





Preview text:
lOMoAR cPSD| 58490434 18/01/2016 GENERAL CHEMISTRY 1 Contents ➢ Definitions
➢ Covalent bonds • VSEPR theory • MO theory ➢ Ionic bond
➢ Metallic bond
➢ Hydrogen force
➢ Van de Wall bond 2 lOMoAR cPSD| 58490434 18/01/2016 Lewis Symbols
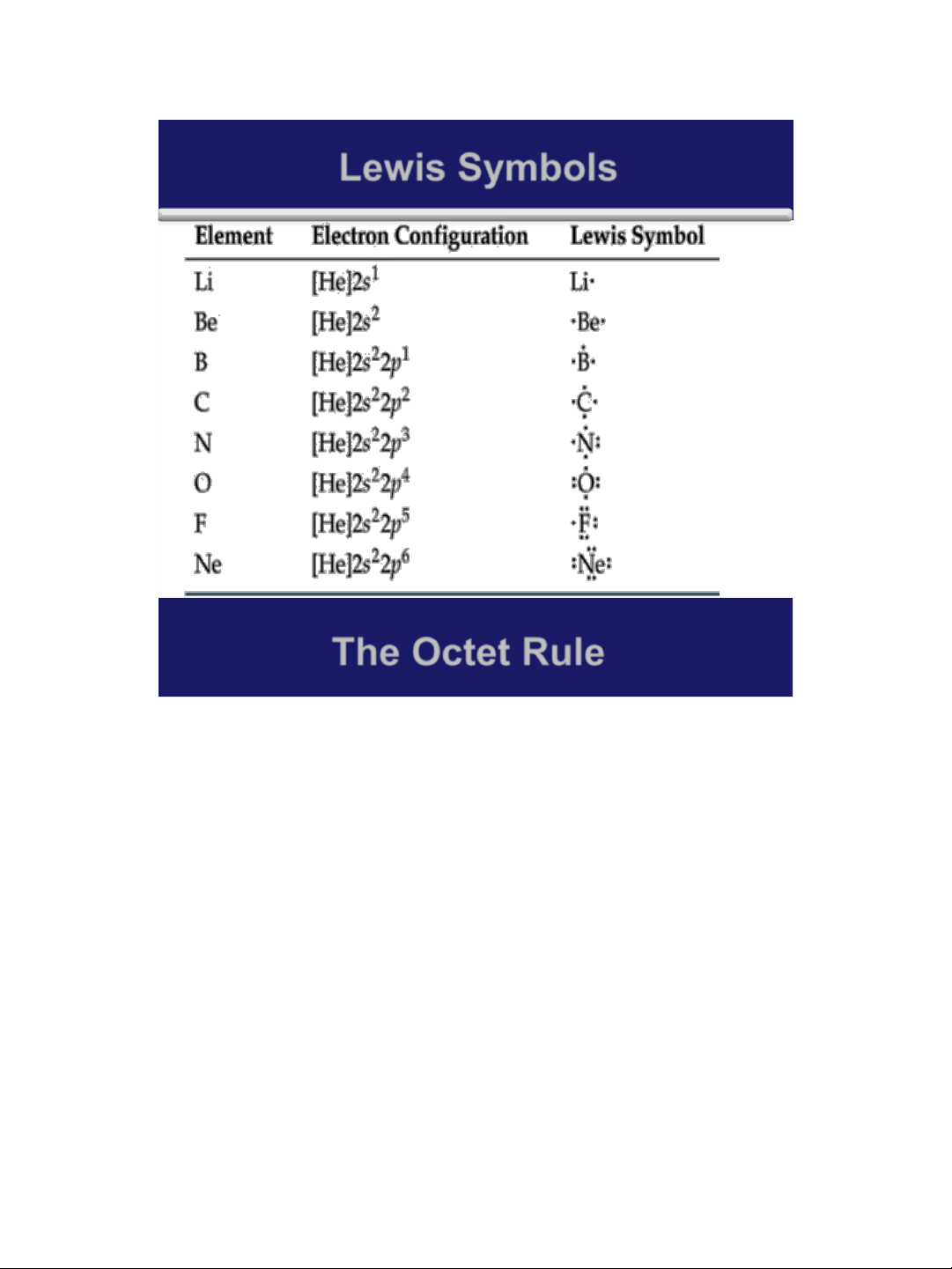
➢ Lewis symbol, Lewis electron-dot symbol, is a symbol in
which electrons in the valence shell of an atom or ion are
represented by dots placed around the letter symbol of the element.
➢ Dots are placed one to each side of a letter symbol until all
four sides are occupied. Then the dots are written two to
a side until all valence electrons are accounted for.
➢ The number of electrons available Cl for bonding are indicated by unpaired dots. 3 lOMoAR cPSD| 58490434 18/01/2016 Lewis Symbols 4 The Octet Rule ➢ All noble gases (except He) has an
ns2np6 configuration.
➢ Octet rule: Atoms tend to gain, lose, or share electrons until
they are surrounded by 8 valence electrons (4 electron pairs).
➢ Caution: There are many exceptions to the Octet rule. 5 lOMoAR cPSD| 58490434 18/01/2016 The Octet Rule
➢ How to place the electrons around the bonded atoms.
➢ How many of the available valence electrons are bonding electrons (shared). ➢ And how many are unshared electrons
(associated with only one atom).
