




Preview text:
lOMoAR cPSD| 59085392
School of Biotechnology, International University-HCMC
HO CHI MINH CITY NATIONAL UNIVERSITY
INTERNATIONAL UNIVERISTY ***** EXPERIMENT 4
QUANTITATIVE DETERMINATION OF CALCIUM IN POWDERED MILK
Lecturer: MSc. LE NGUYEN THIEN PHUC Group 1:
Nguyễn Võ Mai Tú – BTFTIU18214
Kiều Thị Ánh Tuyết – BTFTIU18197
Nguyễn Quang Huy – BTBTUN17054
Nguyễn Nhật Thịnh – BTBTIU17162
Lê Nguyễn Khánh Kha – BTBTIU18357
Võ Thị Thảo Uyên – BTFTIU18191 lOMoAR cPSD| 59085392
School of Biotechnology, International University-HCMC ABSTRACT
Calcium is an important component of a healthy diet and a mineral necessary for life.
It is a mineral that people need to build and maintain strong bone and teeth. It is also
very important for other physical functions, such as muscle control and blood
circulation. In this report calcium quantity in milk powder is determined via
precipitation reaction between oxalate ammonium and calcium ions. After that, the
experiment is continued by titrating the solution with potassium permanganate to
determine precipitate calcium in milk.
Oxalate ammonium will precipitate all calcium ion in any solution when the
experimenter set up all following conditions:
• pH of the solution in which the reactions occur is larger than 4 (from 5 to 5.2).
• (COONH4)2 solution should be hot, saturated and is filled one time only.
• The solution must be chilled immediately after a 1-minute heating period.
Besides, methyl red which is a pH indicator (turning red under pH 4.4, yellow over
pH 6.2, and orange in between 4.4 and 6.4), is used to determine the ability of an
organism to produce ang maintain stable acid and products from glucose fermentation. INTRODUCTION
In this experiment, a new method of quantifying calcium concentration is used, which
is titration. Titration is carried out by gradually adding a standard solution (potassium
permanganate) until reaction reaches the end point (the color of the solution just turns
pale pink). Base on the volume of potassium permanganate that is used, the
concentration of calcium ion is determined. Oxalate ammonium, which is an important
solution in this experiment, is used to directly precipitate calcium in milk. Precipitant lOMoAR cPSD| 59085392
School of Biotechnology, International University-HCMC
under presence of excess sulfuric acid forms oxalic acid which is further
oxidized by potassium permanganate. Notably, we need to eliminate all the (COO) 2- 2
ion out of the calcium because the exceeding (COO) 2- 2 remain in the solution will
affect the titration process and result in a large concentration of calcium. These
reactions are represented by three specific chemical equations: Ca2+ + (COONH +
4)2 → 2NH4 + Ca(COO)2↓ (1)
Ca(COO)2 + H2SO4 → CaSO4 + (COOH)2 (2)
5(COOH)2 + 2KMnO4 + 3H2SO4 → K2SO4 + 2MnSO4 + 10CO2 + 8H2O (3) CHEMICALS
About chemical, we need powdered milk, saturated (COONH4)2, concentrated HCl,
methyl red, 0.1M NH4OH, Acetic acid, Saturated Calcium chloride, 1N H2SO4 and 0.02N KMnO4. PROCEDURES
We take two milk ash samples contained in two separated racemic crucibles out of the
desiccator and add 5mL of distilled water, and then 5 drops of concentrated HCl. After
that mixing well and transfer these solutions separately into 2 different beakers of
250mL to adjust pH. Adding 10-15 drops of methyl red and carry out the neutralization
by 0.1 ammonium solution. We adjust the pH of the solution to from 5 to 5.2 by acetic
acid of weak concentration. At that point, the solution color is orange-pink. While
heating theses beakers by water bath, stirring these solutions and fill 2-3mL of
saturated (COONH4)2 solution. Continue to provide heat to these beakers and then mix
these solutions well for 30 seconds and put these beakers in cool basin of water lOMoAR cPSD| 59085392
School of Biotechnology, International University-HCMC
immediately. Keep the beaker in the basin of water for about 30 minutes. Then
we use filter paper to collect all the precipitate and put them into Erlenmeyer flask.
