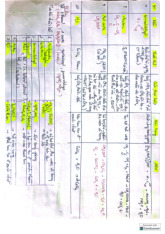


Preview text:
3⁄2 N2(g ) + H+ + e − HN3(aq ) −3.09 [1][2] − + 2 H+ + 2 e +0.6992 [6] −
H2O2(aq ) + 2 H+ + 2 e 2 H2O +1.78
*Au(CN)2+− + e− Au(s) + 2 CN− −0.60 − − − [AuBr +0.96
2] + e Au(s ) + 2 Br − − − [AuBr +0.85
4] + 3 e Au(s ) + 4 Br − − − [AuCl +1.15
2] + e Au(s ) + 2 Cl − − − [AuCl +0.93
4] + 3 e Au(s ) + 4 Cl − − − [AuI +0.58
2] + e Au(s ) + 2 I − − − [AuI +0.56
4] + 3 e Au(s ) + 4 I 3− − 4− [Fe(CN) +0.36 6] + e [Fe(CN)6] − − − [HXeO +1.24 [12]
4] + 3 H2O + 6 e Xe(g ) + 7 OH 3− − − − [HXeO +0.99 [12] 6]
+ 2 H2O + 2 e + [HXeO4] + 4 OH 3− − − [HXeO +1.18 [12] 6]
+ 5 H2O + 8 e Xe(g ) + 11 OH 2 H2O + 2 e− H2(g) + 2 OH− −0.8277 [6] − −
2 BrO3 + 12 H+ + 10 e Br2(l ) + 6 H2O +1.48 −
2 CO2(g ) + 2 H+ + 2 e HOOCCOOH(aq ) −0.43 −
2 H+ + 2 e H2(g ) 0.0000 ≡ 0 −
2 HClO(aq ) + 2 H+ + 2 e Cl2(g ) + 2 H2O +1.63 − 2 Hg2+ + 2 e Hg 2+ +0.91 [9] 2 −
2 HIO(aq ) + 2 H+ + 2 e I2(s ) + 2 H2O +1.44 − −
2 IO3 + 12 H+ + 10 e I2(s ) + 6 H2O +1.20 − 2 NH +
3O H+ + H+ + 2 e N2H5 + 2 H2O +1.42 [1] −
2 TiO2(s ) + 2 H+ + 2 e Ti2O3(s ) + H2O −0.56 − −
2ClO3 + 12 H+ + 10 e Cl2(g ) + 6 H2O +1.49
2FeO42− + 5 H2O + 6 e− Fe2O3(s) + 10 OH− +0.81 [8] Ac3+ + 3 e− Ac(s) −2.20 Ag+ + e− Ag(s) +0.7996 [6] − Ag2+ + e Ag+ +1.98 [9] −
Ag2O(s ) + 2 H+ + 2 e 2 Ag(s ) + H2O +1.17 −
Ag2O3(s ) + 6 H+ + 4 e 2 Ag+ + 3 H2O +1.67 AgBr(s) + e− Ag(s) + Br− +0.07133 [6] AgCl(s) + e− Ag(s) + Cl− +0.22233 [6] AgI(s) + e− Ag(s) + I− −0.15224 [6] −
AgO(s ) + 2 H+ + e Ag+ + H2O +1.77 − − Al(OH) −2.31
3(s ) + 3 e Al(s ) + 3 OH − − − Al(OH) −2.33
4 + 3 e Al(s ) + 4 OH Al3+ + 3 e− Al(s) −1.66 [3] −
As(s ) + 3 H+ + 3 e AsH3(g ) −0.23 [9] − Au+ + e Au(s ) +1.83 [9] −
Au3+ + 3 e Au(s ) +1.52
B(OH)3(aq) + 3 H+ + 3 e− B(s) + 3 H2O −0.89 Ba2+ + 2 e− Ba(s) −2.912 [2] Be2+ + 2 e− Be(s) −1.85 −
Bi(s ) + 3 H+ + 3 e BiH −0.8 [6] 3 −
Bi3+ + 3 e Bi(s ) +0.308 [6] − − Br +1.0873 [6]
2(aq ) + 2 e 2 Br − − Br +1.066 [6]
2(l ) + 2 e 2 Br − −
BrO3 + 5 H+ + 4 e HBrO(aq ) + 2 H2O +1.45 − − −