➢ A pair of unshared electrons in the same orbital is called a lone pair. 6 The Octet Rule
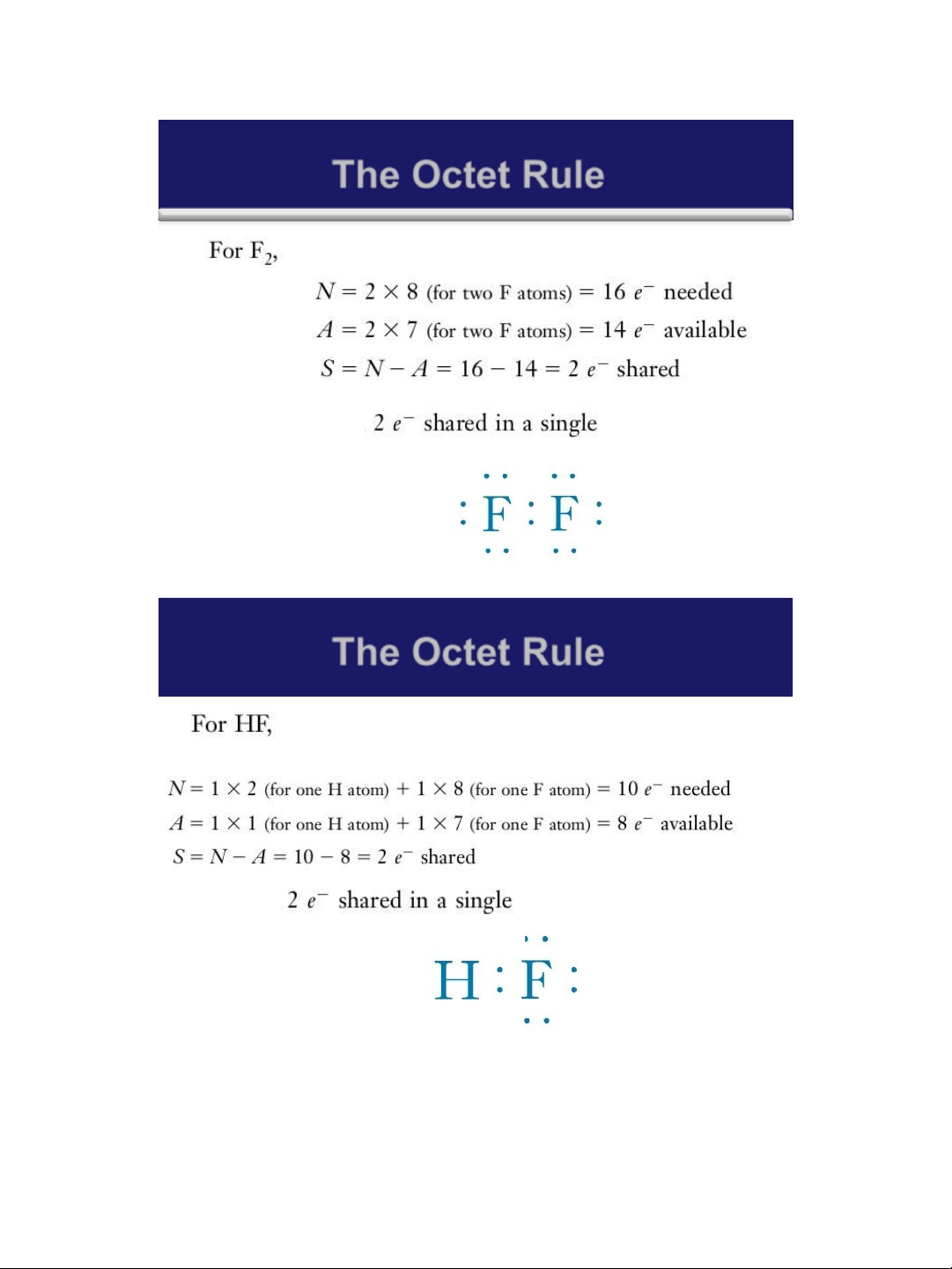
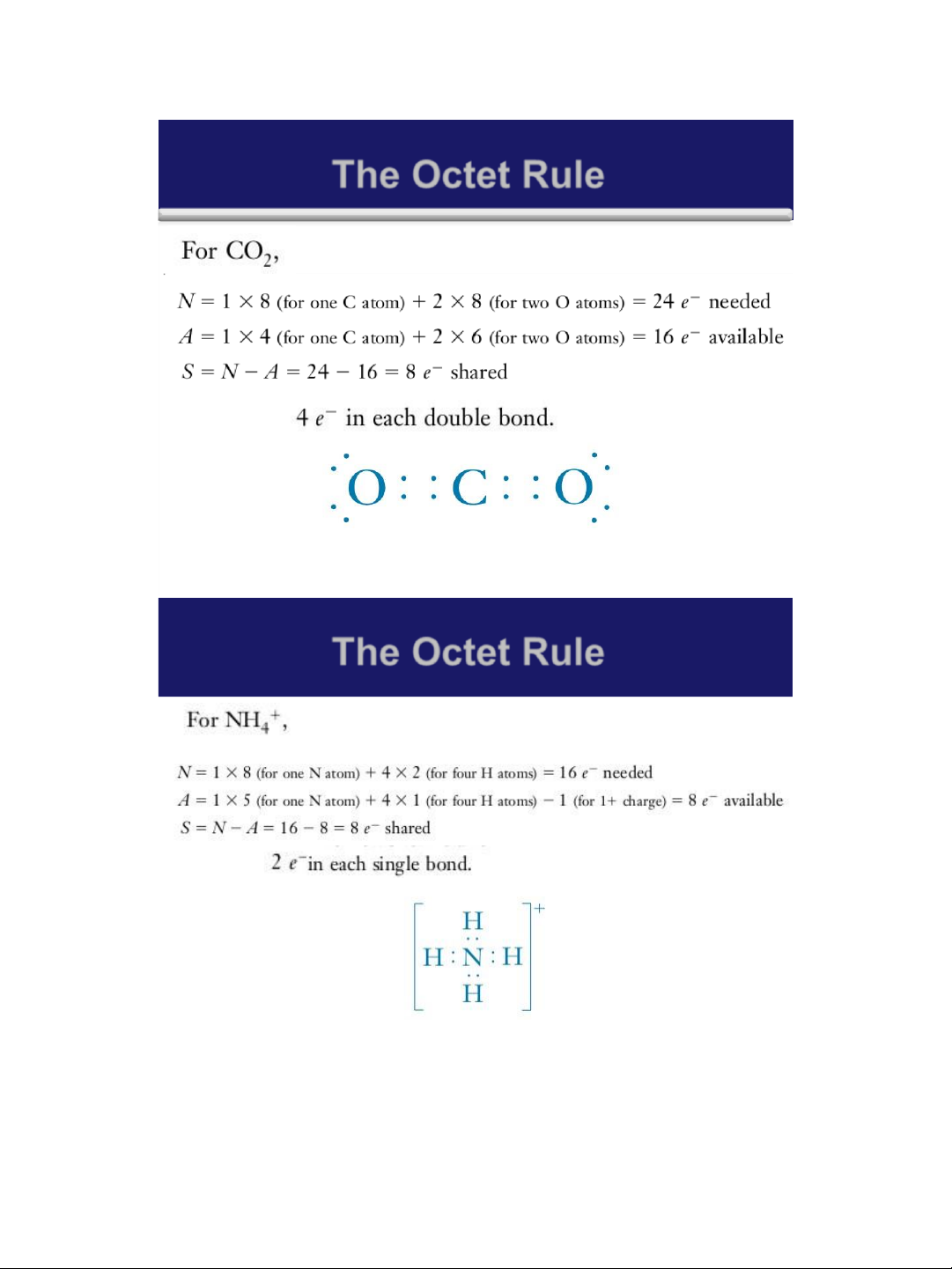
➢ A simple mathematical relationship: S = N - A
✓ S is the total number of electrons shared in the molecule or polyatomic ion.
