
















Preview text:
lOMoAR cPSD| 58490434 1/18/2016 GENERAL CHEMISTRY Chapter 5 Chemical Equilibrium 1 OUTLINE ✓Basic concepts
✓The equilibrium constant
✓LeChatelier’s Principle
✓Relationship between Kp &Kc
✓Relationship between G0rxn and K
✓Evaluation of K at difference temperature. 2 lOMoAR cPSD| 58490434 1/18/2016 Basic concepts
❖Reversible reactions:
✓Reactions that do not go to completion and that can occur in either direction. ✓Both the forward and reverse reactions occur simultaneously. aA+bB cC+ dD
❖In the balanced equation:
✓“a, b, c, d” represent the stoichiometric coefficients
✓A, B are called the “reactants”
✓C, D are called the “products.” ✓The double arrow (
) indicates that the reaction is reversible 3 Basic concepts
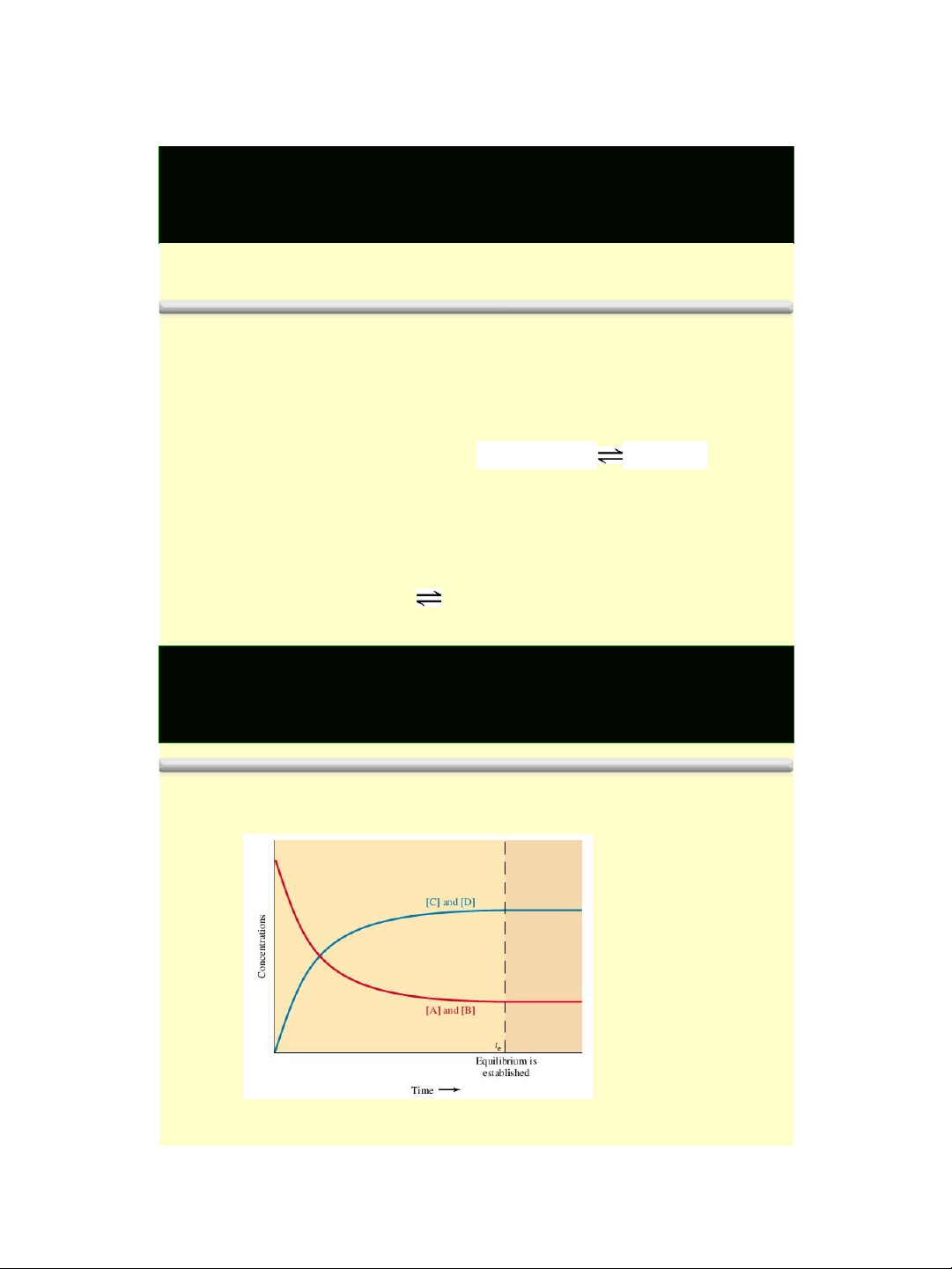
❖Chemical equilibrium exists when two opposing reactions occur
simultaneously at the same rate. 4 lOMoAR cPSD| 58490434 1/18/2016
The equilibrium constant
❖ For a general reaction in the gas phase: aA (g)+bB (g) cC (g)+ dD (g)
✓ The equilibrium constant expression is: Pc Pd Keq Ca PDBb PA
✓Keq is the equilibrium constant.
