
Preview text:
REPORT
EXPERIMENT 5: FACTORS AFFECTING REACTION RATE Semester: 2 Year: 2023 - 2024 Group: 4 Date: Group members: Seq. Full name Student ID % contribution Signature Score (total = 100%) 1 Lê Nhật Dũng IELSIU24023 2
Nguyễn Thị Sông Thu IELSIU24164 3 Huỳnh Kim Tin IELSIU24174 4
Phạm Thị Thuỳ Trang IELSIU24182 Total score: ________/100 I. Introduction (10 pts) II. Experimental (5 pts) III. Results and discussion
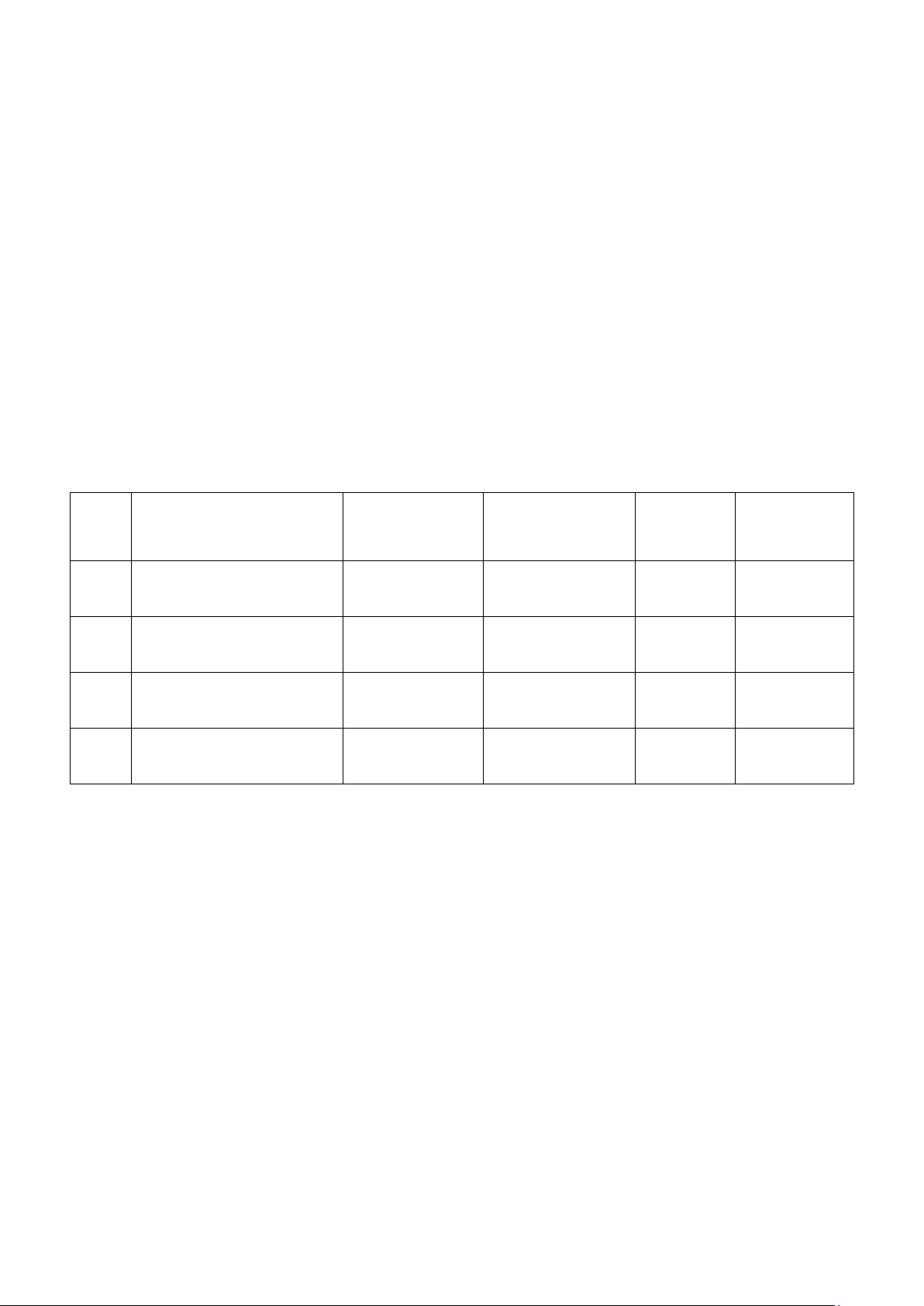
1. EFFECT OF CONCENTRATION ON REACTION TIME (40 pts) Reaction 1: Reaction 2:
__________________________________________________
Calculate the initial concentrations of I- and S 2- 2O8 ions: Mixture # 5: [I-] = [S2O82-] = Mixture Iodide ion (M) Peroxydisulfate (M) Time in seconds 1 