







Preview text:
Chapter 5 Yogurt Production Seiji Nagaoka Abstract
Yogurt is a popular fermented dairy product produced by lactic acid bacteria, including Streptococcus
thermophilus and Lactobacillus delbrueckii subsp. bulgaricus. During yogurt production, these bacteria
produce lactic acid, decreasing pH and causing milk protein to coagulate. Their metabolites, such as
carbonyl compounds, nonvolatile or volatile acids, and exopolysaccharides, strongly affect the quality of
yogurt. In this chapter, the general methods for yogurt production are summarized.
Key words Yogurt, Fermentation, Lactic acid bacteria, Starter culture, Streptococcus thermophilus,
Lactobacillus delbrueckii subsp. bulgaricus, Microbiological analysis, Texture analysis 1 Introduction
Yogurt is a popular dairy product that is widely favored for its
healthy and nutritious quality, as well as for its sensory properties.
According to the Codex standard [1] published by the FAO/
WHO, yogurt is obtained from milk by lactic acid fermentation,
through the action of Streptococcus thermophilus and Lactobacillus
delbrueckii subsp. bulgaricus. There is a symbiotic relationship
between these two species of bacteria; S. thermophilus rapidly pro-
pagates at the start of fermentation and produces pyruvic acid,
formic acid, and carbon dioxide [2]. These substances promote
the growth of L. bulgaricus, while L. bulgaricus hydrolyses milk
proteins into peptides and amino acids that stimulate the growth of
S. thermophilus [3, 4].
Through the fermentation process, lactic acid is produced from
lactose (the main carbohydrate in milk) by the bacterial cultures,
decreasing pH and causing the coagulation of milk protein to give it
a viscous gel-like structure. The components of milk are converted
into carbonyl compounds, nonvolatile and volatile acids such as
acetaldehyde, acetone, acetoin, diacetyl, and acetate that give
yogurt its characteristic flavor [5, 6]. In addition, certain strains
of yogurt starter culture produce an abundance of
Makoto Kanauchi (ed.), Lactic Acid Bacteria: Methods and Protocols, Methods in Molecular Biology, vol. 1887,
https://doi.org/10.1007/978-1-4939-8907-2_5, © Springer Science+Business Media, LLC, part of Springer Nature 2019 45 46 Seiji Nagaoka
exopolysaccharides (EPS), which reduce syneresis and enhance
product texture and viscosity [7].
The main quality characteristics of yogurt, including texture,
taste, and flavor, can vary depending on lactic acid bacteria starters
and their metabolites. Subsequent ingredients and various
manufacturing conditions (e.g., standardization, homogenization,
heat treatment, cooling, etc.) can also affect the product quality of
yogurt. This chapter aims to summarize a general method for
laboratory-scale yogurt production. 2 Materials
Use only analytical-grade reagents and only distilled or deionized
water. Prepare all ingredients for yogurt using distilled water and
food-grade materials. Prepare and store all reagents and ingredients
at room temperature unless otherwise specified. 2.1 Starter Culture
1. Bacterial Strains: S. thermophilus and L. bulgaricus strains, (See Note 1) freeze-dried.
2. Skim milk and yeast extract (SMY) medium: Autoclave a 10%
skim milk powder solution supplemented with 0.1% yeast
extract at 121 ◦C for 7 min.
3. Skim milk (SM) medium: Pasteurize a 10% skim milk powder
solution at 95 ◦C for 10–30 min with mixing. 2.2 Ingredients
1. Milk and milk products: Raw milk, skim milk, skim milk pow-
der, and concentrated skim milk are chiefly used (see Note 2).
Whey powder, whey protein concentrate (WPC), whey protein
isolate (WPI), and milk protein concentrate (MPC) can be used
to fortify the milk solids-not-fat (MSNF; mainly lactose, pro-
tein, and mineral) of the milk base. Cream and butter can be
used to fortify the fat content of the milk base.
2. Sugars and sweeteners, if necessary: Sugar, sucralose, aspartame, etc.
3. Stabilizers, if necessary: Gelatin, pectin, starch, agar, etc.
4. Flavors and aroma, if necessary: Strawberry, apple, pineapple, etc.
5. Fruits preserves, if necessary: Sterilized fruit jam, puree, juice, or a mixture of these. 2.3 Titratable Acidity 1. Sodium hydroxide, standard volumetric solution: c (NaOH) = 0.1 mol/L.
