

Preview text:
MEASUREMENT -
Is the comparison of a physical quantiy to be measured with a unit of measurement
1. Precision : refers to the closeness of the set of values obtained from identical measurements of a quantity
2. Accuracy : refers to the closeness of a single measurement to its true value
3. Precision and Accuracy : are both achieved when results are close to each other and to the desired value
4. Significant figures : Are those digits in a measured number (or result of a calculation with
measured numbers) that include all certain digits plus
a final digit having some uncertainty. -
The number of significant figures in a measurement depends on the measuring device -
Normally to one tenth of a smallest unit -
Greater number of significant figures, higher precision -
Number of significant figures refers to the numbers of digits reported for the value of a
measured or calculated quantity, indicating the precision of the value -
Decimal notation ( kí hi u th ệ p phân ) : + commo ậ
n, - awkward to represent very large and/or
very small numbers – easy mistakes
Rule 1 : Disregard all initial zeros, all remaining digits including terminal zeros and zeros
between nonzero integers are significant
Examples: 0.03050 => 4 significant figures
Rule 2: for addition and subtraction, the smallest number of digits to the right of the
decimal set the significant
Rule 3: for multiplication and division, the smallest number of significant digits determines the significance Example question: -
Number of significant figures refers to the number of digits reported for the value of a
measured or calculated quantity, indicating the precision of the value -
A/An exact number is a number that arises when you count items or sometimes when you define a unit.
One dozen : 1 tá – 12 đ n v ơ ị MATTER
Matter Is anything that has mass and occupies space
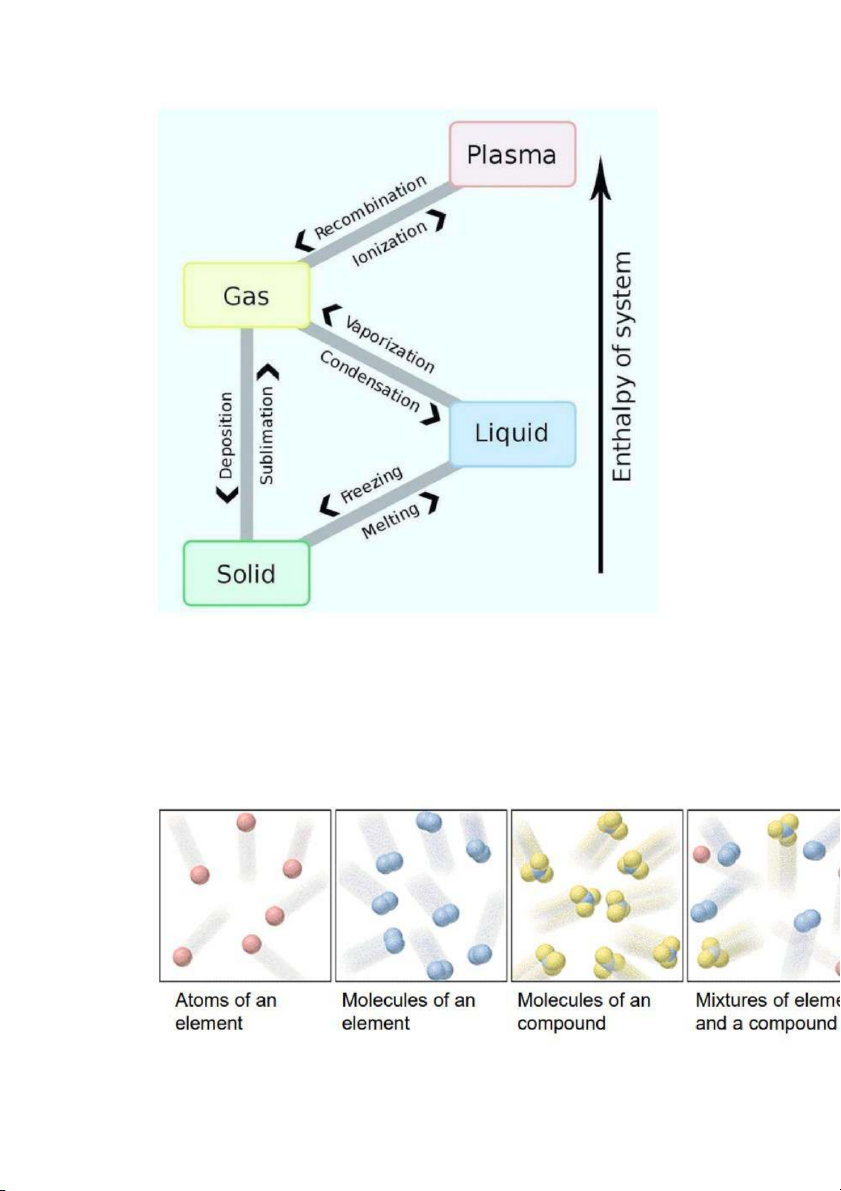
States of matter: 4 states ( according temperature increasing) : solid -> liqid -> gas -> plasma - Solid :
High density (tightly packed close together)
Fixed in position ( can vibrate only)
Retains a fixed volume and shape Not easily compressible Does not flow easily
They are difficult to be pressed, volume and shape are stable, and theirs particles do not float easily
+ Crystalline solids (châất rắấn kếất tinh) : particles arranged in an orderly geometric pattern ex: salt and diamonds
+ Amorphous solids ( châất rắấn vô đ nh hình) : ị
randomly distributed without any long-range
pattern ex: plastic, glass, charcoal - Liqids
Closely packed ( medium desnsity)
Some ability to move around and slided over each other Retains a fixed volume
Takes the shape of the container Not easily compressible Flow easily
They are not easily compressed, their volume is maintained, flow easily and
become the shape of its container. Solid Liquids
Highly density – tightly packed close
Medium density – closely packed Fix volume and shape Fix volume Not easily compressible Not easily compressible Does not flow easily Flow easilly - Gases + complete freedom
Constantly flying around, bumping into each other and container
+ a lot of empty space between particles