0,069 0,034 52,13 2 0,059 0,034 62,34 3 0,048 0,034 76 4 0,038 0,034 84 5 0,028 0,034 96 6 0,017 0,034 107 Mixture Iodide ion Peroxydisulfate Time in seconds 7 0,069 0,029 43,41 8 0,069 0,024 62,60 9 0,069 0,019 76 10 0,069 0,014 94 11 0,069 0,009 145
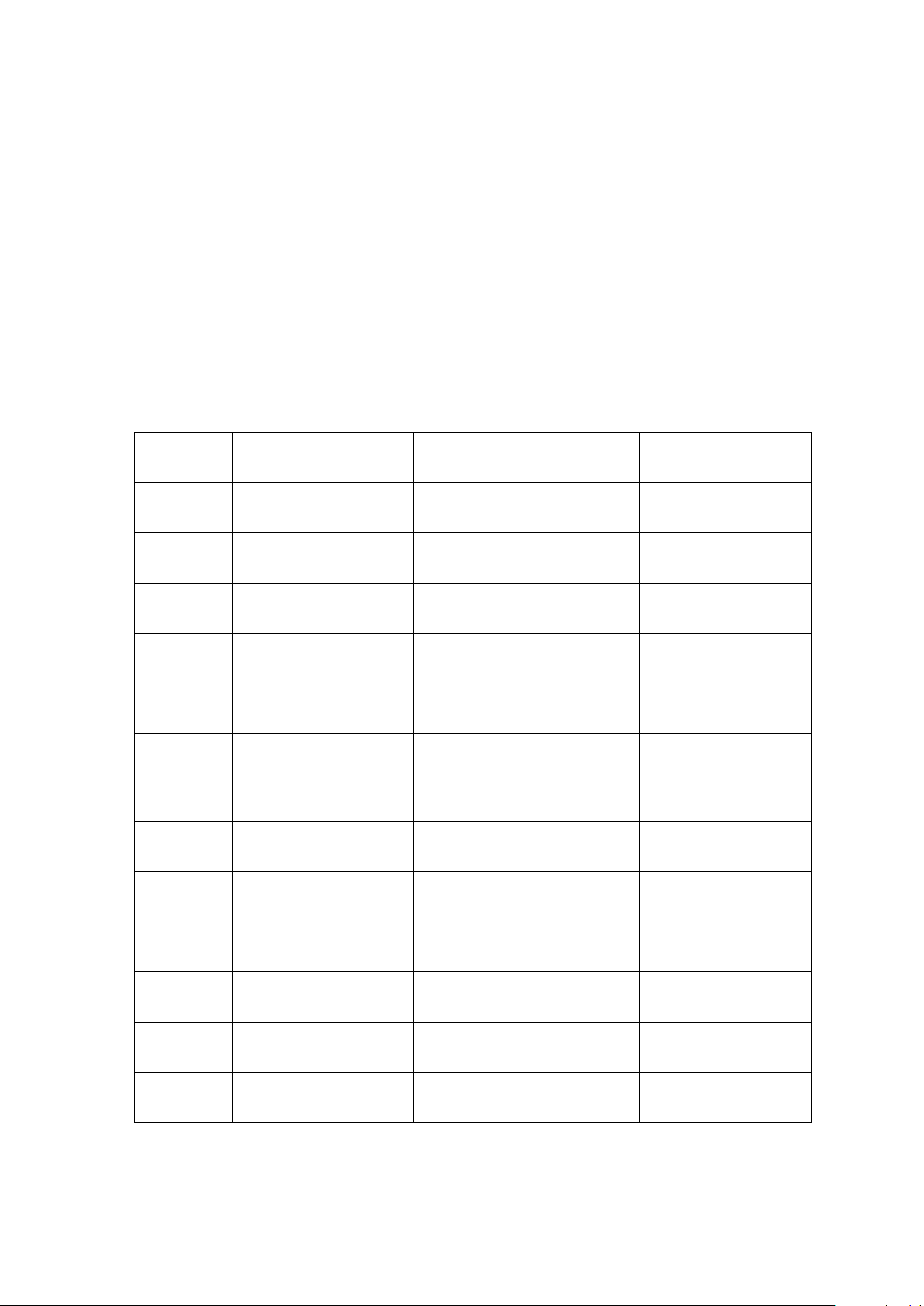
Plotting the concentration of iodide ion versus time: [Note: X – axis: time; Y – axis: concentrations]. - Mixtures # 1-6:
Graph (The correct graph should have adequate information regarding the data: the
name or title of the graph, title of the axes and the unit, the concentration of each data point.)
Describe the order of the reaction with respect to iodide ion? (How does different
concentration of the iodide ion affect the rate of the reaction? Explain.)