After that, we add 20mL of 1N sulfuric acid solution into each Erlenmeyer flask and
heat them in water bath with temperature of 70oC for 1 minute. We titrate the solution
with 0.02 N potassium permanganate solution to determine the concentration of (COO) 2-
2 ion in the solution and use the rule of three to calculate the amount of calcium
ion in the solution and in the sample. RESULT AND DATA ANALYSIS
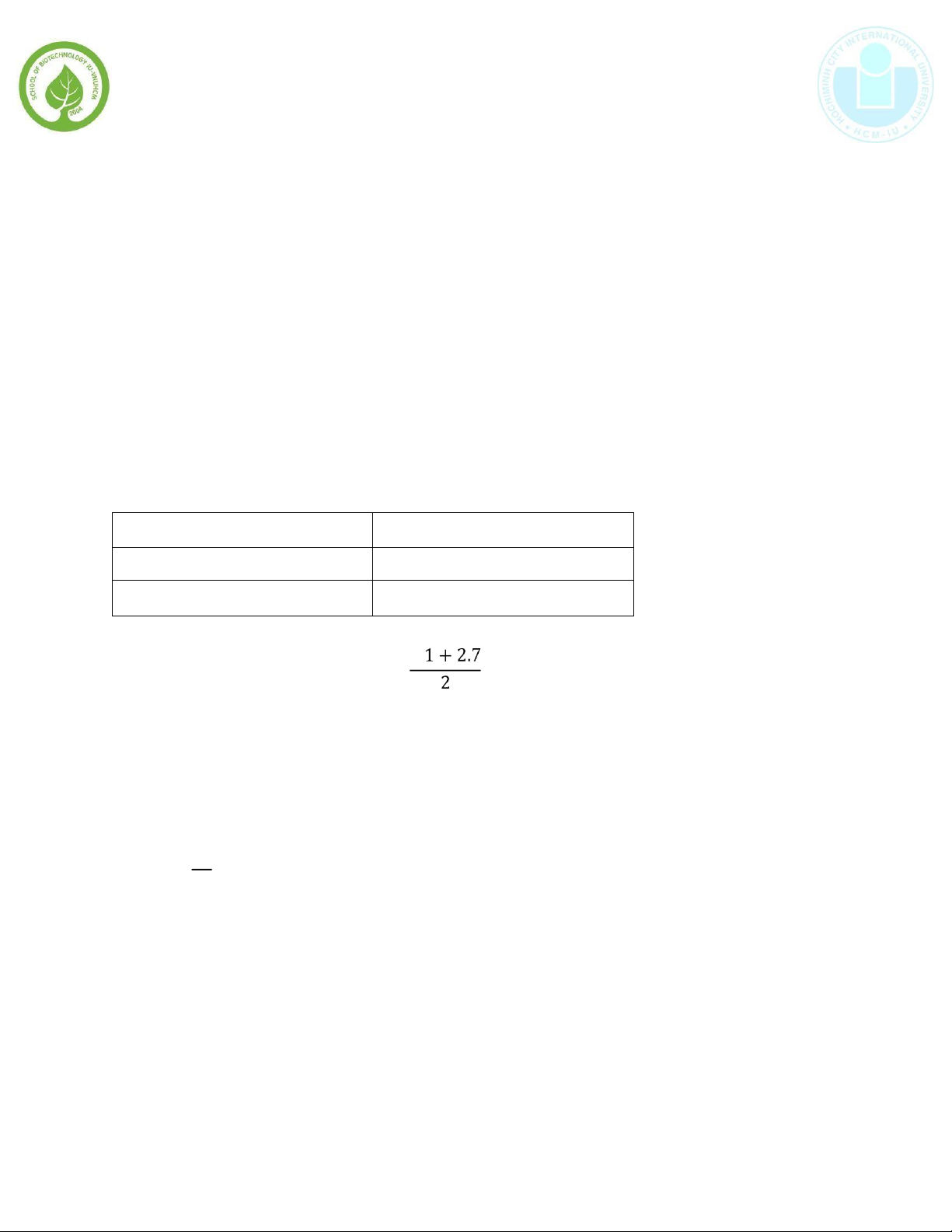
The measured volumes of KMnO4 obtained by titration are shown in table: Trial Volume (mL) 1 2.1 2 2.7
The average volume is calculated by 2. = 2.4𝑚𝐿
2.4 mL is equivalent to 2.4 x 10-3L
The relationship between molarity (M) and normality (N) can be expressed by the formula below: 𝑁 𝑀 = 𝑛𝑒
“ne” is the quantity of electron used for redox reaction
According to the formula, molarity of 0.02N KMnO4 is 0.02/5 = 4 x 10-3 M
Moles of 0.02 N is calculated by Molarity times the volume used for titration:
𝑛𝐾𝑀𝑛𝑂4 = 4 × 10−3 × 2.4 × 10−3 = 9.6 × 10−6 𝑚𝑜𝑙 lOMoAR cPSD| 59085392
School of Biotechnology, International University-HCMC
Based on the reaction, it can be explained that the relationship between amount of calcium ion and KMnO4 is:
2𝑛𝐶𝑎2+ = 5𝑛𝐾𝑀𝑛𝑂4 Therefore, the moles of calcium ion is: 𝑛𝐶𝑎2+ = 5 −5𝑚𝑜𝑙
× 𝑛𝐾𝑀𝑛𝑂4 = 2.4 × 10 2
The molar mass of Ca2+ is 40g/mol, therefore the mass of Calcium in 0.5g of milk powder is:
𝑚𝐶𝑎2+ = 2.4 × 10−5 × 40 = 9.6 × 10−4𝑔
9.6 x 10-4 g is equivalent to 0.96mg
The amount of calcium ion that contains in 100g of powder milk is 192.0mg 0.96 × = 192.0𝑚𝑔 DISCUSSION
The aims of experiment is to determine the amout of Ca2+ based on equation (3) when
used the amout of KMnO4 has known concentration reacts with (COOH)2. Based on