2. Phenolphthalein ethanol solution of 1% (w/v), if necessary. Yogurt Production 47 2.4 Microbiological
1. M17 medium [8]: 2.5 g tryptic digest of casein, 2.5 g peptic Analysis
digest of meat, 5.0 g papain digest of soya, 2.5 g yeast extract
powder, 5.0 g meat extract, 19.0 g β-glycerophosphate diso-
dium salt, 0.25 g magnesium sulfate heptahydrate, 0.50 g
ascorbic acid, 9.0–18.0 g agar, water up to 950 mL. Heat to
boiling temperature to dissolve the medium completely. Adjust
the pH to 6.8 0.1 at 25 ◦C. Autoclave at 121 ◦C for 15 min.
Dissolve the lactose in water at a concentration of 10%, and
autoclave at 121 ◦C for 15 min. Mix the medium and the
lactose solution at a 9:1 ratio at 50 ◦C (see Note 3).
2. Acidified MRS medium [9]: 10.0 g tryptic digest of casein,
10.0 g meat extract, 5.0 g yeast extract powder, 20.0 g glucose,
1.0 mL tween 80, 2.0 g dipotassium hydrogen orthophos-
phate, 5.0 g sodium acetate trihydrate, 2.0 g diammonium
citrate, 0.2 g magnesium sulfate heptahydrate, 0.05 g manga-
nese sulfate tetrahydrate, 9.0–18.0 g agar, water up to
1000 mL. Heat until boiling to dissolve the medium
completely. Adjust the pH to 5.4 0.1 at 25 ◦C by adding
acetic acid. Autoclave at 121 ◦C for 15 min.
3. Yeast extract dextrose chloramphenicol agar medium [10]:
5.0 g yeast extract powder, 20.0 g dextrose, 0.1 g chloram-
phenicol, 12–15 g agar, water up to 1000 mL. Heat until
boiling to dissolve the medium completely. Autoclave at 121 ◦C for 15 min.
4. Peptone-salt solution (diluent) [11]: 1.0 g peptone of enzy-
matic digest of casein, 8.5 g sodium chloride. Dissolve each
component in 1000 mL water, and adjust the pH with sodium
hydroxide or hydrochloric acid solution at 7.0 0.2 at 25 ◦C.
Autoclave at 121 ◦C for 15 min. 3 Methods
Carry out all procedures at room temperature unless otherwise specified. 3.1 Preparation
Carry out in an aseptic environment to avoid microbial of Bulk Starter (See contamination. Note 1)
1. Stock culture: Sub-cultivate freeze-dried S. thermophilus and L.