Comments: (How does the effect of concentration play a role in this experiment?)
- Mixtures # 1, 7, 8, 9, 10, and 11:
Graph (The correct graph should have adequate information regarding the data: the
name or title of the graph, title of the axes and the unit, the concentration of each data point.)
The order of reaction with respect to peroxydisulfate ion? (How does different
concentration of the iodide ion affect the rate of the reaction? Explain.)
Comments: (How does the effect of concentration play a role in this experiment?)
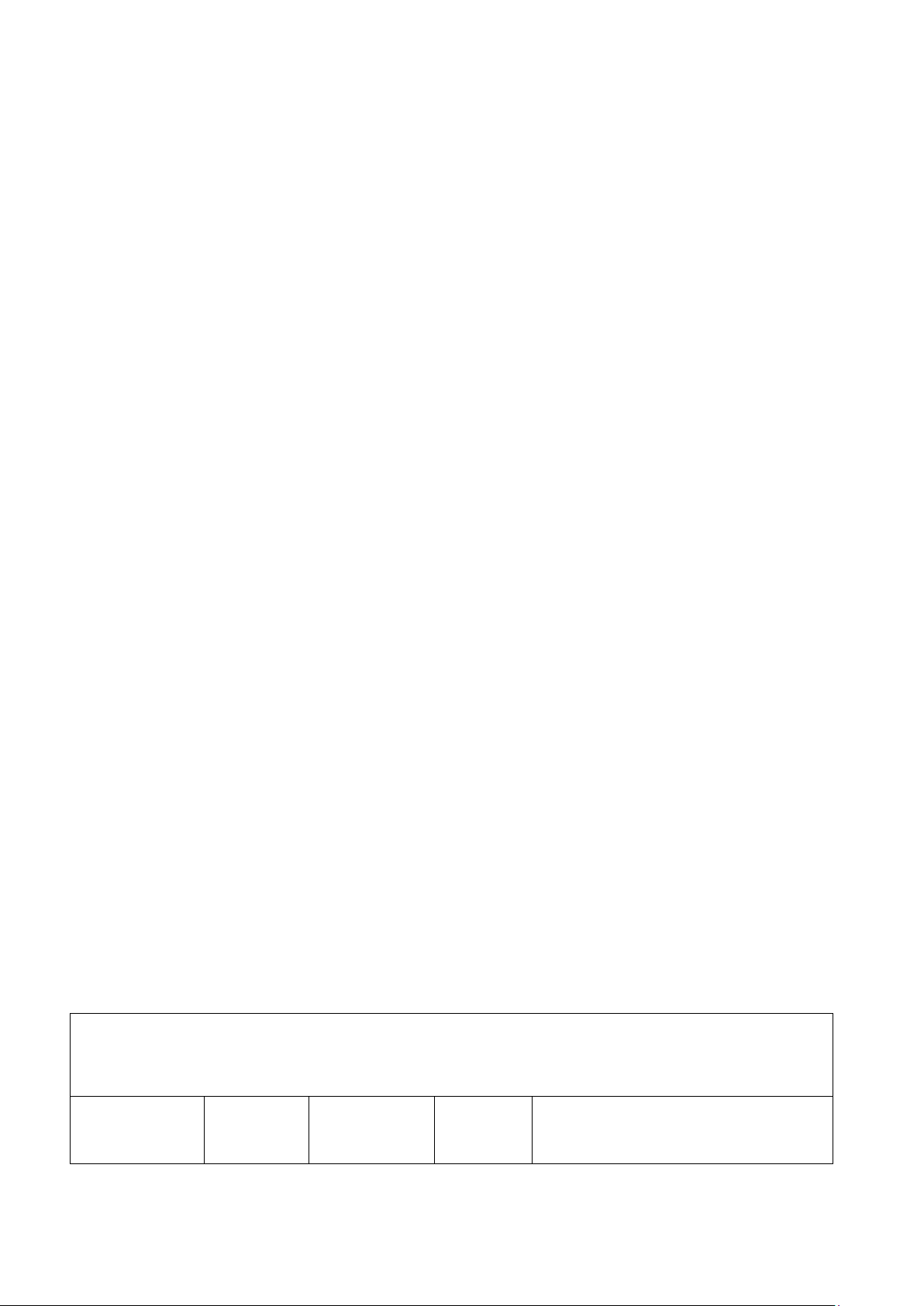
2. EFFECT OF TEMPERATURE ON THE REACTION RATE (20 pts) Reaction System: Description of Predicted Observation Reaction Explanation conditions outcome time Room colorless The solution 65,53 temperature 50 °C colorless 4,31 90 °C colorless 1,25
Comment: (What is the color of the initial reactants? What is the color of the end of the reaction?