BrO4 + 2 H+ + 2 e BrO3 + H2O +1.85 −
C(s ) + 4 H+ + 4 e CH4(g ) +0.13 [9] Ca2+ + 2 e− Ca(s) −2.868 [2] Cd2+ + 2 e− Cd(s) −0.40 [3] − Ce4+ + e Ce3+ +1.44 − − Cl +1.36 [3]
2(g ) + 2 e 2 Cl −
ClO2(g ) + H+ + e HClO2(aq ) +1.19 − −
ClO3 + 2 H+ + e ClO2(g ) + H2O +1.18 − − −
ClO4 + 2 H+ + 2 e ClO3 + H2O +1.20
CO(g) + 2 H+ + 2 e− C(s) + H2O +0.52 −
CO2(g ) + 2 H+ + 2 e CO(g ) + H2O −0.11 −
CO2(g ) + 2 H+ + 2 e HCOOH(aq ) −0.11 Co2+ + 2 e− Co(s) −0.28 [6] − Co3+ + e Co2+ +1.82 −
CoO2(s ) + 4 H+ + e Co3+ + 2 H2O +1.42 2− −
Cr2O7 + 14 H+ + 6 e 2 Cr3+ + 7 H2O +1.33 Cr3+ + 3 e− Cr(s) −0.74 − Cr3+ + e Cr2+ −0.42 Cs+ + e− Cs(s) −3.026 [2] − Cu(NH 2+ + +0.10 [9] 3)4 + e Cu(NH3)2 + 2 NH3 − Cu+ + e Cu(s ) +0.520 [9] −
Cu2+ + 2 e Cu(s ) +0.340 [9] Cu2+ + e− Cu+ +0.159 [9]
Cu2O(s) + H2O + 2 e− 2 Cu(s) + 2 OH− −0.360 [6] −
CH3OH(aq ) + 2 H+ + 2 e CH4(g ) + H2O +0.50 Eu2+ + 2 e− Eu(s) −2.812 [2] Eu3+ + e− Eu2+ −0.35 [5] F2(g) + 2 e− 2 F− +2.87 [9][3] −
F2(g ) + 2 H+ + 2 e 2 HF(aq ) +3.05 Fc+ + e− Fc(s) +0.641 [11]
Fe(CN)64− + 6 H+ + 2 e− Fe(s) + 4HCN(aq) −1.16 [8] − − Fe(OH) −0.89 [8]
2(s ) + 2 e Fe(s ) + 2 OH Fe2+ + 2 e− Fe(s) −0.44 [3] − − Fe −0.86 [8]
2O3(s ) + 3 H2O + 2 e 2Fe(OH)2(s ) + 2 OH −
Fe3+ + 3 e Fe(s ) −0.04 [8] − Fe3+ + e Fe2+ +0.77
Fe3O4(s) + 8 H+ + 8 e− 3 Fe(s) + 4 H2O +0.085 [10] 2− −
FeO4 + 3 e + 8 H+ Fe3+ + 4 H2O +2.20 [14] Ga3+ + 3 e− Ga(s) −0.53 Ge(s) + 4 H+ + 4 e− GeH4(g) −0.29 −
Ge4+ + 4 e Ge(s ) +0.12 −
GeO(s ) + 2 H+ + 2 e Ge(s ) + H2O +0.26
GeO2(s) + 2 H+ + 2 e− GeO(s) + H2O −0.37 H2(g) + 2 e− 2 H− −2.25 −
H2MoO4 + 6 H+ + 3 e Mo3+ + 2 H2O +0.43 −
H2MoO4(aq ) + 2 H+ + 2 e MoO2(s ) + 2 H2O +0.65
H2MoO4(aq) + 6 H+ + 6 e− Mo(s) + 4 H2O +0.11 −
H2SeO3(aq ) + 4 H+ + 4 e Se(s ) + 3 H2O +0.74 −
H3AsO3(aq ) + 3 H+ + 3 e As(s ) + 3 H2O +0.24 −
H3AsO4(aq ) + 2 H+ + 2 e H3AsO3(aq ) + H2O +0.56 −
H3PO2(aq ) + H+ + e P(white )[note 2] + 2 H2O −0.508 [6] −
H3PO3(aq ) + 2 H+ + 2 e H3PO2(aq ) + H2O −0.499 [6] −
H3PO3(aq ) + 3 H+ + 3 e P(red )[note 2] + 3 H2O −0.454 [6]
H3PO4(aq) + 2 H+ + 2 e− H3PO3(aq) + H2O −0.276 [6] −
