaker.
• Let the sample stand for 30 min at room temperature.
• Gently shake the beaker every 10 min.
• Take supernatant and dilute 2 times. Do in duplicate.
2. Prepare rutin standard at different concentration
Concentration (μg/ml) 0 20 40 60 80 100 ml rutin stock 0 2 4 6 8 10 ml distilled water 10 8 6 4 2 0
Table 1: Rutin Standard preparation
a. Prepare standard curve and determine total flavonoid contents of tea For each test tube:
• Add 0.5 ml of prepared rutin standard (for construct Standard curve) or diluted tea (for determine TFC of tea). • Add 1.5ml of ethanol 95%. • Add 0.1 ml of AlCl3 10%. • Add 0.1 ml of CH3COOK 1M.
• Add 2.8 ml of distilled water. Do in duplicate.
Let these test tubes stand for 30 min and measure absorbance at 415 nm. IV. Questions
1. What are flavonoids? What are their health effects on human? List some food sources for flavonoids. (20%)
2. What is the principle of aluminum chloride colorimetric method? (20%)
1. Construct the Standard curve for your TFC determination. (20%)
2. Based on your Standard curve, caculate TFC of different tea in term of µg rutin equivalent per g of tea. Discuss your results. (30%)
3. Lab-work behavior and participation. (10%) V. References
1. Van Hung, P., & Morita, N. (2008). Distribution of phenolic compounds in the graded flours milled
from whole buckwheat grains and their antioxidant capacities. Food chemistry, 109(2), 325-331.
2. Yao, L. H., Jiang, Y. M., Shi, J., Tomas-Barberan, F. A., Datta, N., Singanusong, R., & Chen, S. S.
(2004). Flavonoids in food and their health benefits. Plant foods for human nutrition, 59(3), 113-122.
3. Pham, H. V. (n.d.). Food analysis lab manual (Vol. 2). Ho Chi Minh, International University.
Vietnam National University HCMC lOMoARcPSD|47206417
Determination of Vitamin C in foods I. Objective
Determine the Vitamin C content of orange juice using the indicator dye iodine in a titration method. II. Materials • Orange • Sweet leaf (Rau ngót) • Soluble starch • KI • KIO3 • H2SO4, 3M • Beaker, 150 ml • Beaker, 250 ml • Burette, 25 ml • Graduated cylinder, 25 ml
