












Preview text:
lOMoARcPSD|47206417 Microbiology Lab report
Submitted to: Ms. Xuân By group 1 Tuesday :
Nguyễn Phương Tuyền BTBTIU12124 Lê Hồ Thi BTBCIU14044 Thái Đức Thi BTBTIU14220
Nguyễn Ngọc Anh Khoa BTBTIU14100 LABORATORY 1
1/ 10 main rules when working in microbiology lab?
- Only lab manual, lab notebooks be brought to your laboratory work space- Lab -coats,
gloves,comforters, lab-hats, or safety- glasses must be worn at all time
- Disinfecting your work area at the beginning and before leaving the lab-room-
Unauthorizedexperiments are not allowed
-Be very careful with hot plates, Bunsen burners, stirring motors, high-voltage power supplier
- All contaminated material must be disinfected before disposal reuse
- In the event of any accident or injury, report immediately to the laboratory instructors
- Working alone in the laboratory, eating, drinking, or smoking is strictly prohibited
- After the lab-session, observe good hygiene by washing your hands before leaving thelaboratory
- Never “horse around” or play practical jokes in laboratory
2/ Tell different kinds of medium referring to medium’s liquid status and shapes?
-Media can be classified into three kinds of media depending on their liquid status (physical
state):liquid (broth) media, semisolid media and solid media.
Liquid media, such as nutrients broth, tryptic soy broth, or brain-heat infusion broth, can be used
to propagate large numbers of microorganism in fermentation studies and for various
biochemical test. This is the most common type of medium.
•Semisolid media are also used in fermentation studies, motility of bacteria and anaerobic growth.
It usually contains less than 1% of agar.
•Solid media are commonly used for observation of microorganism’s appearance, isolation of
pure culture, cultures storage and biochemical reactions observation. It is made by letting agar
solution cool down to become solid form.
-Media can be poured into test tube or in Petri dish. The shape of the solid medium varies
depending on how we pour the medium.
•When agar in the test tube is allowed to harden at angle, a slanted position, the tube is called an agar slant
•If the agar is allowed to harden at an upright position, the tube is called agar deep tube.
•If the agar is poured into a Petri plate, the plate is called an agar plate. 2 | P a g e lOMoARcPSD|47206417
3/ Why do we have to incubate medium before storing in a fridge?
-We have incubators before storing in a fridge that are used to maintain optimal temperature at
37C because E.coli grow best at 37C and to make sure there is no contamination in our cultures
and all moisture is dried out completely.
Lysogeny broth (LB), a nutritionally rich medium, is primarily used for the growth of bacteria. The
initialism is also commonly, albeit incorrectly, taken to mean Luria broth, Lennox broth, or Luria-
Bertani medium. According to its creator Giuseppe Bertani, the abbreviation LB was actually
intended to stand for lysogeny broth
There are several common formulations of LB. Although they are different, they generally share
a somewhat similar composition of ingredients used to promote growth, including the following: • Peptides and casein peptones •
Vitamins (including B vitamins) •
Trace elements (e.g. nitrogen, sulfur, magnesium) • Minerals
Peptides and peptones are provided by tryptone. Vitamins and certain trace elements are
provided by yeast extract. Sodium ions for transport and osmotic balance are provided by sodium
chloride. Tryptone is used to provide essential amino acids to the growing bacteria, while the
yeast extract is used to provide a plethora of organic compounds helpful for bacterial growth.
5/ Why is 70% Ethanol used as disinfectant?
This has to do with the process by which it kills microorganisms. It works by denaturing their
proteins and dissolving their lipids. The water in the ethanol solution is the portion that actually
does the denaturing. Using higher concentration makes the ethanol less effective because there
cells cannot be denatured by the water. Lower concentrations do not allow the ethanol to be as
effective because it cannot break down the all lipids or allow the water to get into the cells.
The other reasons are more on the use side of things. Firstly higher concentrations of ethanol
evaporate very quickly. Concentrations like 95% or 90% may evaporate before than can come in
contact with most of the microbial life. Also, water is pretty cheap and while ethanol is not
worth its weight in gold, there is a cost attached to it. It is pointless to have extra ethanol in
your solution that is not doing anything. LABORATORY 2
1/ What is the purpose of flaming in the aseptic technique?