✓ N is the total number of valence shell electrons
needed by all the atoms in the molecule or ion to
achieve noble gas configurations
✓ A is the number of electrons available in the
valence shells of all of the atoms. 7 lOMoAR cPSD| 58490434 18/01/2016 The Octet Rule 8 The Octet Rule 9 lOMoAR cPSD| 58490434 18/01/2016 The Octet Rule 10 The Octet Rule 11 lOMoAR cPSD| 58490434 18/01/2016 The Octet Rule 12 The Octet Rule 13 lOMoAR cPSD| 58490434 18/01/2016 The Octet Rule 14 The Octet Rule Home work:
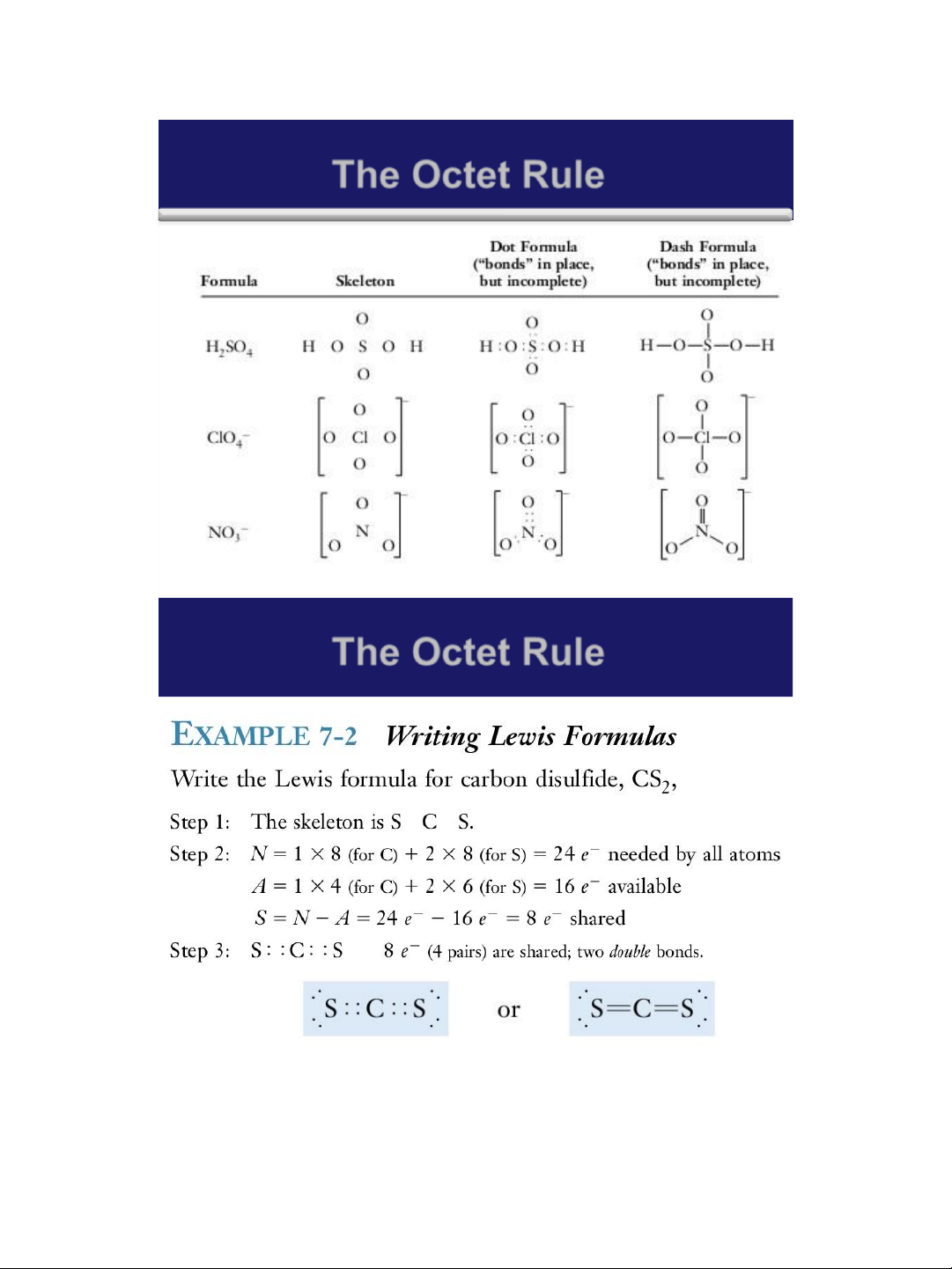
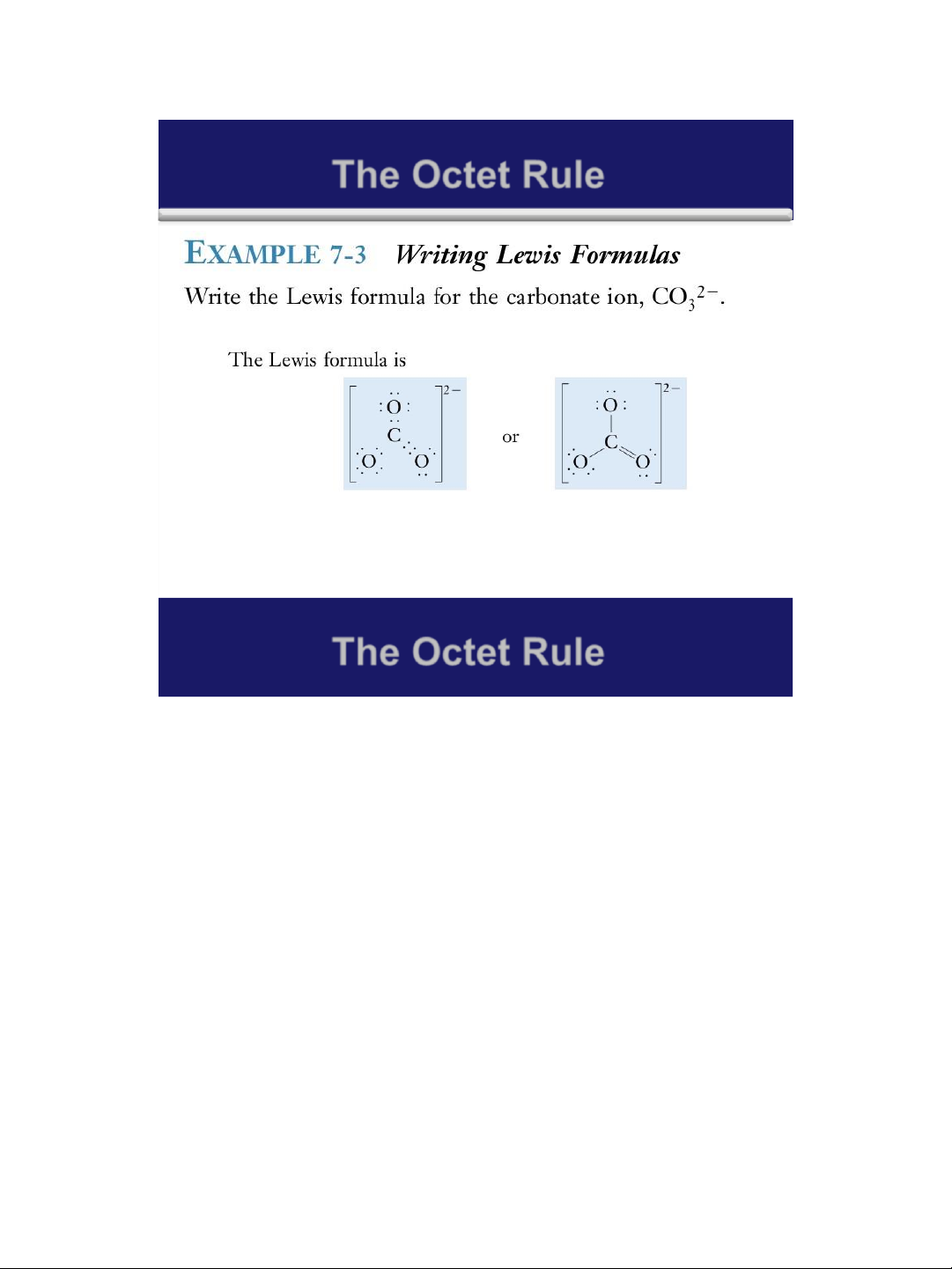
1. Write Lewis formulas for the following molecules: a. Br2 ; b. H2S ; c. NF3
