

Preview text:
lOMoAR cPSD| 49981208
Unit 1 Review – Show work, units & significant figures.
1. For the following forms of matter:
• Indicate the form of matter (element, compound, homogeneous mixture or heterogeneous mixture)
• Explain how you identified it as such
• If a mixture, identify the separation technique you would use to separate into components and the
property(s) that technique utilizes
a. water – compound, the formula for water is H2O. 2 or more elements written together indicate a compound
b. air – a homogeneous mixture, air is composed of molecules of nitrogen gas and oxygen gas not
chemically combined with each other, they can be separated into the 2 gases using condensation
which uses the property of boiling points.
c. soil – a heterogeneous mixture, soil is composed of dirt, sand, small rocks and plant materials. You
can visually see the components. It can be separated into its components using filtration (a sieve)
which utilizes particle size.
d. iron (Fe) – an element, iron’s symbol and name are found on the Periodic Table
e. rust (Fe2O3) – a compound, 2 or more elements written together indicate a compound
f. Salt Water – a homogeneous mixture, composed of sodium chloride and water. It can be separated
into its components utilizing crystallization which relies on the solubility of the solute in the
solvent and the solvent’s boiling point.
g. Vinegar – a homogeneous mixture, composed of acetic acid and water, both are liquids and
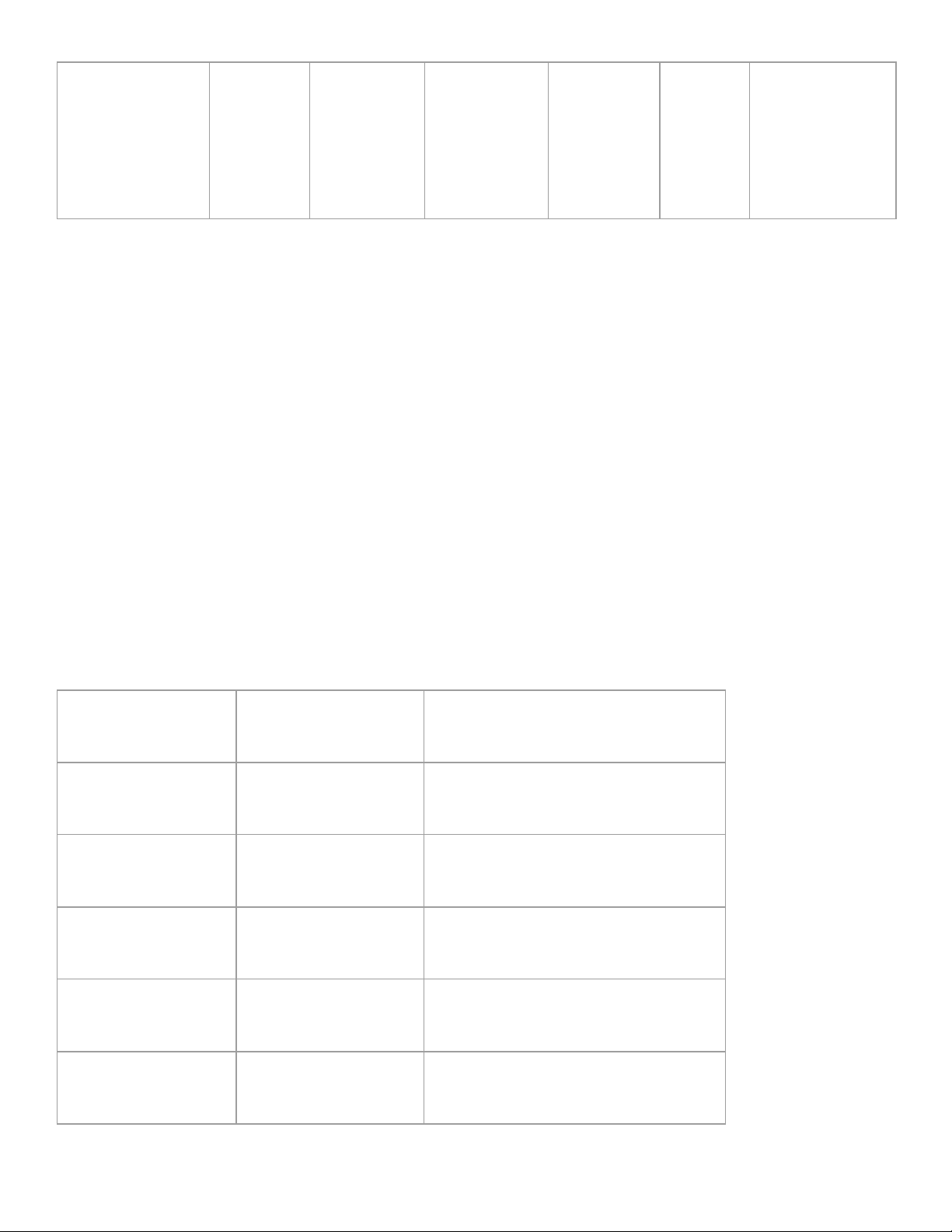