-To sterilize or re-sterilize an item that may have come in contact with contaminants in the air
(i.e: the mouth of a culture tube) or the culture ( like the inoculating loop/ needle ).
2/ In all routine laboratory work, petri plates are labeled on the bottom. Why ?
- Petri plates are labeled on bottom that there is no confusion as to the sample number
andprevent the culture to get mixed up.
- To avoid condensation, so by labeling on the bottom it can be seen easily.
3/ In the streak-plate technique, how are microorganisms diluted and spread out to form individual colonies?
- Bacterial mixture is transferred to the edge of an agar plate with an inoculating loop.- Spread
the loop over the agar surface the cell fall off the loop. Many at first, decreasing number with
spreading. Eventually single cells fall off. The important thing is to produce good spacing between colonies.
- When spreading the microorganisms, we must be careful not to cut the agar or put the loop
deep inside the agar. Gently spread the microorganism on the surface of the agar.
4/ Which area of a streak plate will contain the greatest amount of growth? The least amount of
growth? Explain your answers.
The first streaking area will have maximum growth but the colonies will be crowded together because it
has the highest content of mixture. The last quadrant streaked should have the least number of
colonies, but they should be well separated and easily sampled, since it has least amount of mixture.
5/ Draw your streak patterns. Did you obtain isolated colonies? If not, what event wrong?
Take the picture from the Lab Manual: Quadrant Streak.
In our group: some obtain isolated colonies and there is no contamination in the streak, but
some get the contamination. Reasons for this contamination may be:
- If the lid is removed too far from the agar, air or body contaminants may seed the agar.
- If the loop is not properly sterilized, it may be a source of contamination. 6/ •
Does each discrete colony represent the growth of one cell?
No. A colony represents the growth of descendants of single parent cell. •
Why can a single colony on a plate be used to start a pure culture?
To identify the organism easier, can be use as a control into comparing other bacteria and to reduce contamination. 4 | P a g e lOMoARcPSD|47206417
7/ How can a streak plate become contaminated?
We can accidently contaminate the streak plate by forgetting sterilize the loop while streaking
and leaving the lid off to let the pathogens outside move in. • Not burning the loop • Breathe on sample • Leave lid off • Not burn other utensils
8/ What is the purpose of sub-culturing?
Cell lines and microorganisms cannot be held in culture indefinitely due to the gradual
rise in toxic metabolites, use of nutrients and increase in cell number due to growth.
Subculture is therefore used to produce a new culture with a lower density of cells than the
originating culture, fresh nutrients and no toxic metabolites allowing continued growth of the
cells without risk of cell death.
Subculture is important for both proliferating (e.g. a microorganism like E. coli) and
nonproliferating (e.g. terminally differentiated white blood cells) cells. It is needed to provide
nutrients to the medium the culture which are deprived after some time. The sub-culturing
provides proper nutrients to the microbes so that it can grow easily and form colonies which
then can be used to obtain the desired products.
9/ In sub-culturing, when do you use the inoculating loop?
Using inoculating loop when culture in liquid medium and help to pick up enough media to make a good smear.
An inoculation loop is a thin metal device with a handle at one end and a looped wire at
the other end. The looped end is useful for obtaining bacterial samples from colonies growing
on media plates or from liquid media, as the loop can hold a drop of liquid, somewhat like a
bubble wand holds liquid soap.
The advantage of an inoculation loop is that the instrument can be used and sterilized
repeatedly, reducing the amount of contaminated lab waste generated. It is possible to injure
and infect an eye with an inoculating needle. Loops are safer. A needle is used for stabbing the bacteria into agar deep.
10/ How is it possible to contaminate a subculture?
Doing aseptic techniques incorrectly cause contamination. By not practicing aseptic
technique (i.e. not flaming the mouth of the tube or inoculation tools).
- leaving the lid off too long, leaving the lid off test tube
- unsterilized instruments, double dipping
- breathing directly on specimen, operator contamination
11/ How would you determine whether culture media given to you by the laboratory
instructor are sterile before you use them?