2. Write Lewis formulas for the following: a. BCl3 b. TiCl2 c. BeBr2 15 lOMoAR cPSD| 58490434 18/01/2016 Covalent Bonding
❖ A covalent bond is formed when two atoms
share one or more pairs of electrons . ❖ Covalent bonding occurs when the
electronegativity difference between elements
( atoms ) is zero or relatively small . 16
Covalent Bonding Illustration
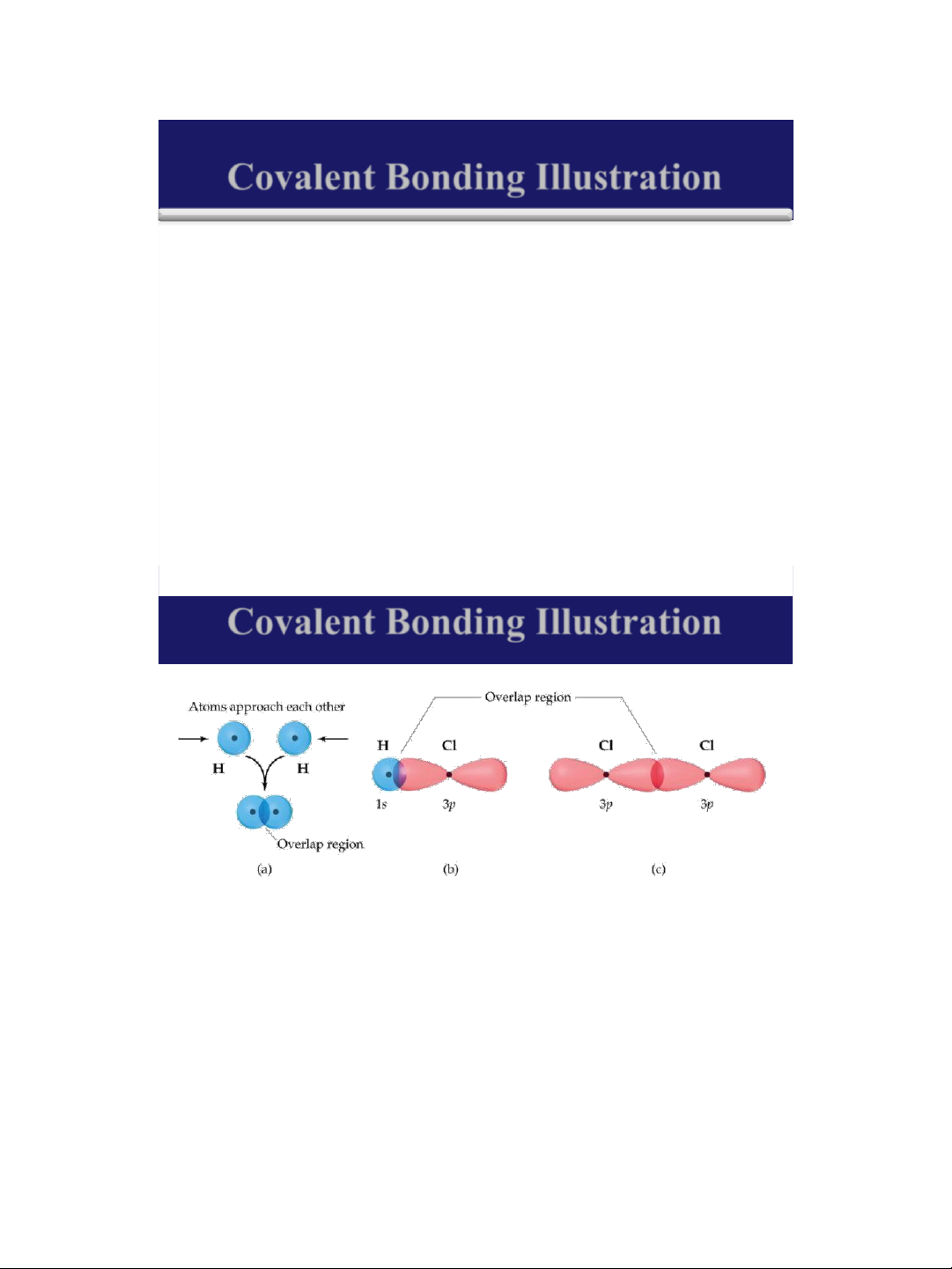
➢ As two hydrogen atoms approach, the electron of
each hydrogen atom is attracted by the nucleus of the other hydrogen atom.
➢ If these two electrons have opposite spins so that
they can occupy the same region (orbital).
➢ The electrons are shared between the two hydrogen
atoms, and a single covalent bond is formed.