✓The subscript “eq” to emphasize that partial pressure in
the equilibrium constant expression are those at equilibrium. 5
The equilibrium constant lOMoAR cPSD| 58490434 1/18/2016 ❖ For a general reaction:
K c values always involve equilibrium values of concentrations. 6
The equilibrium constant lOMoAR cPSD| 58490434 1/18/2016
❖ The equilibrium constant Keq has no units. ❖ The value of Kc:
✓ Is constant at a given temperature,
✓ Changes if the temperature changes,
✓ Does not depend on the initial concentrations.
✓ Kc>1: most of the reactants would be converted into products,
we called a reaction “product-favored.”
✓ Kc<1: most of the reactants remain and only small amounts of products are formed. 7
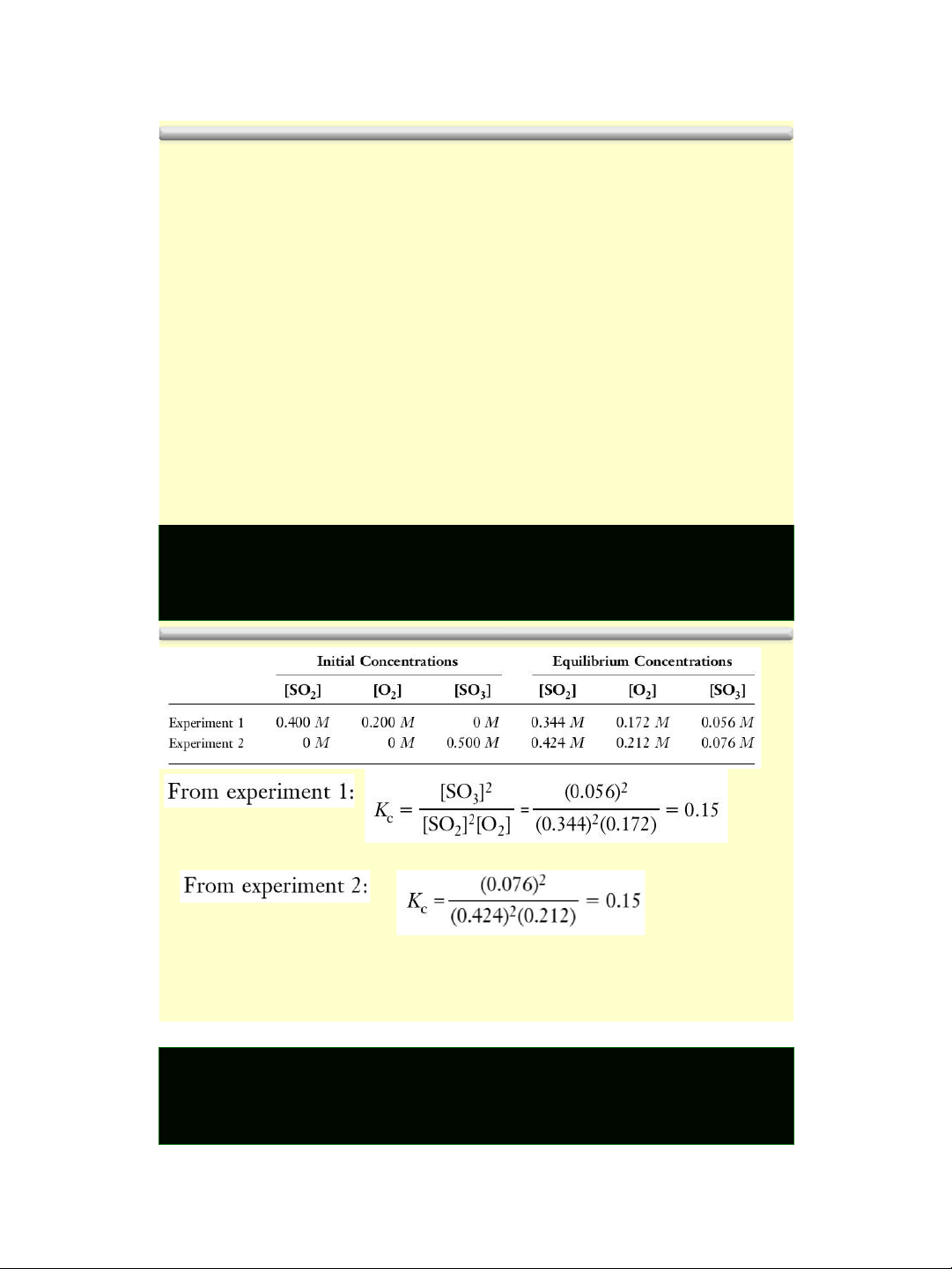
The equilibrium constant Example: 8
The equilibrium constant lOMoAR cPSD| 58490434 1/18/2016 9 Variation of Kc
❖ The value of Kc depends on the form of the balanced equation for the reaction.