It is hard to know instructor’s culture media are sterile. However, we can check visually
by looking for any strange in medium. In addition, we can use microscope or inoculate in the
incubator and see if there is any bacteria growth. LABORATORY 3
1/ Why is the low-power objective placed in position when the microscope is stored or carried?
Because high-power objective lens are bigger and can be broken or damaged easily
while low-power objective is smaller and easier to carry and store.
2/ Why is oil necessary when using the 90x to 100x objective?
The most powerful lens of the light microscope is the 100x oil immersion objective.
Because light is refracted every time it passes through a medium with a different refractive
index, (air to glass or vice versa) the quality of the image is reduced with each passage. Thus, by
reducing the number of such passages to a minimum, the clarity, brilliance and resolving power is preserved.
3/ What is the function of the iris diaphragm? The substage condenser?
The iris diaphragm controls the amount of light passing through the slide or specimen,
while the substage condenser focuses a cone of light on the slice or specimen.
4/ In microbiology, what is the most commonly used objective?
Using commonly 100x objective because microorganisms have super small size and observing by using 100x is more useful.
5/ In microbiology, what is the most commonly used ocular? explain your answer.
The most commonly used ocular for microbial observation is 10x, if you got a higher ocular you
would just have a higher magnification but not be able to see detail. this is the kind
microscopes use most often because it results in the best image. that is the initial ocular you
always start out with and always finish with.
6/ Why are unstained bacteria more difficult to observe than stained bacteria? Some reasons:
• Bacteria, like most life, are largely composed of water. Water is clear. 6 | P a g e lOMoARcPSD|47206417
• Bacteria are very small; their size, generally, is only barely within the resolution range of typical bright-field scopes
• Bacteria are very small, and so reflect very little light. A transmission scope depends on reflection to form images.
• Also, some bacteria can move around fast making it hard to see them under the microscope.
Staining adds contrast and makes them easier to see.
7/ Describe the following type of bacteria movement: Brownian movement Flagella motion Gliding motion
a. Brownian movement—bombardment by water molecules leading to erratic movement.
b. Flagellar motion—a linear or circular motion consistently directed toward a specific site.
c. gliding motion—movement over a solid surface of an individual cell (pseudopodal) or a colony (swirling motion).
8/ Which of the bacteria exhibited true motility on the slides?
All of these bacteria are motile, but each will show a distinct pattern of flagellar activity
9/ How does true motility differ from Brownian movement?
Motile bacteria are capable of moving freely. Brownian motion is simply non-motile bacteria
that look like they are moving due to the collisions with water molecules.
Brownian movement is not a reponse of the microorganism to stimuli, ưhile true motility is.
Brownian movement is also due to the random movement of molecules based on molecular weight and thermal activity.
10/ What are the two purposes of heat fixation?
1. To attach the organisms on the slide
2. It is a way to kill bacteria without destroying it
11/ How would you define a properly prepare bacteria smear?
A dried preparation of bacterial cells on a glass side -just right staining
-don't want to thick of a smear
It should be thin enough so that the microorganisms are clearly separated but thick enough so
that several hundred bacteria in a typical field of view.
12/ Why should you use an inoculating needle when making smears from solid media? An
inoculating loop from liquid media.
The inoculating needle picks up less culture than the loop, and with solid cultures this avoids
putting too much on the slide. With liquid media (which usually contains a lower density of
bacteria than solid cultures), one or two loopfuls is required to obtain sufficient numbers of
bacteria on the slide. It generally requires greater than 105 cells per loopful to obtain easily
observed stained cells in one field of view under 1000x magnification.
13/ Name the reagent used and state the purpose of each of the following in the Gram stain:
a. Mordant: attaches the crystal violet tightly to the wall of gram- positive cells.
b. Primary stain: initially stains the cell wall of both gram- positive and gram- negative bacteria
c. Decolorizer: removes the crystal violet from gram- negative cell walls by using ethanol.
d. Counterstain: stains cells that at this point are colorless ( gram- negative cells)
14/ Which step is the most crucial or most likely to cause poor results in the Gram stain? Why?
The decolorization step- too much, and all cells appear to be gram negative; too little, and all
cells appear to be gram positive. By using the decolorizer species will differ from one another in
regard to which ease of crystal violet, iodine complex is on the move.