➢ Both electrons are now in the orbital s of both hydrogen atoms. 17 lOMoAR cPSD| 58490434 18/01/2016 18
Covalent Bonding Illustration
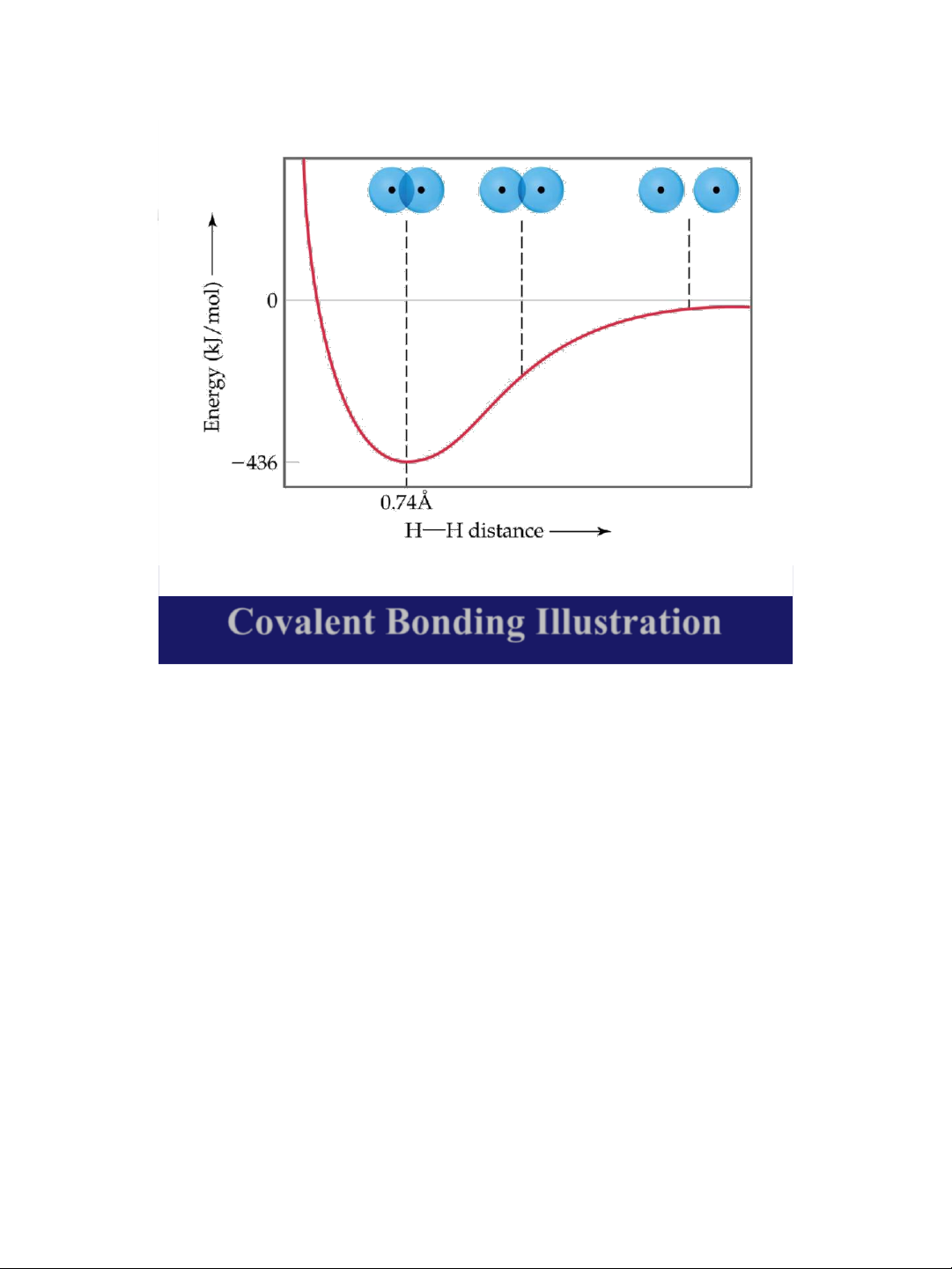
➢ At some distance the minimum energy is reached.
➢ The minimum energy corresponds to the bonding distance (or bond length).
➢ As the two atoms get closer, their nuclei begin to repel and the energy increases.
➢ At the bonding distance, the attractive forces between
nuclei and electrons just balance the repulsive forces
(nucleus-nucleus, electronelectron). 19 lOMoAR cPSD| 58490434 18/01/2016
Covalent Bonding Illustration
➢ Conclusion :
✓ Bonds form when orbitals on atoms overlap .
✓ There are two electrons of opposite spin in the overlap region .
✓ As the amount of overlap increases , the energy
of the interaction decreases . 20
Covalent Bonding Illustration 21 lOMoAR cPSD| 58490434 18/01/2016 Molecular Shapes
➢ Lewis structures give atomic connectivity: they tell us which
atoms are physically connected to which. However, do not show their overall shape.
➢ A molecule’s shape is determined by its bond angles.
➢ Consider CCl4: experimentally we find all Cl-C-Cl bond angles are 109.5 .
▪ The molecule cannot be planar.
▪ All Cl atoms are located at the vertices (đỉnh) of a
tetrahedron with the C at its center. 22 Molecular Shapes
❖ Molecular Shapes of CCl4 space-filling model ball-and-stick model 23 lOMoAR cPSD| 58490434 18/01/2016 Molecular Shapes
❖ We discuss how to explain the geometries of
molecules in terms of their electronic structures .
❖ We also explore two theories of chemical bonding : ✓ Valence Bond theory .
✓ and Molecular Orbital theory . 24 VSEPR Theory
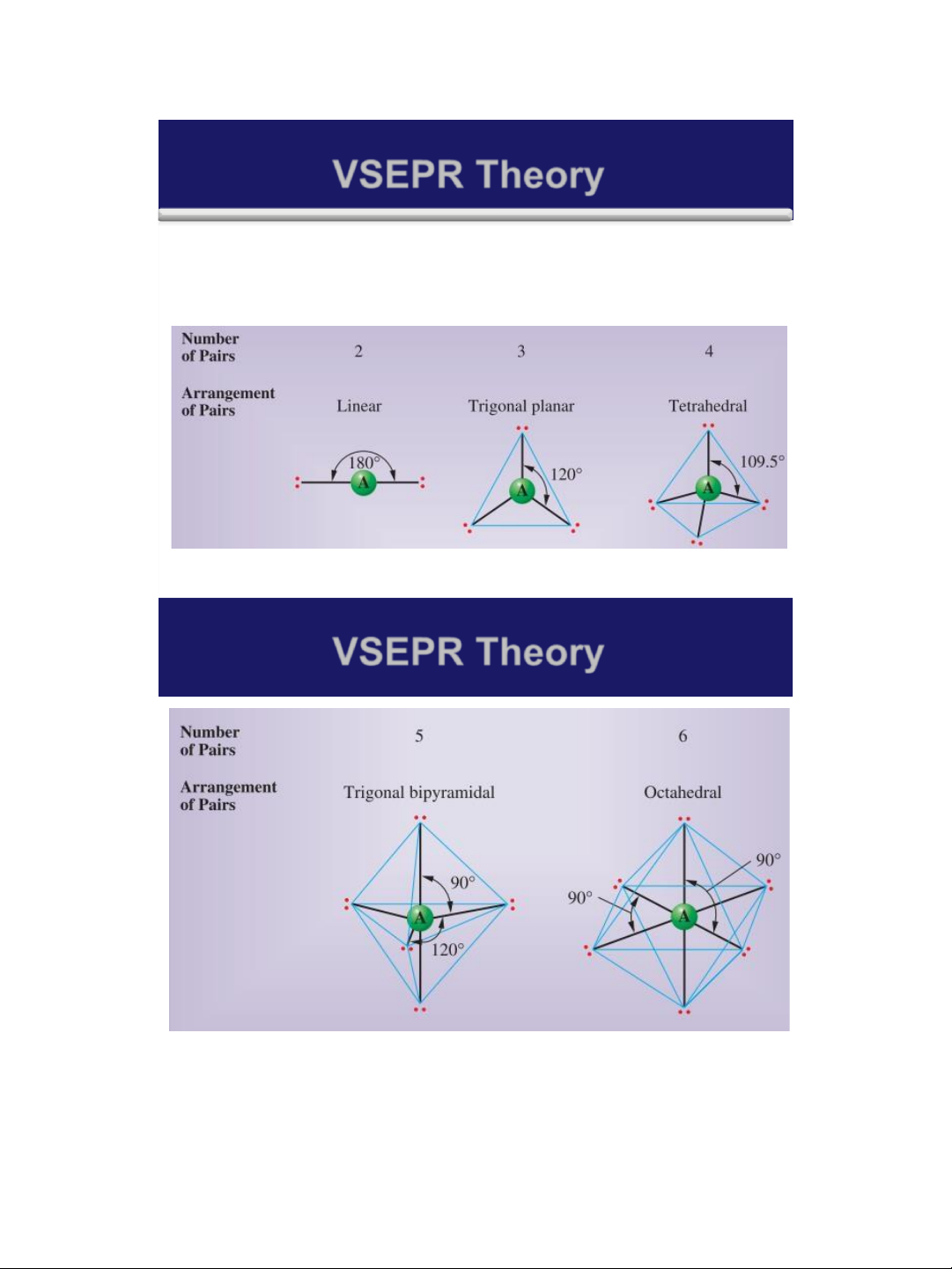
➢ The Valence-Shell Electron-Pair repulsion (VSEPR) model
predicts the shapes of molecules and ions by assuming that
the valence-shell electron pairs are arranged about each
atom so that electron pairs are kept as far away from one
another as possible, thus minimizing electron-pair repulsions.