❖ If an equation for a reaction is multiplied by any factor, n, then
the original value of Kc is raised to the nth power. 10 Variation of Kc lOMoAR cPSD| 58490434 1/18/2016 11 Calculating Keq
❖ Proceed as follows:
✓ Tabulate the initial and the equilibrium concentrations (or partial pressures) that are given.
✓ If an initial and equilibrium concentration is given for a species,
calculate the change in concentration.
✓ Use stoichiometry on the change in concentration line only to
calculate the changes in concentration of all other species in the equilibrium .
✓ Use initial concentrations and the changes in concentration to
calculate the equilibrium concentration. These are used to evaluate the equilibrium constant. 12 Calculating Keq lOMoAR cPSD| 58490434 1/18/2016
❖ In one of their experiments, Harber and co-workers
introduced a mixture of hydrogen and nitrogen into a
reaction vessel and allowed the system to attain chemical
equilibrium at 472oC. The equilibrium mixture of gases
was analyzed and found to contain 0.1207 M H2, 0.0402
M N2, and 0.00272 M NH3. From these data,
calculate the equilibrium constant, Keq, for N2(g) + 3H2(g) 2NH3(g) 13 Calculating Keq
❖ Gaseous Hydrogen iodide is placed in a closed container
at 425 oC, where it partially decomposes to hydrogen
and iodine: 2HI (g) H2(g) + I2(g). At equilibrium, it
is found that [HI] = 3.35 10-3M; [H2] = 4.79 10-4M; [I2]
= 4.79 10-4M. What is the value of Keq at this temperature.
❖ A mixture of 0.100 mole of NO, 0.050 mole of H2, and
0.050 mole of H2O is placed in a 1.00-L vessel. The
following equilibrium is established:
2NO(g) + 2H2(g) N2(g) + 2H2O(g) Calculate the Keq for the reaction. 14 lOMoAR cPSD| 58490434 1/18/2016 Calculating Keq
❖Enough ammonia is dissolved in 5.00 liters of water at
25oC to produce a solution that is 0.0124 M in ammonia.
The solution is then allowed to come to equilibrium.
Analysis of the equilibrium mixture shows that the
concentration of OH- is 4.64 x 10-4M. Calculate Keq at 25 oC for the reaction. NH + 3(aq) + H2O(l)
NH4 (aq) + OH-(aq) 15 Calculating Keq
❖ A mixture of 5.00 x 10-3 mol of H2 and 1.00 x 10-2 mol of
I2 is placed in a 5.00 L container at 448 oC and allowed to
come to equilibrium. Analysis of the equilibrium mixture
shows that the concentration of HI is 1.87 x 10-3 M. Calculate
the Kc at 448 oC for the reaction. H2(g) + I2(g) 2HI(g) Keq=50.51 16 lOMoAR cPSD| 58490434 1/18/2016 Calculating Keq
❖ Sulfur trioxide decomposes at high temperature in a sealed container: 2SO3(g)
2SO2(g) + O2(g).
Initially the vessel is charged at 1000K with SO3(g) at a
concentration of 6.09 x 10-3 M. At equilibrium, the SO3
concentration is 2.44 x 10-3 M. Calculate the value for Keq at 1000 K. 17
Calculating Equilibrium Concentrations
❖ The same steps used to calculate equilibrium constants are used.
❖ Generally, we do not have a number for the change in concentration line.
❖ Therefore, we need to assume that x mol/L of a species is produced (or used).