+ assumes the shape and volume of its container + is compressible + flow easily - Plasma + nuclei + electrons - Atoms (nguyến t ):
ử the smallest particle that an element can be subdivided into and retain
the chemical properties of that element -
Compound (h p châất) : a subst ợ
ance with constant composition that can be broken down into elements by chemical processes -
Molecules ( phân t ) : a bonded c ử
ollection of two or more atoms of the same or different elements
The smallest particle of a compound having the properties of a compound. ATOMS AND MOLECULES -
Physical properties are the characteristics of matter that can be changed without changing its composition
+ characteristics that are directly observable (quan sát tr c tiếếp)
+ Changes that alter the of the matter
+ State changes : boiling / condensing , melting / freezing , Subliming + dissolving :
Ex : The boiling of water: the water molecules are separated from each other, but their
structure(cấếu trúc) and composition( thành phấần) do not change. -
Chemical properties are the characteristics that determine how the composition of matter
changes as a result of contact with other matter or the influence of energy
o characteristics that describe the behavior of matter
o composition change of the matter: the atoms that are present rearrange into new
molecules, but all of the original atoms are still present + rusting
+ processes that release losts of energy (ex burning) -
Chemistry = Matter and its changes Mass is conserved
Energy is required to affect change -
Energy is anything that has the capacity to do work genera or te heat.
There are things that do not have mass and volume A category of such things is called energy -
Although chemistry is the study of matter, matter is effected by energy. -
“Matter is neither created nor destroyed in a chemical reaction” -
“Energy can neither be created nor destroyed.” -
The combined amount of matter and energy in the universe is constant. -
Potential energy is energy that is stored by virtue of position above ground ( ref ≡ erence point) -
Chemical potential energy: energy is due to chemical interaction (bonding: nuclei-nuclei,
nuclei-electrons, electronselectrons) -
Kinetic energy is energy of motion, or energy that is being transferred from one object to another. -
Temperature = a measure of the average kinetic energy of the particles that make up the system. -
Thermometer = device for the measurement of temperature. -
When a change results in the release of energy it is called an exothermic process.
An exothermic chemical reaction occurs when the reactants have more chemical potential
energy than the products.
When a change requires the absorption of energy it is called an endothermic process.
An endothermic chemical reaction occurs when the products have more chemical potential energy than the reactants.
Heat capacity is the amount of heat a specific substance must absorb to raise its temperature by 1 °C.
Specific heat (capacity) = heat capacity of 1 gram of the substance.
Specific heat is the amount of energy required to raise the temperature of one gram of a substance by 1 °C.
Ch màu xanh : chú ý, có phâần điếần t ừ các bài t ở p t l ậ àm trến m ng ạ
Ch màu đ / bôi vàng : có trong quiz c a thâầy Lâm ủ