➢ There are simple shapes for AB2, AB3 and AB4 molecules.
The acronym VSEPR is pronounced “vesper.” 25 lOMoAR cPSD| 58490434 18/01/2016 VSEPR Theory
➢ There are five possible arrangements of electron pairs
about an atom as shown in the following Figure : 26 VSEPR Theory 27 lOMoAR cPSD| 58490434 18/01/2016 Molecular geometry General rule:
➢ When you determine the geometry of a molecule
experimentally, you locate the positions of the
atoms, not the electron pairs.
➢ To predict the relative positions of atoms around a
given atom using the VSEPR model.
➢ The direction in space of the bonding pairs gives you the molecular geometry. 28
The Prediction of Geometry by the VSEPR Model
The step to follow in order to predict the geometry of an
AXn molecule or ion by the VSEPR method, 4 steps:
➢ Write the electron-dot formula (Lewis structure) from the molecular formula..
➢ Count the total number of electron pairs around the central atom.
➢ Determine the arrangement of these electron pairs about the central atom.
➢ Obtain the molecular geometry from the directions of the
bonding pairs for this arrangement. 29 lOMoAR cPSD| 58490434 18/01/2016
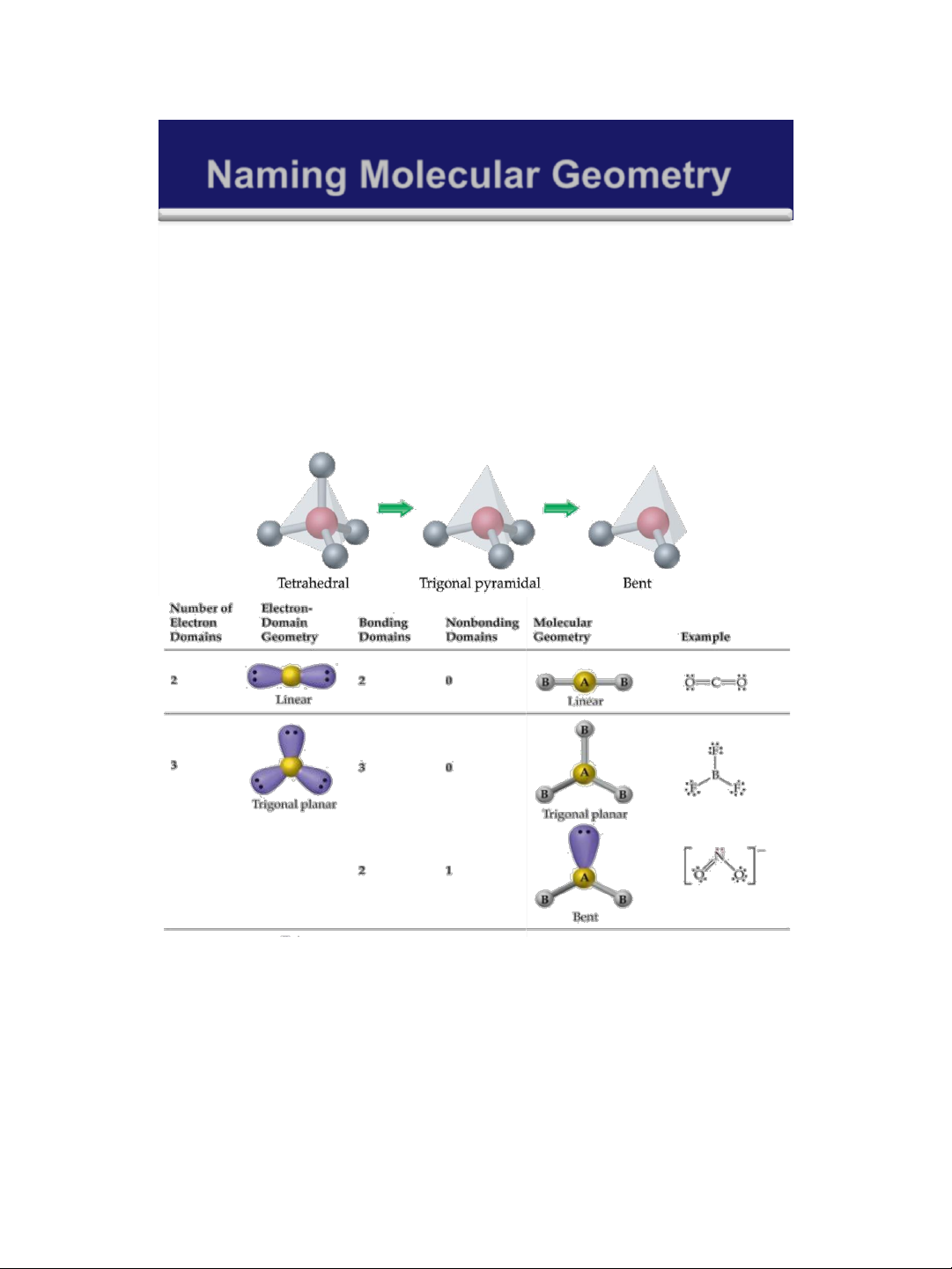
Naming Molecular Geometry Note:
➢ When considering the geometry about the central atom ,
we consider all electrons ( lone pairs and bonding pairs ).