❖ The equilibrium concentrations are given as algebraic expressions. 18 lOMoAR cPSD| 58490434 1/18/2016
Calculating Equilibrium Concentrations 19
Calculating Equilibrium Concentrations 20 lOMoAR cPSD| 58490434 1/18/2016 The reaction quotient
❖ The reaction quotient, Q, for the general reaction is given as follows: 21 The reaction quotient
✓ Q < Kc: Forward reaction predominates until equilibrium is established.
✓ Q = Kc: System is at equilibrium.
✓ Q > Kc: Reverse reaction predominates until equilibrium is established. 22 lOMoAR cPSD| 58490434 1/18/2016 The reaction quotient Q < Kc
✓ The system is not at equilibrium the forward reaction must occur to a
greater extent than the reverse reaction;
✓ Some HI must react to form more H2 and I2 to reach equilibrium 23 The reaction quotient
❖ At 448 oC the equilibrium constant, Keq, for the reaction: H2(g) + I2(g)
2HI (g) is 50.5. Predict how the reaction
will proceed to reach equilibrium at 448 oC if we start with
2.0 x 10-2 mol of HI, 1.0 x 10-2 mol of H2, and 3.0 10-2
mol of I2 in a 2.0L container. 24 lOMoAR cPSD| 58490434 1/18/2016
LeChatelier’s Principle
❖LeChatelier’s Principle:
If a change of conditions (stress) is applied to a system at
equilibrium, the system shifts in the direction that reduces the stress
to move toward a new state of equilibrium.
❖Three types of changes can disturb the equilibrium of a reaction: 1.Changes in concentration
2.Changes in pressure or volume (for reactions that involve gases)
3.Changes in temperature.
LeChatelier’s Principle (is pronounced “le-SHOT-lee-ay.”) 25
FACTORS THAT AFFECT EQUILIBRIA
❖ Changes in Concentration: 26 lOMoAR cPSD| 58490434 1/18/2016
FACTORS THAT AFFECT EQUILIBRIA
❖ Changes in Concentration:
✓ Adding a reactant or product shifts the equilibrium away from the increase.
✓ Removing a reactant or product shifts the equilibrium towards the decrease.
✓ To optimize the amount of product at equilibrium, we need
to flood the reaction vessel with reactant and continuously
remove product (LeChatelier’s Principle). 27
FACTORS THAT AFFECT EQUILIBRIA 28 lOMoAR cPSD| 58490434 1/18/2016
FACTORS THAT AFFECT EQUILIBRIA
❖ Changes in Volume and Pressure: 29
FACTORS THAT AFFECT EQUILIBRIA
❖ Changes in Volume and Pressure:
✓If there is no change in the total number of moles of gases, a
volume (pressure) change does not affect the position of equilibrium.
✓If changing in the total number of moles of gases:
▪ A decrease in volume (increase in pressure) shifts a
reaction in the direction that produces the smaller total number of moles of gas.
▪ An increase in volume (decrease in pressure) shifts a
reaction in the direction that produces the larger total number of moles of gas. 30 lOMoAR cPSD| 58490434 1/18/2016
FACTORS THAT AFFECT EQUILIBRIA
❖ Changes in Temperature:
✓ Adding heat (i.e. heating the vessel) favors away from the increase:
▪ if H > 0, adding heat favors the forward reaction.
▪ if H < 0, adding heat favors the reverse reaction.
✓ Removing heat (i.e. cooling the vessel), favors towards the decrease:
▪ if H > 0, cooling favors the reverse reaction.
▪ if H < 0, cooling favors the forward reaction. 31
FACTORS THAT AFFECT EQUILIBRIA
❖ Addition of a Catalyst:
✓ A catalyst lowers the activation energy barrier for the reaction.
✓ Therefore, a catalyst will decrease the time taken to reach equilibrium.
✓ A catalyst does not effect the composition of the equilibrium mixture. 32 lOMoAR cPSD| 58490434 1/18/2016
RELATIONSHIP BETWEEN Kp & Kc
In general, the relationship between Kc and KP is:
Be Careful About the Value of R L.atm R 0.082 mol.K 33
HETEROGENEOUS EQUILIBRIA 34 lOMoAR cPSD| 58490434 1/18/2016
HETEROGENEOUS EQUILIBRIA
✓Heterogeneous equilibria involve species in more than one phase. ✓Example:
✓Pure liquids and pure solids do not appear in the K expressions for heterogeneous equilibria. 35
RELATIONSHIP BETWEEN G0rxn & K 36 lOMoAR cPSD| 58490434 1/18/2016
RELATIONSHIP BETWEEN G0rxn & K 37
RELATIONSHIP BETWEEN G0rxn & K 38