• Graduated cylinder, 100 ml • Erlenmeyer flasks, 125 ml
• Fluted filter paper, 2 pieces • Filtering cloth
• Funnel, approximately 6 – 9 cm diameter (to hold filter paper)
• Funnel, approximately 2 – 3cm diameter (to fill burette) • Glass stirring rods • Pipette bulb or pump • Ring stand • Spatulas • Volumetric flask, 50 ml • Volumetric flask, 200 ml • Volumetric flask, 250 ml • Volumetric pipettes, 2 ml • Volumetric pipettes, 5 ml
• Volumetric pipette, 10 or 20 ml • Analytical balance III. Method
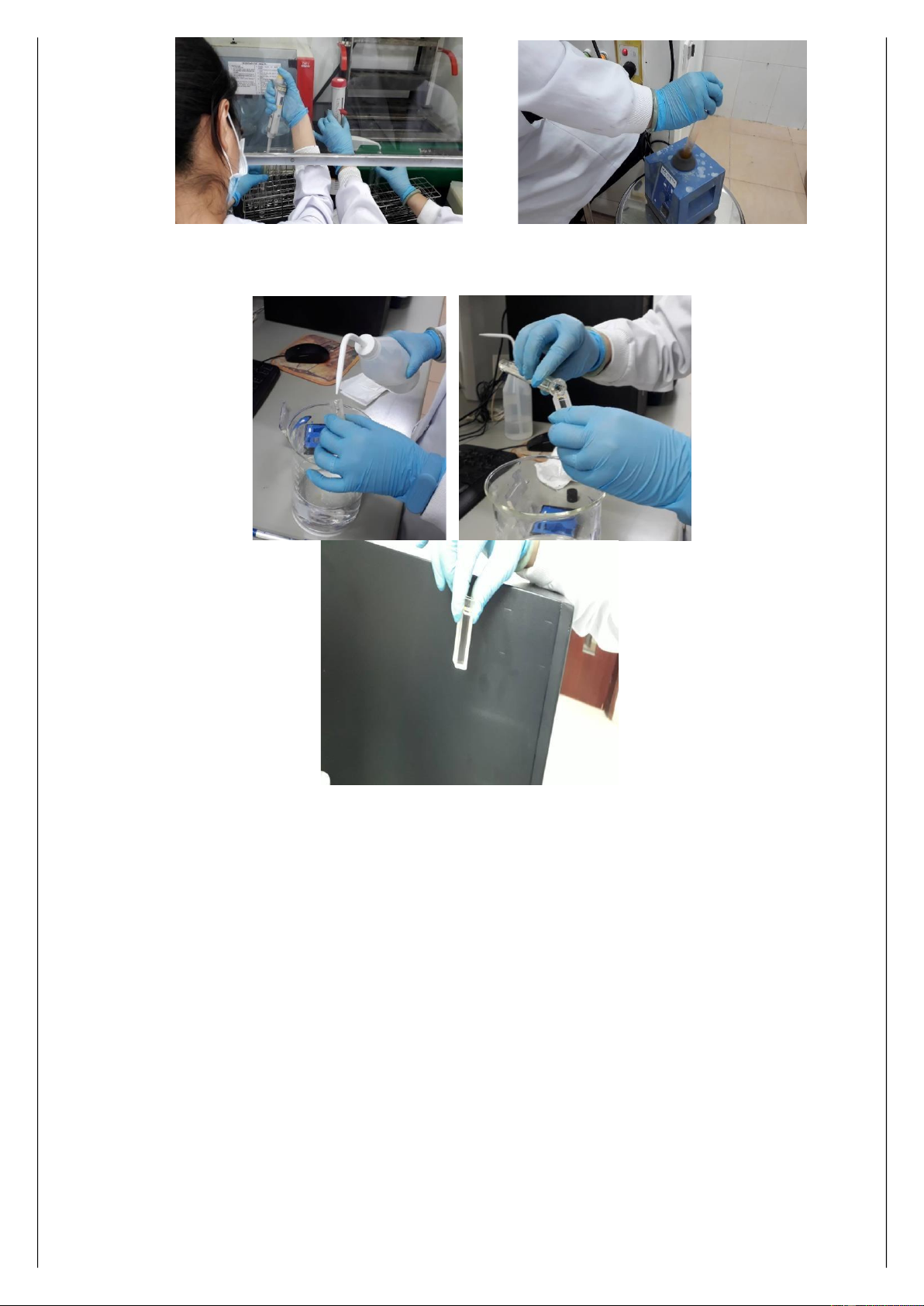
1. Prepare 1% starch indicator solution
• Add 1 g soluble starch to 100 ml distilled water
• Mix the solution well on a hot stirring plate until the solution is clear
• Allow the solution to cool 2. Prepare iodine solution
• Dissolve 5 g KI and 0.27 g KIO3 in 200 ml of distilled water
• Add 30 ml of 3 M sulphuric acid in a hood with fan on
• Dilute the solution with distilled water to 500 ml in a 500 ml graduated cylinder • Vortex 17 lOMoARcPSD|47206417
3. Prepare vitamin C standard solution
• Dissolve 500 mg vitamin C tablet in 200 ml distilled water
• Dilute to 500 ml with distilled water
4. Standardize vitamin C solution
• Add 25 ml of vitamin C solution to a 250 ml flask
• Add 10 drops of 1% starch solution
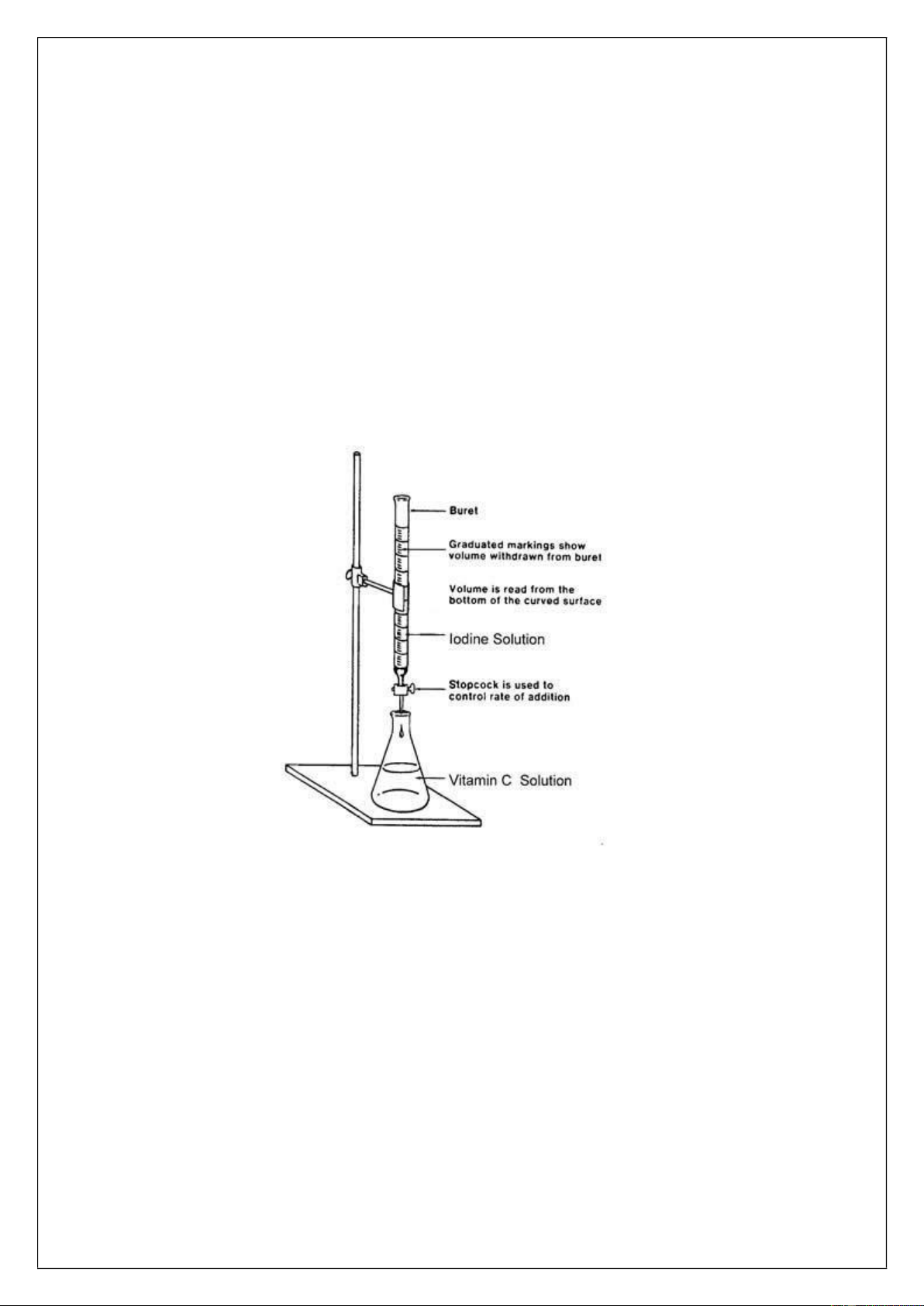
• Rinse your burette with a small volume of the iodine solution and then fill it. Record the initial volume.
• Titrate with iodine solution until the color of solution changes into blue and persists for at least 20 seconds
• Record the final volume of iodine solution. The volume that was required is the starting volume minus the final volume.
• Do triplicate. The results should agree within 0.1 ml.
5. Prepare orange juice solution
• Cut an orange and swirl the pieces to extrude the juice out
• Strain the mixture through cheesecloth
• Pipette 10ml of filtered juice into a centrifugal tube
• Centrifuge at room temperature, 3000 rpm for 10 mins
6. Prepare sweet leaf solution
• Blend a 100 g sample with ~ 50 ml of distilled water