15/ What part of the bacterial cell is the most involved with Gram staining, and why? The
cell wall is most involved because this is the site where gram-positive xells retain crystal
violet and where gram- negative cells are decolorized by a mixture of isopropyl alcohol and acetone.
16/ Why must young cultures be used when doing a Gram stain? To get more accurate results
Old cells tend to stain gram negative due to cell wall deterioration regardless of their origin.
17/ What is meant by gram variable?
Some cells in the same cultures will be gram negative and some will be gram positive
18/ Label the compound microscope below. 8 | P a g e lOMoARcPSD|47206417 LABORATORY 4
1/ Why do we need to do preparation and care of stock culture?
Although the aseptic culture has been carried out, the more frequently we use the cultures, the
higher chance they are contaminated. Moreover, the cultures are aging also have bad effect on
inoculations. During a long period of inoculation (2-3 weeks) and at room temperature, loss of
organisms is unavoidable. Thus, to ensure against the hazard of contamination or death of
organisms, we need to prepare and care for stock cultures.
2/ What culture stock tube do we use to identify the unknown bacteria?
Working stock tube will be used to identify our unknown bacteria, since we need to make slides and routine inoculations.
Reservoir stock tube is only stored in refrigerator after incubation until sometime later. In case
that the working stock becomes too old or damaged, we will replace it with fresh culture that is
made from the reservoir stock.
3/ Why cannot strict anaerobes survive when oxygen is present?
Strict anaerobes cannot survive with oxygen presence because they have oxygen labile Fe-S
centers and do not have mechanism to protect them from oxidizing power of oxygen.
Organisms that cannot deal with the problems presented by oxygen cannot survive in air and
are killed. In fact, oxygen has a tendency to form very reactive by-products (H2O2 and O2-
superoxide) inside a cell. These byproducts create havoc by reacting with protein and DNA, and thus inactivating them.
4/ What are reactive oxygen species (ROS) and what are their effects to cells and cellular
activities? Give an example for radical ROS and an example for non-radical ROS. Reactive
oxygen species (ROS) were initially recognized as toxic by-products of aerobic metabolism,
removed by means of antioxidants and antioxidative enzymes. In recent years, it has become
apparent that ROS play an important signaling role in plants controlling processes such as
growth, development, response to biotic and abiotic environmental stimuli, and programmed cell death.
For example, addition of superoxide or hydrogen peroxide to a variety of cultured cells leads to
an increased rate of DNA replication and cell proliferation - in other words, these radicals function as mitogens.
Despite their beneficial activities, reactive oxygen species clearly can be toxic to cells. By
definition, radicals possess an unpaired electron, which makes them highly reactive and thereby
able to damage all macromolecules, including lipids, proteins and nucleic acids. Free Radicals:
Any species capable of independent existence that contains one or more unpaired
electrons . A molecule with an unpaired electron in an outer valence shell. R3C. Carbon-centered R3N. Nitrogen-centered R-O. Oxygen-centered R-S. Sulfur-centered Non-Radicals:
Species that have strong oxidizing potential.
Species that favor the formation of strong oxidants (e.g., transition metals). Examples: H2O2 Hydrogen peroxide HOCl- Hypochlorous acid O3 Ozone 1O2 Singlet oxygen ONOO- Peroxynitrite 10 | P a g e lOMoARcPSD|47206417 Men+ Transition metals
5/ Name 2 strategies that living organisms can use to scavenge excessive ROS out of cells.
Scavenging or detoxification of excess ROS is achieved by an efficient antioxidative system
comprising of the nonenzymic as well as enzymic antioxidants .
The enzymic antioxidants include superoxide dismutase (SOD), catalase (CAT), guaiacol
peroxidase (GPX), and glutathione reductase (GR)…
Superoxide dismutases (SOD) are enzymes that catalyze the conversion of two superoxides into hydrogen peroxide and oxygen.
Three non-enzymatic antioxidants of particular importance are:
Vitamin E is the major lipid-soluble antioxidant, and plays a vital role in protecting membranes
from oxidative damage. Its primary activity is to trap peroxy radicals in cellular membranes.
Vitamin C or ascorbic acid is a water-soluble antioxidant that can reduce radicals from a variety
of sources. Interestingly, vitamin C also functions as a pro-oxidant under certain circumstances.