H4XeO6(aq ) + 2 H+ + 2 e XeO3(aq ) + H2O +2.42 [12] −
H4XeO6(aq ) + 8 H+ + 8 e Xe(g ) + 6 H2O +2.18 [12]
H6TeO6(aq) + 2 H+ + 2 e− TeO2(s) + 4 H2O +1.02 [13] −
HClO2(aq ) + 2 H+ + 2 e HClO(aq ) + H2O +1.67
HCOOH(aq) + 2 H+ + 2 e− HCHO(aq) + H2O −0.03 −
HCHO(aq ) + 2 H+ + 2 e CH3OH(aq ) +0.13 −
Hg2+ + 2 e Hg(l ) +0.85 Hg22+ + 2 e− 2 Hg(l) +0.80
HgO(s) + H2O + 2 e− Hg(l) + 2 OH− +0.0977 − −
HMnO4 + 3 H+ + 2 e MnO2(s ) + 2 H2O +2.09 • −
HO2 + H+ + e H2O2(aq ) +1.51 − −
HSeO4 + 3 H+ + 2 e H2SeO3(aq ) + H2O +1.15 − −
HSO4 + 3 H+ + 2 e SO2(aq ) + 2 H2O +0.16 I2(s) + 2 e− 2 I− +0.54 [3] − − − I +0.53 [3] 3 + 2 e 3 I In3+ + 3 e− In(s) −0.34 [9] − −
IO3 + 5 H+ + 4 e HIO(aq ) + 2 H2O +1.13 K+ + e− K(s) −2.931 [2]
La(OH)3(s) + 3 e− La(s) + 3 OH− −2.90 [2] La3+ + 3 e− La(s) −2.379 [2] Li+ + e− Li(s) −3.0401 [2] Mg2+ + 2 e− Mg(s) −2.372 [2] Mn2+ + 2 e− Mn(s) −1.185 [6] −
MnO2(s ) + 4 H+ + 2 e Mn2+ + 2 H2O +1.23 −
MnO2(s ) + 4 H+ + e Mn3+ + 2 H2O +0.95 − − − MnO +0.59
4 + 2 H2O + 3 e MnO2(s ) + 4 OH − −
MnO4 + 4 H+ + 3 e MnO2(s ) + 2 H2O +1.70 − −
MnO4 + 8 H+ + 5 e Mn2+ + 4 H2O +1.51 − − − MnO +0.90 4 + H+ + e HMnO4
MoO2(s) + 4 H+ + 4 e− Mo(s) + 2 H2O −0.15 −
N2(g ) + 2 H2O + 6 H+ + 6 e 2 NH4OH(aq ) +0.092
N2(g) + 4 H2O + 2 e− 2 NH2OH(aq) + 2 OH− −3.04 [1]
N2H4(aq) + 4 H2O + 2 e− 2 NH4+ + 4 OH− +0.11 [1] Na+ + e− Na(s) −2.71 [2][3] Nb3+ + 3 e− Nb(s) −1.099 Ni2+ + 2 e− Ni(s) −0.25 − − NiO +1.59
2(s ) + 4 H+ + 2 e Ni2+ + 2 OH − −
NO3 (aq ) + 2 H+ + e NO2(g ) + H2O +0.80 −
O2(g ) + 2 H+ + 2 e H2O2(aq ) +0.70 − −
O2(g ) + 2 H2O + 4 e 4 OH (aq ) +0.40 [3] −
O2(g ) + 4 H+ + 4 e 2 H2O +1.229 [3] O2(g) + H+ + e− HO2•(aq) −0.13
O3(g) + 2 H+ + 2 e− O2(g) + H2O +2.075 [5] −
P(red ) + 3 H+ + 3 e PH3(g ) −0.111 [6] −
P(white ) + 3 H+ + 3 e PH3(g ) −0.063 [6] Pb2+ + 2 e− Pb(s) −0.13 [3] − Pb4+ + 2 e Pb2+ +1.69 [9] − − PbO(s ) + H −0.58
2O + 2 e Pb(s ) + 2 OH − 2− PbSO −0.3505 [6]
4(s ) + 2 e Pb(Hg ) + SO4 − 2− PbSO −0.3588 [6]