• Strain the mixture through cheesecloth
• Wash the filter with a few milliliters of distilled water
• Collect all filtrate and washings in the volumetric flask
• Add distilled water to make a final solution of 100 ml in a volumetric flask.
• Pipette 10ml of filtered juice into a centrifugal tube 18 lOMoARcPSD|47206417
• Centrifuge at room temperature, 3000 rpm for 10 mins
7. Titrate juice/ sweet leaf samples
• Add 25 ml of juice/ sweet leaf samples to a 250 ml flask
• Perform the same as standardizing vitamin C solution above • Do triplicate 8. Titrate blank solution
• Add 25 ml of water to a 250 ml flask
• Perform the same as standardizing vitamin C solution above • Do triplicate IV. Questions
1. Using the data obtained in standardization of the dye, calculate the titer. (20%)
2. Calculate the ascorbic acid content of the juice sample and the sweet leaf sample in mg/ ml (20%)
3. By comparing results obtained for different groups, did heat and/or oxygen exposure during
processing and storage of the samples analysed seem to affect the vitamin C content? (30%)
4. Why was it necessary to standardize the iodine solution? (20%)
5. Lab-work behavior and participation. (10%) V. References
1. AOAC International (2007) Official methods of analysis, 18th ed. (2005) Current through revision 2,
2007 (On-line). AOAC International, Gaithersburg, MD
2. Nielsen SS (2010) Vitamin C determination by indophenol method, Ch.7.In: Nielsen SS, Food
analysis laboratory manual, 2ndedn. Springer, New York
3. Pegg RG, Landen WO, Eitenmiller RR (2010) Vitamin analysis. Ch. 11. In: Nielsen SS (ed) Food
analysis, 4thedn. Springer, New York
4. Chem Projects Research Team (2019) Assignment directions for a project measuring the amount of
vitamin C in fresh squeezed fruit juice verses frozen concentrate. CheMystery Labs
5. Pham, H. V. (n.d.). Food analysis lab manual (Vol. 2). Ho Chi Minh, International University. Vietnam National Univers
XÁC NHẬN CỦA GIẢNG VIÊN PHỤ TRÁCH
XÁC NHẬN CỦA TRƯỞNG BỘ MÔN Nguyễn Hồng Long 19