➢ When naming the molecular geometry , we focus only on
the positions of the atoms . We ignore lone pairs in the molecular geometry 30
Trigonal pyramidal: tháp tam giác
Tetrahedral: tứ diện 31 lOMoAR cPSD| 58490434 18/01/2016
Trigonal pyramidal: tháp tam giác
Tetrahedral: tứ diện 32 33 lOMoAR cPSD| 58490434 18/01/2016 34
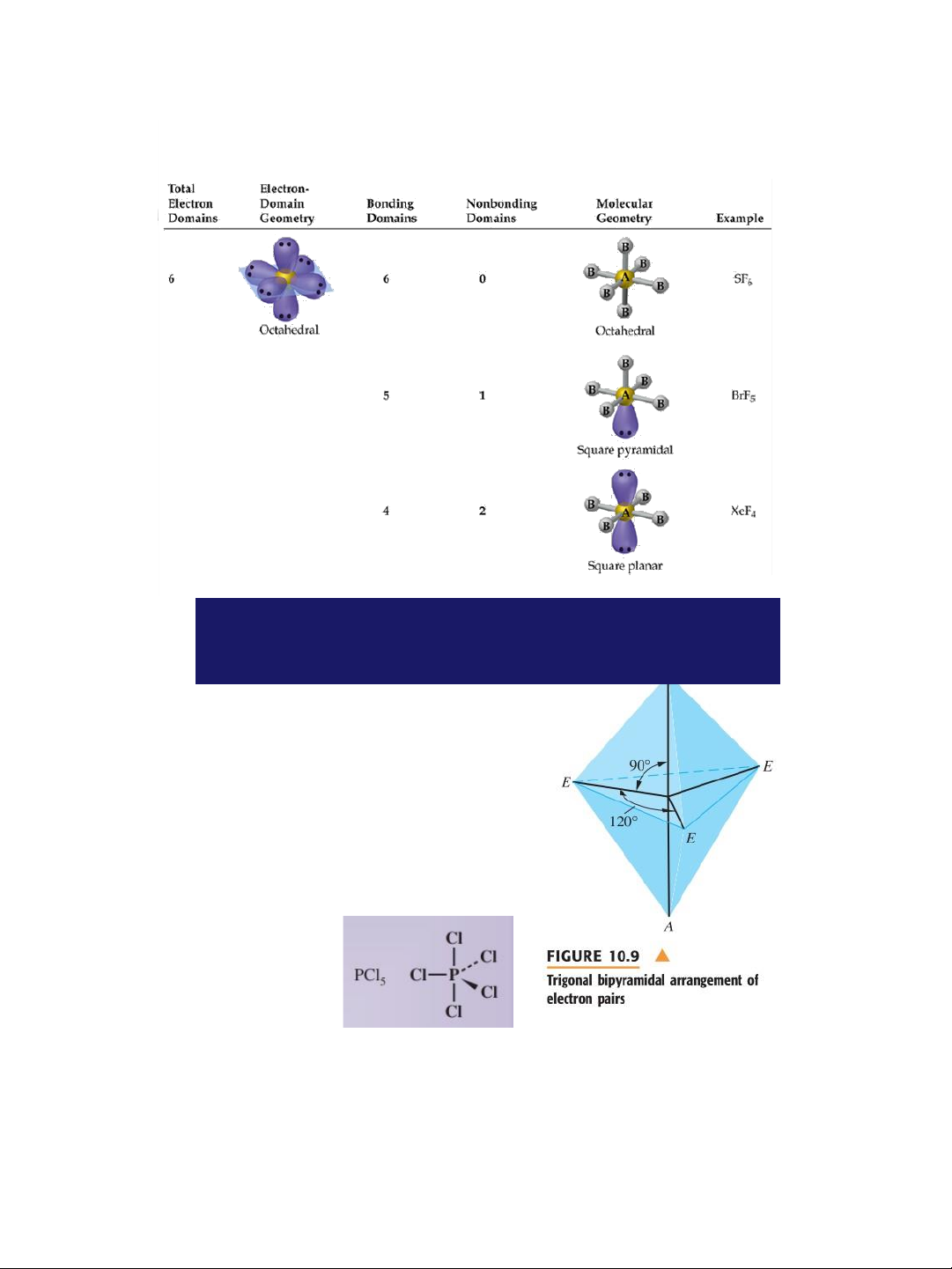
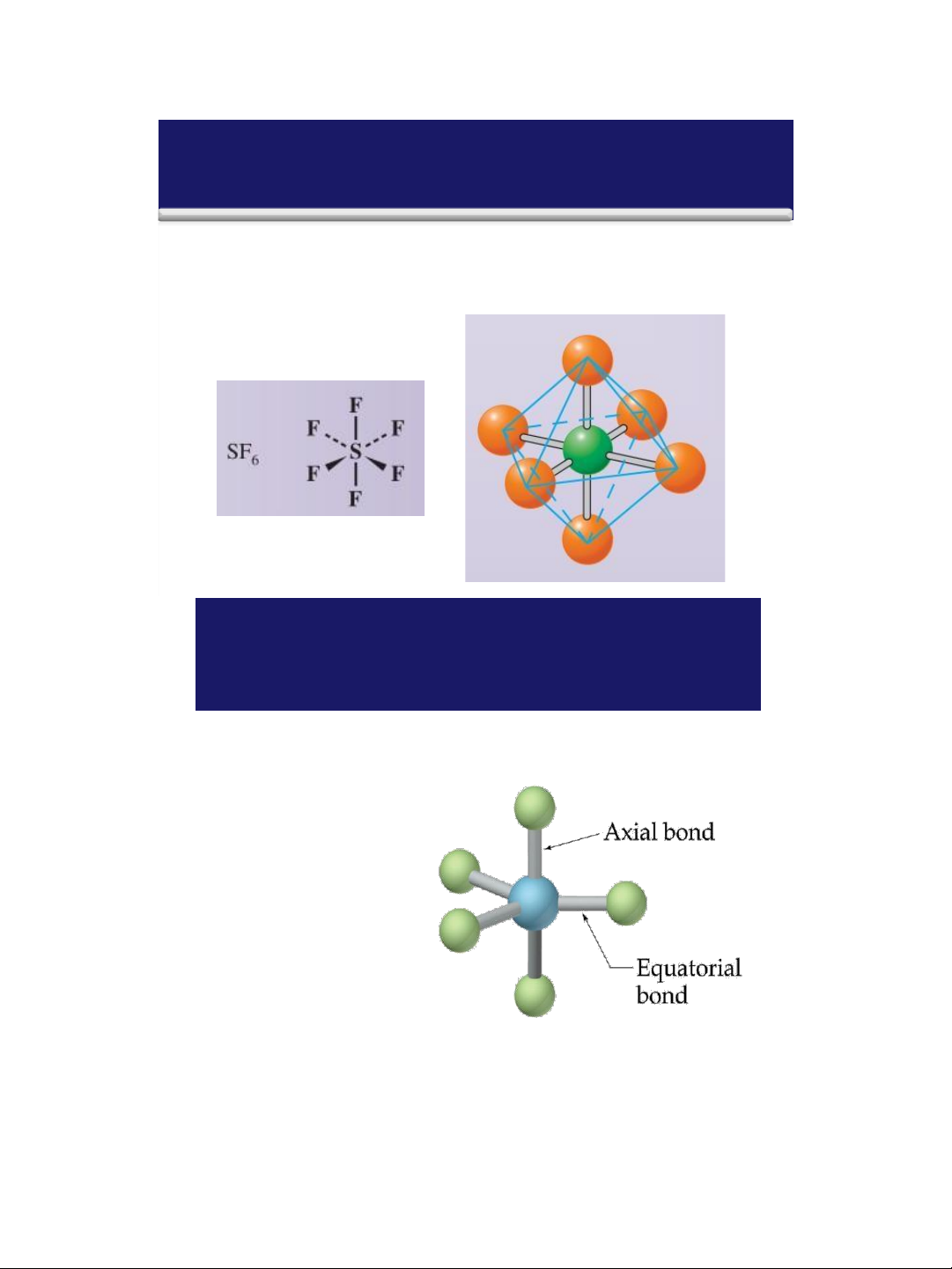
Molecules with Expanded Valence Shells Atoms that have expanded ➢ octets have b AB ipy ramidal) or 5 ( trigonal AB (octahedral) electron pair 6 geometries. ➢ For trigonal strubipy ctu ram res idal
there is a plane containing three
electrons pairs. The fourth and
fifth electron pairs are located above and below this plane. 35 lOMoAR cPSD| 58490434 18/01/2016
Molecules with Expanded Valence Shells