KMnO4 volumetric data that we have recorded in titrations twice. We realize that we
only consume a small amount of KMnO4 compared to the beginning, because the
amount of Calcium initially used is small. We have some errors:
- The amount of (COONH4)2 added was not enough, only 2-3 drops compared to
2-3mL in the laboratory manual, so it was not enough to creat create a
Ca(COO)2 precipitate as a precondition for the appearance of (COOH)2.
- The flasks are not shaken well, so Ca(COO)2 does not react completely to H2SO4
in reaction (2) error in determining the amount of Ca(COO)2. lOMoAR cPSD| 59085392
School of Biotechnology, International University-HCMC
- In reaction (1), the flasks are contain Ca2+ and (COONH4)2 which not be left
in the water long enough for the complete reaction to occur.
- Error in filling the amount of KMnO4 into burret and reading the number in burret. CONCLUSION
In this experiment 4, we practiced calcium quantification in powder milk. The method
was used is titration by using potassium permanganate solution to determine the
concentration of Ca2+. After titrating, according to volume of KMnO4, we calculate
the quantities of Calcium ion.
Oxalate ammonium plays an important role in this experiment when it can react all
Ca2+ contained in powdered milk forming precipitate, since then indicate Calcium quantification by titration.
After doing this experiment, we make some notes:
- Adding enough the amount of (COONH4)2 to create a Ca(COO)2 precipitate as a precondition.
- Shaking flasks well to Ca(COO)2 react completely to H2SO4, thereby avoiding
error in determining the amount of Ca(COO)2.
- Making sure the amount of KMnO4 are filled into buret and reading number on buret exactly. REFERENCES Biochemistry lab manual 2003.