bulgaricus strains independently at 37 ◦C for 16–20 h in SMY medium at least three times.
2. Mother starter: Inoculate 1% of the stock culture indepen-
dently in SMY medium and cultivate at 37 ◦C for 16–20 h.
3. Inoculate 1% of both mother starters to SM medium at the
ratio of S. thermophilus to L. bulgaricus of 50:50–90:10. 48 Seiji Nagaoka
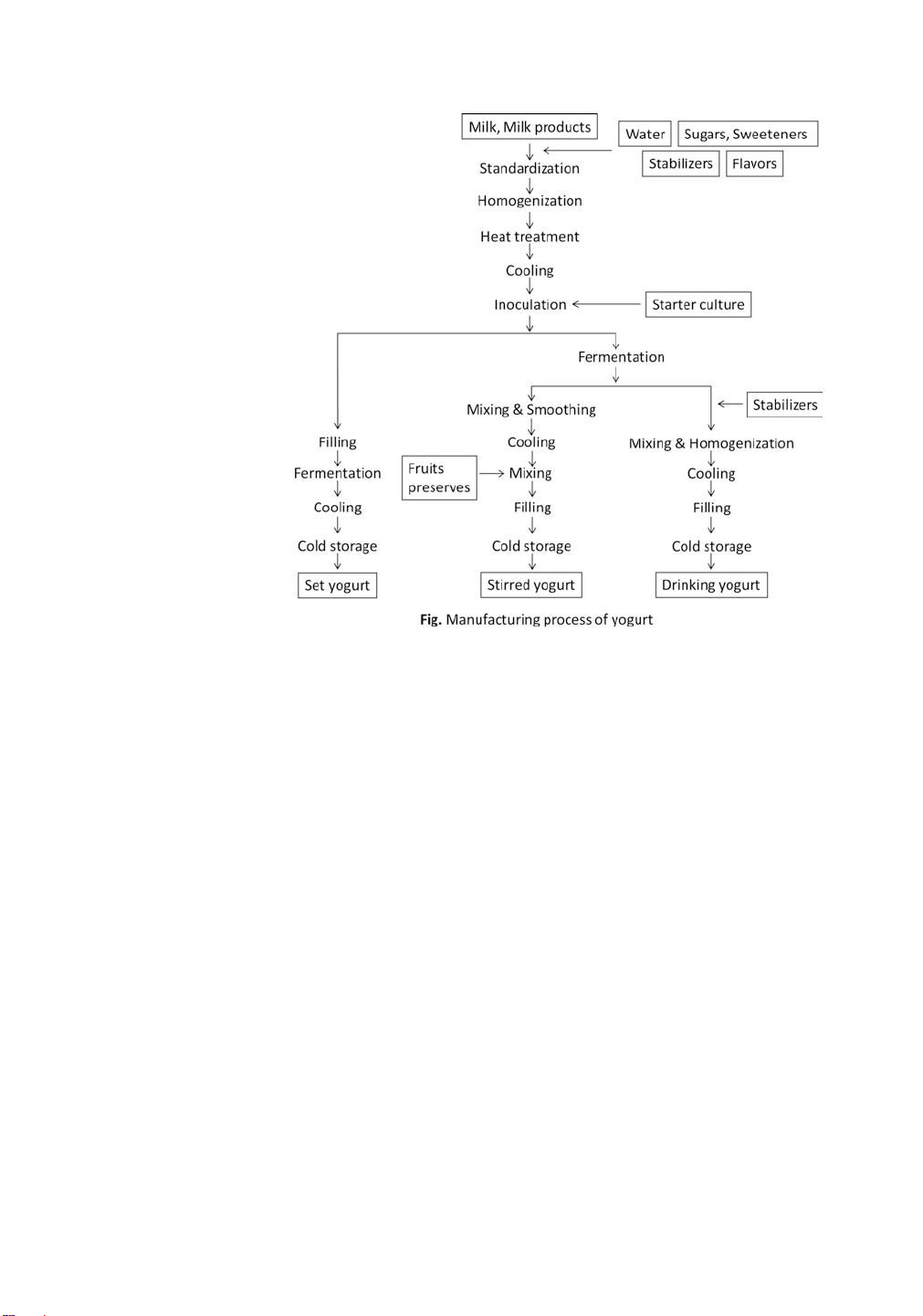
Fig. 1 Manufacturing process of yogurt
4. Cultivate at 37–43 ◦C until the titratable acidity reaches to 0.8% (≈pH 4.6).
5. Cool the culture medium in a cold-water bath, and keep in a refrigerator. 3.2 Preparation
The Codex standard [12] stated that yogurt contains a protein of Yogurt
content greater than 2.7% and a fat content below 15%. Generally,
commercial yogurts include fat at a rate of about 0–3.5% and
MSNF of 8–14%. The process of yogurt production at laboratory
scale is summarized in Fig. 1. Three classes of method for produc-
ing representative yogurts are described in the text that follows. 3.2.1 Set Yogurt
Set yogurt is produced by filling the milk base into individual
containers before fermentation and is characterized by a firm texture.
1. Standardization: Dissolve all ingredients in ambient or hot
water at less than 60 ◦C in stainless steel vessels.
2. Homogenization: Heat the milk base to around 65–70 ◦C and
homogenize at 10–20 MPa pressure by using double-stage
homogenizer to reduce the diameter of the milk fat globules (see Note 4). Yogurt Production 49
3. Heat treatment: Heat the milk base to 85 ◦C for 20–30 min or
90–95 ◦C for 5 min [13] to kill all pathogenic microorganisms
and denature whey protein (see Notes 2 and 5).
4. Cooling to incubation temperature: Cool milk base in a cold-
water bath to 40–45 ◦C immediately (see Note 6).
5. Inoculation with starter: Add 2–4% of bulk starter or specified
amount of commercial starter culture for direct vat set (DVS)
or direct vat inoculation (DVI) to the milk base kept at 40– 43 ◦C, and mix it gently.