Glutathione may well be the most important intracellular defense against damage by reactive oxygen species.
Various workers have reported increased activities of many enzymes of the antioxidant defense
system in plants to combat oxidative stress induced by various environmental stresses.
6/ Give description about your studied micro-organisms (i.e.: based on oxygen and
temperature requirement).
Our group study about temperature conditions.
The rate at which chemical reactions take place in a cell is determined by the enzyme activity.
That temperature at which a cell’s enzyme function optimally is referred to as optimal growth
temperature. As the temperature of the cell is decreased from its optimum, the rate of the
enzymatic activity will slow. Increased temperature can result in the irreversible denaturing of
the enzyme and therefore the cessation of all activity.
The minimum growth temperature is the lowest temperature at which the species will grow;
the maximum growth temperature is the highest temperature at which it can grow. The
optimal temperature is the temperature at which it grows best.
Bacteria are divided into three different major groups based on the temperature at which they grow optimally.
Psychrophiles are organisms which can grow at the temperature range between -5oC and 20oC.
The optimum temperature is around 15oC.
Mesophiles are those organisms with optimum growth temperature between 25oC and 40oC
and many of them grow optimally at 37oC.
Thermophiles are group of organisms that can grow at the temperature range between 45oC
and 65oC although some are able to grow in temperature greater than 90oC. These organisms
are often found in hot springs, compost piles...
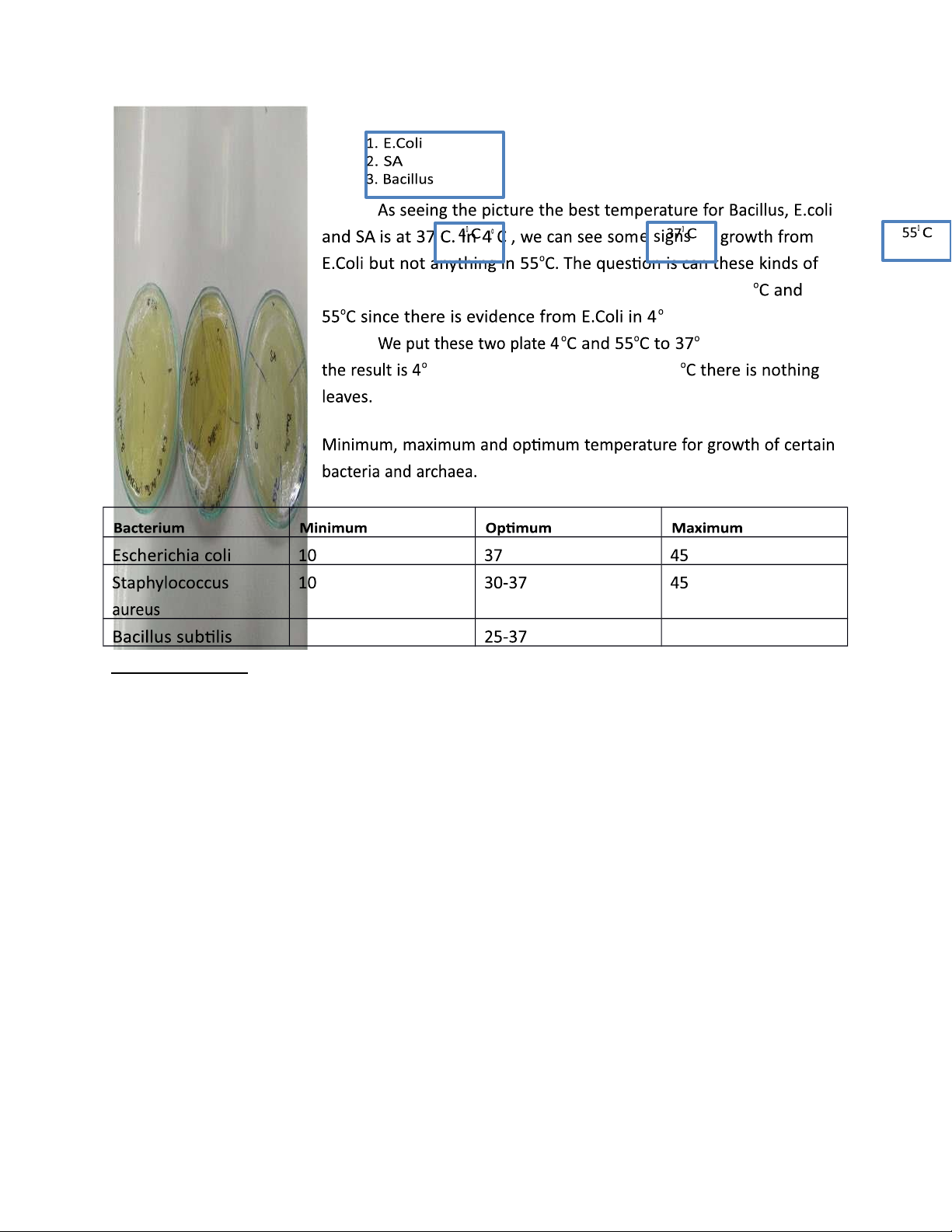
7/ Include a picture of your results. Discuss the effects of temperatures/oxygen to the growth
of the studied micro-organisms. Compare your results with the theory and make a discussion.