How does the effect of temperature play a role in this experiment?)
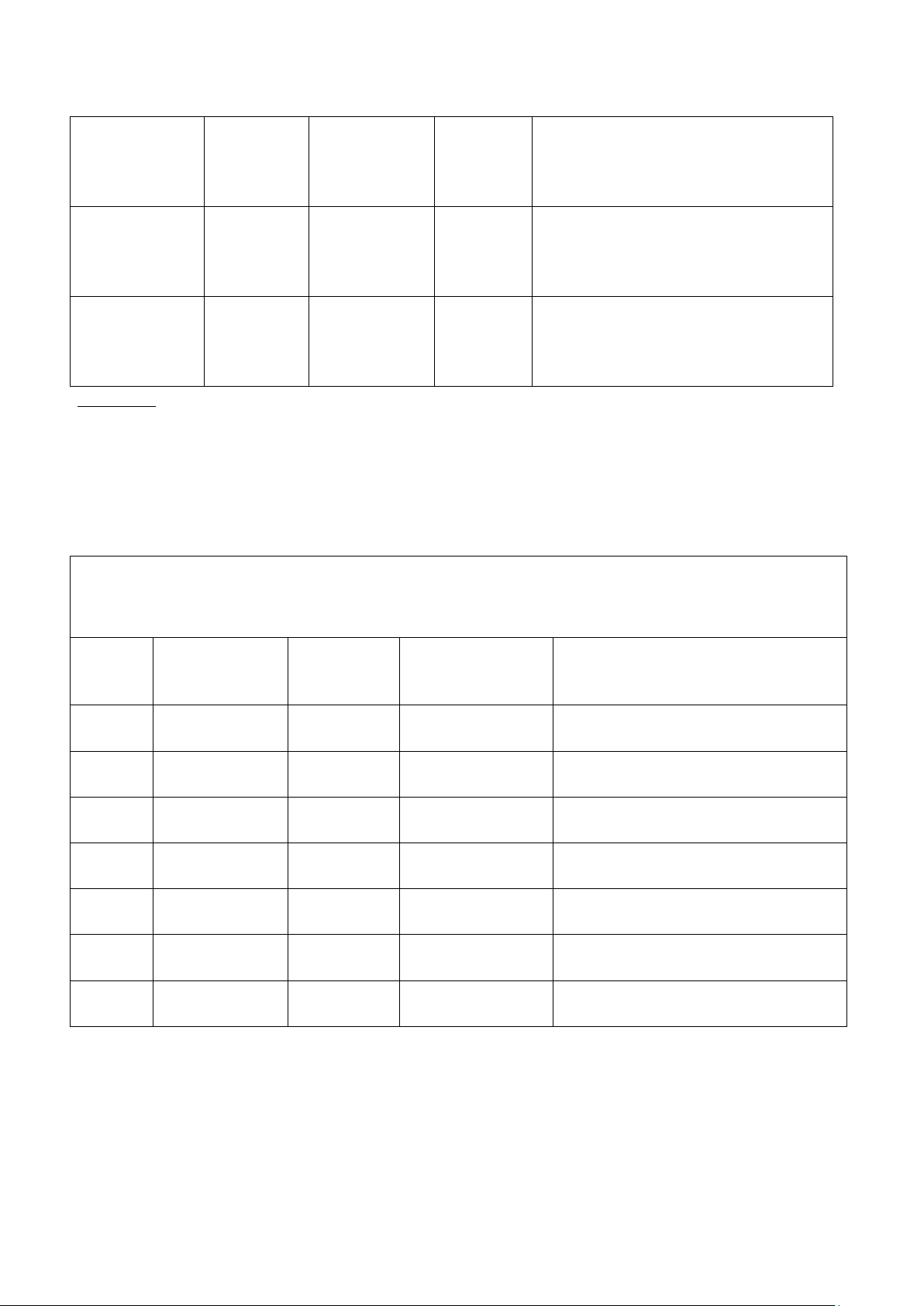
3. EFFECT OF A CATALYST ON THE REACTION RATE (15 pts) Reaction System: Trial Description of Predicted Observation Explanation conditions outcome (Reaction rate) 1 + MnCl2 Slow 25,3 2 + MnO2 Very fast 1,65 3 + NaCl Slow 54,65 4 + CaCl2 Very fast 6,24 5 + Zn Very slow 59,61 6 + KNO3 7 + Fe(NO3)3
The order of the calatylic activity:
Comment: (Which is the best and the worst candidate for the catalytic decomposition of hydrogen
peroxide? What is the mechanism of action of the above catalysts in increasing the decomposition rate
of hydrogren peroxide? How can catalysts be employed to benefit human consumption?) IV. Conclusions (10 pts)
(Conclude all your performance in this report.)