6. Filling: Fill the milk base into cup.
7. Fermentation: Cultivate at 40–43 ◦C until the titratable acidity
reaches to 0.8% (≈pH 4.6) (see Note 7).
8. Cooling: Cool the fermented milk base in a cold room or
refrigerator to below 15 ◦C immediately.
9. Cold storage: Store the product in a refrigerator at around 5 ◦C. 3.2.2 Stirred Yogurt
Stirred yogurt is incubated in a tank, and the final coagulum is
broken by stirring prior to cooling and filling. The texture will be
less firm than a set yogurt. Most of these yogurts are supplemented
with fruits, sugar, sweeteners, stabilizers, flavors, etc.
1. Carry out the same procedure until step 5 of set yogurt.
2. Fermentation: Cover the container, and incubate at 40–45 ◦C
for 3–8 h until a pH of below 4.6 is reached (see Note 7).
3. Mixing and smoothing: Break down the curd by stirring with
impeller. Smooth the aggregates by using pump filtration or
back pressure valve, if necessary.
4. Cooling: Cool the fermented milk base in a cold-water bath to
10–25 ◦C, mixing immediately.
5. Addition of fruit preserve, if necessary: Mix the fermented base with fruit preserve.
6. Filling: Fill the product into a cup.
7. Cold storage: Store the product in a refrigerator at around 5 ◦C. 3.2.3 Drinking Yogurt
Drinking yogurt is a liquid type of yogurt in which stabilizers,
sugars, flavor, and other ingredients are mixed with plain yogurt and homogenized.
1. Carry out the same procedure as for stirred yogurt, until step 3 of stirred yogurt.
2. Addition of stabilizer: Add the sterilized stabilizer solution,
such as high methoxyl pectin or carboxymethyl cellulose 50 Seiji Nagaoka
(CMC), to the fermented base at a final rate of 0.2–0.5% to prevent whey syneresis.
3. Mixing and homogenization: Mix well and then homogenize
at 10–20 MPa pressure by using single- or double-stage
homogenizer to decrease the viscosity for drinkable texture
and to disperse the stabilizers.
4. Cooling: Immediately cool the product in a cold-water bath to 10 ◦C.
5. Filling: Fill the product into a bottle.
6. Cold storage: Store the product in a refrigerator at around 5 ◦C. 3.3 Measurement
The concentration of lactic acid produced by starter culture in of Titratable Acidity
yogurt is measured by a titratable acidity method according to
International Dairy Federation (IDF) standards [14]. This method
is used to estimate the progress of fermentation and change in
acidity during the shelf life (post-acidification). The Codex stan-
dard [12] requires yogurt to contain more than 0.6% titratable acidity.
1. Mix the sample thoroughly with a spatula, using a rotary motion (see Note 8).
2. Weigh approximately 10 g of the sample into a 50 mL beaker.
3. Add approximately 10 mL of water and mix.
4. Titrate the contents of the beaker, while stirring, using the
sodium hydroxide solution, to a pH of 8.30 (see Note 9).
5. Calculate the grams of lactic acid per 100 g of product (AT) using the following equation:
AT = V × 0.9/m
V, the volume (mL) of sodium hydroxide used; m, the mass
(g) of the sample; 0.9, the conversion factor for the lactic acid. 3.4 Microbiological
The Codex standard [12] requires 1 g of yogurt to contain more Analysis
than ten million colony-forming units (CFU) of lactic acid bacteria,
constituting the starter culture. The numbers of S. thermophilus and 3.4.1 Lactic Acid
L. bulgaricus in yogurt are measured according to the IDF standard Bacterial Count method [15]:
1. Mix the sample using a sterile spatula.
2. Weigh 10 g of sample into a sterile container.
3. Add the 90 g of sterile peptone-salt solution (diluent), and mix
it well to obtain a 101 dilution. Yogurt Production 51
4. Repeat the procedure mentioned above to obtain serial dilutions.
5. Using a sterile pipette, transfer 1 mL of each dilution into Petri dishes.
6. Pour 15 mL of medium (M17 medium for S. thermophilus and
acidified MRS medium for L. bulgaricus), maintained on a
water bath at 45 ◦C, into Petri dishes.
7. Mix the inoculum with the medium by rotating the Petri
dishes. Cool the medium until it solidifies.