The theory table is just estimate approximately the temperature range (especially:
minimum and maximum) of 3 micro-organisms. We can say that 3 types can be listed to
mesophiles. And they can tolerate the extreme temperature at 4oC but die in 55oC. 12 | P a g e lOMoARcPSD|47206417
At 4oC the enzymatic rate is slow so transport processes so slow that growth can not
occur. When putting them back to their optimum temperature they grow fast and we can easily observe them on plate. e signs of
micro-organisms could still survive or completely die at 4 C plate? C in 24hours and
C plate, all 3 types grow but in 55 LABORATORY 5
1/ Why is it necessary to perform a plate count in conjunction with the turbidimetry procedure?
Although the two methods are somewhat parallel in the results they yield, there are distinct differences.
For one thing, the SPC reveals information only as related to viable organisms; that is, colonies
that are seen on the plates after incubation represent only living organisms, not dead ones.
Turbidimetry results, on the other hand, reflect the presence of all organisms in a culture, dead and living.
If the plate count is only performed, we cannot get the exact number of organisms in our sample. 2/ What is a CFU?
CFU stands for colony forming units. It expresses the viable cell counts, which is a measure of living cells in a sample.
When scientists want to know how many microorganisms there are in a solution of bacteria or
fungi, it's usually too time-consuming to count every cell individually under the microscope. By
diluting a sample of microbes and spreading it across a petri plate, microbiologists can instead
count groups of microbes, called colonies, with the naked eye. Each colony is assumed to have
grown from a single colony-forming unit, or CFU.
Scientists can then use the CFU count to determine roughly how many microbes were in the original sample.
For example, if 200 colonies are counted on a plate made with a 1-milliliter sample of a solution
diluted 1,000 times from its original strength, the original solution contains approximately
200,000 CFUs per milliliter. Each CFU doesn't necessarily correspond to a single microbe,
however; if the cells stick together in lumps or chains, the CFU instead refers to these groupings.
3/ Outline some steps that you used to identify your unknown?
The first step in the identification of an unknown bacterial organism is to learn more about its
morphological characteristics, which includes: -
cell shape (rod shaped, spherical, curved or pleomorphic) - size -
gram staining reaction (positive or negative) -
motility (directional or unidirectional) - cellular arrangement -
presence of endospores, capsules or granules - catalase production (bubbles)
4/ Why use serial dilution not single dilution?
Serial dilutions involve diluting a stock or standard solution multiple times in a row. Typically,
the dilution factor remains constant for each dilution, resulting in an exponential decrease in concentration.
Serial dilutions are used to accurately create extremely diluted solutions, as well as solutions for
experiments that require a concentration curve with an exponential or logarithmic scale. Serial
dilutions are widely used in experimental sciences, including biochemistry, pharmacology, microbiology, and physics. 14 | P a g e lOMoARcPSD|47206417
A serial dilution allows a microbiologist to count how many bacteria (for example) are present in a sample.
By diluting the sample down to a point where the amount of bacteria can be counted they can
then approximate the number of bacteria in the whole sample.