distillation can be used to separate it into its components. Distillation uses boiling points for separation. 2. Complete the table: Filtration Decanting Distillation Crystallization Chromato- Centri- graphy fugation Density Density Property(s) used Particle Attractive Solubility & Boiling Points for separation size Forces Boiling Point lOMoAR cPSD| 49981208 Sand & Gravel from Rubbing Ink Blood Sugar Water Identify a Water Ocean water Alcohol & specific mixture Water that can be separated with this technique
3. Describe the separation techniques required to purify a sample of gasoline contaminated with water, sugar and pebbles.
Water & Gasoline are insoluble. The pebbles will sink to the bottom. Sugar will dissolve in the water.
Decant the water from the gasoline. Filter the pebbles from the gasoline. Crystallize the sugar out of the water.
4. Define the following terms:
a. Physical Property – a characteristic that can be observed or measured without changing the identity of the substance.
b. Intensive Physical Property – a physical property that is independent of the amount of substance present.
c. Extensive Physical Property – a physical property that is determined by the amount of substance present.
d. Chemical Property – a characteristic that describes how a substance reacts
e. Chemical Change – atoms are rearranged to form new substances with new properties, can be identified by
observing a change in energy, the production of a gas and/or the formation of a preceipitate
5. Complete the table regarding physical & chemical properties: Property
Physical or Chemical If Physical - Intensive or Extensive? Density Physical Intensive Reacts with Acid Chemical Volume Physical Extensive Boiling Point Physical Intensive Phase of Matter Physical Intensive lOMoAR cPSD| 49981208 Reacts with Oxygen Chemical Combustible Chemical Soluble in Water Physical Extensive
6. Complete the table regarding physical & chemical changes: Physical Or
If Chemical - Identify the evidence of Change Chemical? the change Hydrogen exploding Chemical Energy released Physical Gasoline Evaporating Sugar re-crystallizing Physical Chemical A precipitate forms
A yellow solid forms when mixing 2 solutions Physical Water changing to ice
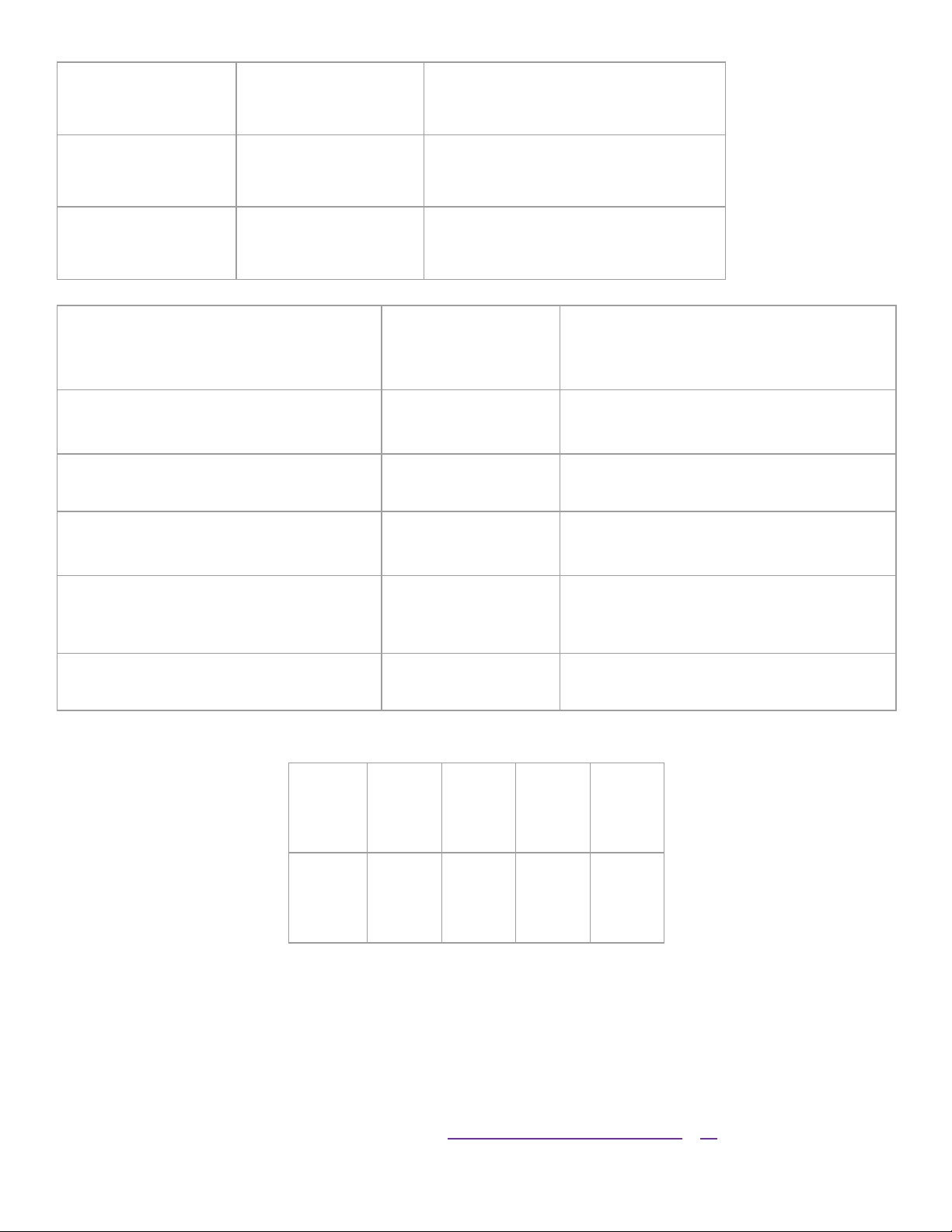
7. A student collected the following data: Accepted value = 5.32 g
Trial 1 Trial 2 Trial 3 Trial 4 Trial 5 5.25 g 5.18 g 5.21 g 5.26 g 5.29 g For the following questions you must: • Show your work •
Use correct significant figures • Use correct unit
a. Does this student have precision? Explain.
No. The degree of uncertainty is in the 0.01 place. The difference between the greatest value and the least
value is 0.11 which is greater than 0.09.
b. Using the average, calculate the percent error. (5.25 + 5.18 + 5.21 + 5.26 + 5.29) = 26 = 5.20 g 5 5 lOMoAR cPSD| 49981208
|5.32 g – 5.20 g| x 100 = 12.0% 5.32 g
c. Does this student have accuracy? Explain.
No. The percent error is greater than 5% therefore the data is not accurate.