8. Incubate the prepared dishes in an inverted position with the
incubator set at 37 ◦C for 48 h for S. thermophilus and in the
anaerobic jar with the incubator set at 37 ◦C for 72 h for L. bulgaricus.
9. Count the colonies on plates with between 15 and 300 colonies.
10. Calculate the CFU/g by the following equation:
CFU/g = number of colonies/total dilution used
For example, if 238 colonies were present on the plate treated
with 106 dilution, the calculation would be as follows: CFU/g = 238/106 = 2.4 × 108 3.4.2 Yeast and Mold
Generally, yeast and mold are recognized as spoilage microorgan- Count
isms of yogurt because of the production of gas and off-flavors
during cold storage. Yeast and molds are not heat-resistant and
should be killed by heat treatment. Therefore, during
manufacturing, fungal contamination generally occurs after heat
treatment. Yeast and mold counts are detected according to IDF standard methods [10].
1. Carry out the same procedure as for lactic acid bacteria, until
step 5 of lactic acid bacterial count.
2. Pour 15 mL of yeast extract dextrose chloramphenicol agar
medium maintained on a water bath at 45 ◦C into Petri dishes.
3. Mix the inoculum with the medium by rotating the Petri
dishes. Cool the medium until it solidifies.
4. Incubate the prepared dishes in an inverted position, with the
incubator set at 25 ◦C for 5 days.
5. Count the colonies on plates, and calculate the CFU/g using the equation described above. 52 Seiji Nagaoka 3.5 Texture Analysis
Curd firmness is measured to evaluate the resistance to the impact
of shaking during transport for set yogurt. Horiuchi [16] suggested 3.5.1 Curd Firmness
the method for evaluating the curd firmness of set yogurt and also and Smoothness
the smoothness by using the Neo Curd meter M302 (Itechno Engineering Co.)
1. Set the yogurt sample at 5–10 ◦C on the stage of curd meter.
2. Penetrate the yogurt sample using the yogurt knife attached with a 100 g weight.
3. Record the maximum weight (g) required until break of the
penetration angle curve, which represents firmness, while the
angle serves as an indicator of smoothness. The angle is a value
up to 90◦; a smaller value represents a smoother texture (see Note 10). 3.5.2 Viscosity
Viscosity is one important factor for evaluating the sensory char-
acteristics, such as thickness, of stirred yogurt or drinking yogurt.
Generally, the viscosity is measured using a rotational (B-type)
viscometer such as Brookfield viscometer (Brookfield Engineering)
or Rheomat (S. I. Instruments).
1. Attach the spindle of the appropriate size, depending on the sample viscosity.
2. Place the yogurt on the counter at 5–10 ◦C, and lower the
spindle through the surface of the yogurt to level the notch on
the spindle to the surface of the yogurt.
3. Turn the motor on at a constant speed of 10–60 rpm.
4. Record the results in centipoises (cp) after 20–50 s of shearing. 3.5.3 Syneresis by
Whey separation of yogurt can be done by the centrifugal method Centrifuging
[16]. This method helped us to estimate rapidly and in advance the
actual whey syneresis of yogurt after storage.
1. Transfer 25–40 g of yogurt sample at 5 ◦C to 50 mL centrifuge tubes.
2. Centrifuge at 2150 × g for 20 min at 5 ◦C.
3. Weigh the quantity (g) of supernatant liquid separated at the
top of the coagulum inside centrifuge tubes.
4. Calculate the degree of whey syneresis of yogurt by the follow- ing equation:
The ratio of syneresis (%) = the supernatant (g)/yogurt sample (g) × 100 Yogurt Production 53 4 Notes
1. If a commercial starter culture for DVS or DVI is used, this procedure is not needed.
2. Milk ingredients or milk-base pasteurized by ultra-high tem-
perature (UHT) should not be used for yogurt production
because UHT lowers the yogurt viscosity and gel firmness [17, 18].
3. If the prepared culture media are not used immediately, they
should be cooled and stored at 4 ◦C for no longer than 1 week.
4. Higher homogenization pressures result in increased firmness
and viscosity due to an increase in surface area caused by the
formation of a larger number of smaller fat particles. Homoge-
nization also helps to reduce whey separation [2].
5. If the whey proteins are not denatured sufficiently by heat
treatment, later they will not react with the casein protein.
This gives the yogurt a weak gel with lower water retention capacity.