4(s ) + 2 e Pb(s ) + SO4 Pd2+ + 2 e− Pd(s) +0.915 [5] Pt2+ + 2 e− Pt(s) +1.188 [5] 2− − − PtCl +0.758 [5] 4
+ 2 e Pt(s ) + 4 Cl 2− − 2− − PtCl +0.726 [5] 6 + 2 e PtCl4 + 2 Cl Ra2+ + 2 e− Ra(s) −2.8 [2] Rb+ + e− Rb(s) −2.98 [2] Re3+ + 3 e− Re(s) +0.300 Ru(NH3)63+ + e− Ru(NH3)62+ +0.10 [5] S(s) + 2 H+ + 2 e− H2S(g) +0.14 − S 2 − 2O3
+ 6 H+ + 4 e 2 S(s ) + 3 H2O +0.60 2− − 2− S +2.010 [6] 2O8 + 2 e 2 SO4 2− − 2− S +0.08 4O6 + 2 e 2 S2O3 −
SbO+ + 2 H+ + 3 e Sb(s ) + H2O +0.20 Sc3++ + 3 e− Sc(s) −2.077 [4] Se(s) + 2 H+ + 2 e− H2Se(g) −0.11 Si(s) + 4 H+ + 4 e− SiH4(g) −0.14
SiO2(s) + 4 H+ + 4 e− Si(s) + 2 H2O −0.91 Sn(s) + 4 H+ + 4 e− SnH4(g) −1.07 −
Sn2+ + 2 e Sn(s ) −0.13 − Sn4+ + 2 e Sn2+ +0.15
SnO(s) + 2 H+ + 2 e− Sn(s) + H2O −0.10
SnO2(s) + 2 H+ + 2 e− SnO(s) + H2O −0.09 −
SO2(aq ) + 4 H+ + 4 e S(s ) + 2 H2O +0.50 2− −
SO4 + 4 H+ + 2 e SO2(aq ) + 2 H2O +0.17 Sr2+ + 2 e− Sr(s) −2.899 [2] −
Ta2O5(s ) + 10 H+ + 10 e 2 Ta(s ) + 5 H2O −0.75 −
Ta3+ + 3 e Ta(s ) −0.6 Te(s) + 2 e− Te2− −1.143 [9] Ti2+ + 2 e− Ti(s) −1.63 [3]
Ti2O3(s) + 2 H+ + 2 e− 2 TiO(s) + H2O −1.23 −
Ti3+ + 3 e Ti(s ) −1.37 [7]
TiO(s) + 2 H+ + 2 e− Ti(s) + H2O −1.31 −
TiO2+ + 2 H+ + 4 e Ti(s ) + H2O −0.86 −
TiO2+ + 2 H+ + e Ti3+ + H2O +0.19 Tl+ + e− Tl(s) −0.34 [9] − Tl3+ + 2 e Tl+ +1.25 −
Tl3+ + 3 e Tl(s ) +0.72 U3+ + 3 e− U(s) −1.66 [5] − U4+ + e U3+ −0.52 [5] − UO +
2 + 4 H+ + e U4+ + 2 H2O +0.273 [5] UO22+ + e− UO2+ +0.163 [5] V2+ + 2 e− V(s) −1.13 [9] − V3+ + e V2+ −0.26 [3] −
VO2+ + 2 H+ + e V3+ + H2O +0.34 −
WO2(s ) + 4 H+ + 4 e W(s ) + 2 H2O −0.12
WO3(aq) + 6 H+ + 6 e− W(s) + 3 H2O −0.09 [9] −
XeF2(aq ) + 2 H+ + 2 e Xe(g ) + 2HF(aq ) +2.32 [12] −
XeO3(aq ) + 6 H+ + 6 e Xe(g ) + 3 H2O +2.12 [12] Y3+ + 3 e− Y(s) −2.372 [2] 2− − − Zn(OH) −1.199 [6] 4
+ 2 e Zn(s ) + 4 OH Zn2+ + 2 e− Zn(Hg) −0.7628 [6] Zn2+ + 2 e− Zn(s) −0.7618 [6] Zr4+ + 4 e− Zr(s) −1.45 [6] − − ZrO(OH) −2.36 [2]
2(s ) + H2O + 4 e Zr(s ) + 4 OH
ZrO2(s) + 4 H+ + 4 e− Zr(s) + 2 H2O −1.553 [6] α-PbO −
2(s ) + 4 H+ + 2 e Pb2+ + 2 H2O +1.468 [9]
β-PbO2(s) + 4 H+ + 2 e− Pb2+ + 2 H2O +1.460 [9]