➢ For octahedral structures, there is a plane containing four
electron pairs . Similarly, the fifth and sixth electron pairs
are located above and below this plane . 36
Molecules with Expanded Valence Shells
➢ To minimize e--e- repulsion, lone pairs are always placed in equatorial positions. 37 lOMoAR cPSD| 58490434 18/01/2016
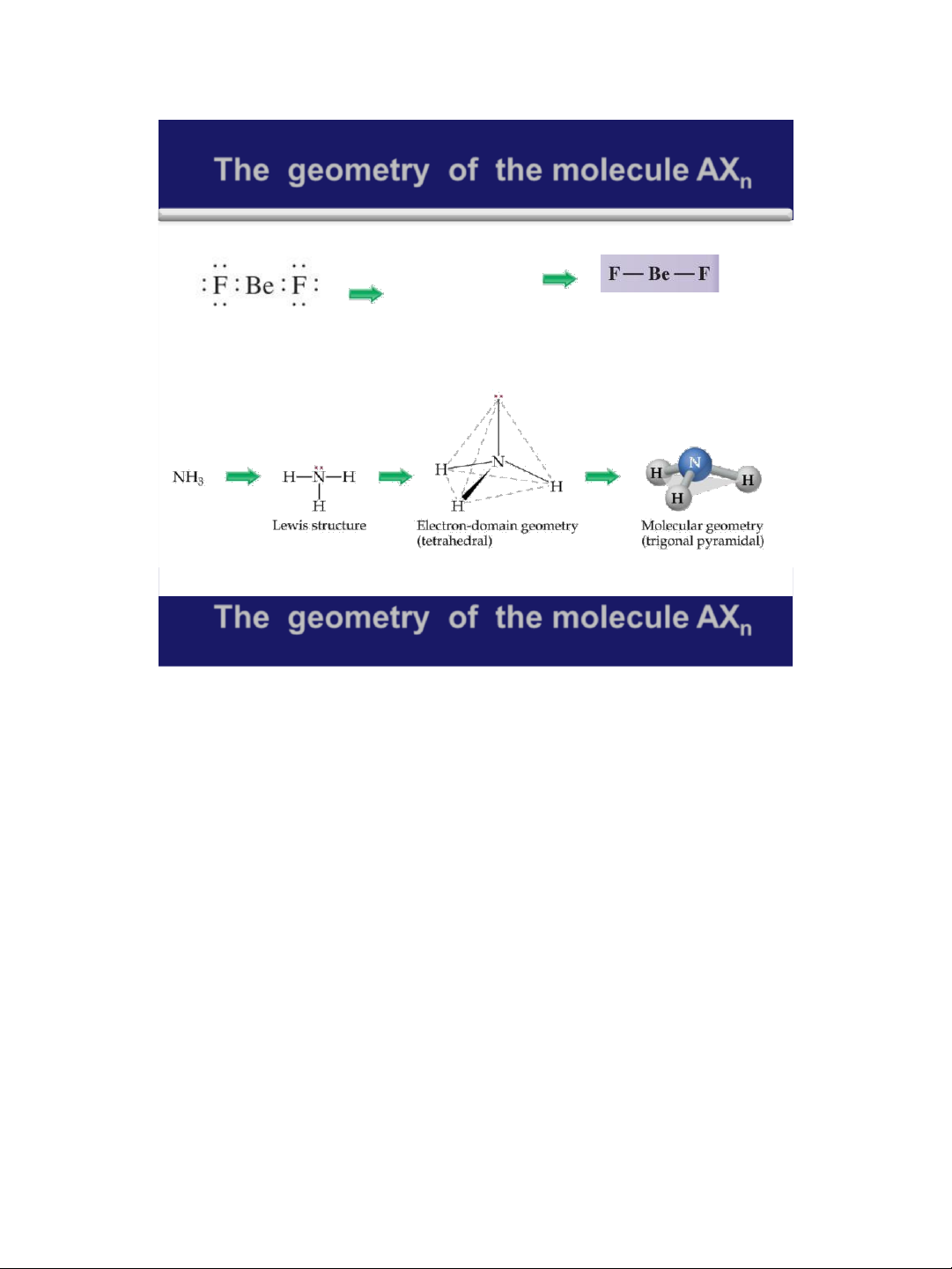
The geometry of the molecule AX n A linear arrangement There are two the V ( SEPR The geometry of electron pairs model) the BeF molecule 2 is linear 38
The geometry of the molecule AXn Home work:
1. Predict the geometry of the following molecules, using the
VSEPR method: a. BeCl2 ; b. SO2 ; c. SiCl4 .
2. Predict the geometry of the following molecules, using the VSEPR method: a. BF3 ; b. PF3 39