6. If the starter culture is inoculated at too high a temperature
(e.g., >50 ◦C), the fermentation time is delayed by heat dam- age to lactic acid bacteria.
7. If the curd is subjected to vibration at pH 5.5 to 4.6 where
gelation is being formed, the curd tends to develop whey
separation or other undesirable changes in texture, which
leads to a defective product. Note that the relationship between
acidity and pH varies depending on MSNF of the milk base.
8. Homogenize the sample using an appropriate device in order
to facilitate the grinding and dispersion of the aggregates,
which inhibit an accurate titration.
9. Titration can be performed using 0.1 M NaOH in the presence
of 0.5 mL of 1% phenolphthalein ethanol solution as an indica-
tor to an end point of faint pink color.
10. The vertical axis of the graph represents the height of the knife,
and the horizontal axis represents the additional weight that is
added, beyond the 100 g weight. In the graph, the length of
10 mm on the vertical axis and 10 g on the horizontal axis are the same. 54 Seiji Nagaoka References 1. Codex Alimentarius Commission (1984)
and milk products - Enumeration of colony-
Codex alimentarius: code of principles
forming units of yeasts and/or moulds -
concerning milk and milk products, interna-
Colony-count technique at 25 ◦C. ISO
tional standards for milk products and interna- 6611:2004 (IDF 94:2004)
tional individual standards for cheese.
11. International Dairy Federation/International FAO/WHO
Organization for Standardization (2001) Milk
2. Tamime YA, Robinson KR (eds) (1999)
and milk products – general guidance for the
Tamime and Robinson’s Yogurt science and
preparation of test samples, initial suspensions
technology, 2nd edn. Woodhead, Cambridge
and decimal dilutions for microbiological
3. Bautista CS, Dahiya RS, Speck ML (1966)
examinations and/or moulds. ISO 8261:2001
Identification of compounds causing symbiotic (IDF 122:2001)
growth of Streptococcus thermophilus and Lacto- 12. Codex Alimentarius Commission (2003)
bacillus bulgaricus in milk. J Dairy Res 33:299–
Codex standard for fermented milks, Codex 307 stan 243-2003
4. Radke-Mitchell L, Sandine WE (1984) Asso-
13. Sfakianakis P, Tzia C (2014) Conventional and
ciative growth and differential enumeration of
innovative processing of milk for yogurt man-
Streptococcus thermophilus and Lactobacillus
ufacture; development of texture and flavor: a
bulgaricus, a review. J Food Prot 47:245–248 review. Foods 3:176–193
5. Beshkova D, Simova E, Frengova G et al
14. International Dairy Federation/International
(1998) Production of flavor compounds by
Organization for Standardization (1991)
yogurt starter cultures. J Ind Microbiol Bio-
Yogurt - determination of titratable acidity. technol 20:180–186 IDF 150:1991
6. Ott A, Fay LB, Chaintreau A (1997) Determi-
15. International Dairy Federation/International
nation and origin of the aroma impact com-
Organization for Standardization (2003)
pounds of yogurt flavor. J Agric Food Chem
Yogurt—enumeration of characteristic micro- 45:850–858
organisms—colony-count technique at 37 ◦C.
7. Folkenberg MD, Dejmek P, Skriver A et al ISO 7889:2003 (IDF 117:2003)
(2006) Sensory and rheological screening of
16. Horiuchi H (2014) A new manufacture
exopolysaccharide producing strains of bacte-
method for set yogurt with low-temperature
rial yoghurt cultures. Int Dairy J 16:111–118
reduced dissolved oxygen fermentation. Dis-
8. Terzaghi BE, Sandine WE (1975) Improved
sertation, Tokyo University of Agriculture
medium for lactic streptococci and their bacter-
17. Parnell-Clunies E, Kakuda Y, Smith AK (1988)
iophages. J Appl Microbiol 29:807–813
Gelation profiles of yoghurt as affected by heat
9. De Man JK, Rogosa M, Sharpe ME (1960) A
treatment of milk. J Dairy Sci 71:582–588
medium for the cultivation of lactobacilli. J
18. Labropoulos EA, Palmer KJ, Lopez A (1981) Appl Bacteriol 23:130–135
Whey protein denaturation of UHT processed
10. International Dairy Federation/International
milk and its effect on rheology of yogurt. J
Organization for Standardization (2004) Milk Texture Stud 12:365–374




