



Preview text:
lOMoAR cPSD| 23136115 Homework 1
1) What is the difference between qualitative and quantitative analysis? What is the purpose
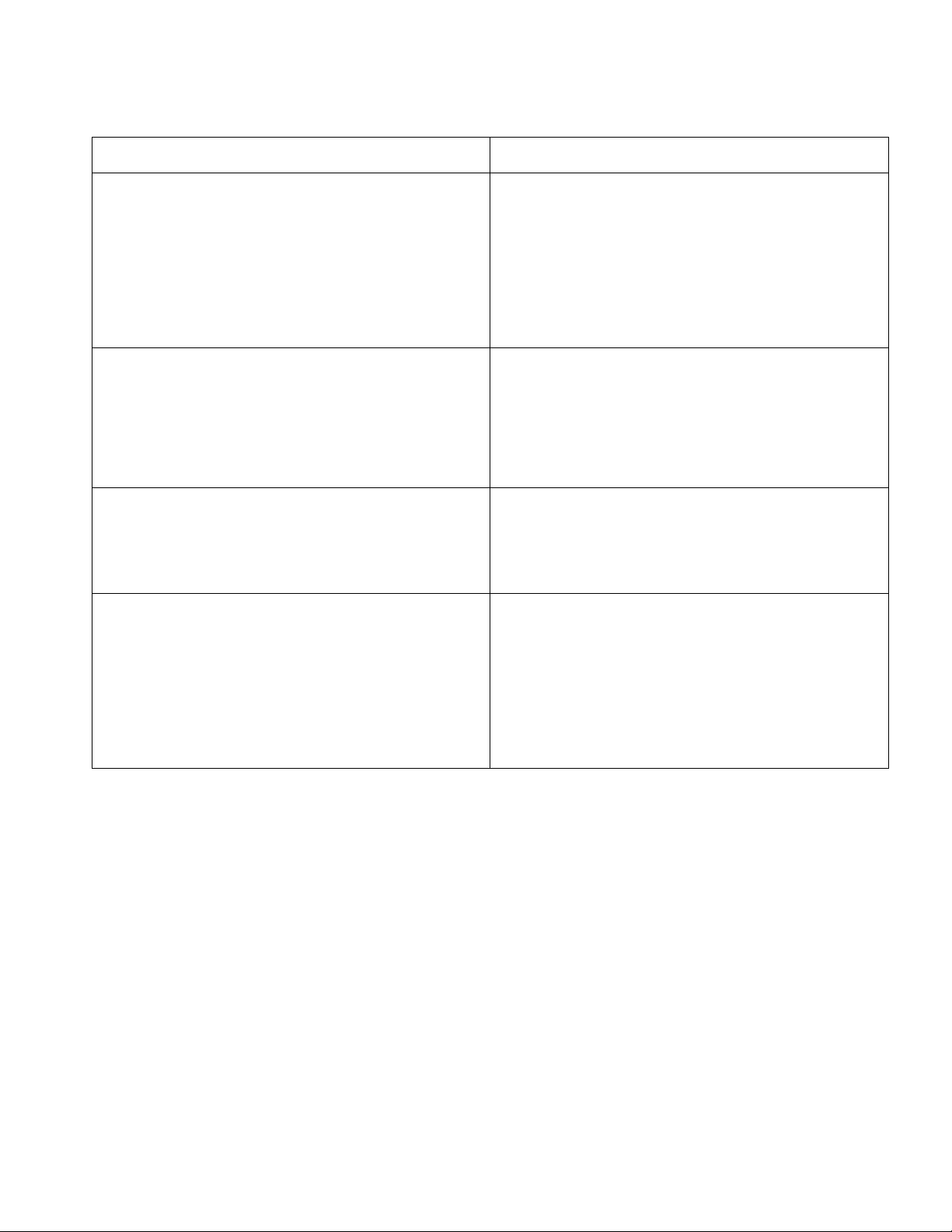
of a calibration curve? Qualitative Analysis Quantitative analysis
The data collected is non - numerical and often The data collected is numerical and typically
in the form of words, images, observations, or involves measurements or counts. It focuses
narratives. It focuses on understanding and
on analyzing the data statistically to uncover
interpreting the underlying meanings, themes, patterns, trends, relationships. and patterns within the data.
Qualitative analysis emphasizes subjective
Quantitative analysis emphasizes an objective
interpretation and the exploration of complex
approach, numerical measurement. It often
phenomena. It often involves in-depth
involves surveys, experiments, or analysis of
interviews focus groups, observations. pre-existing.
Involves techniques such as thematic analysis
Involves techniques such as statistical tests,
content analysis, discourse analysis or
data modeling or hypothesis testing. grounded theory.
Qualitative analysis typically results in rick,
Quantitative analysis aims for generalizability
detailed insights that are context - specific. Its it seeks to make inferences about larger
findings are often context - dependent, making populations based on a representative sample.
it engine to generalize the results to larger populations.
⇒ Both qualitative and quantitative analysis approaches have their strengths and limitations.
The purpose of a calibration curve is to establish the relationship between the concentration of
an analyte (substance or chemical compound) and the signal response produced by a
measurement instrument. It involves preparing standard solutions with known concentration of
the analyte and analyzing each solution using the instrument. Calibration curves are crucial for
accurate quantitative analysis, accounting for variations and providing a basic for comparison.
2) A solution contains 12.6 ppm of dissolved Ca(NO -
3)2 (which dissociates into Ca2+ + 2NO3 ).
Find the concentration of NO -
3 in parts per million (ppm).
The mass fraction of NO₃⁻ in Ca(NO ) :₃ ₂ Ca(NO - 3)2 -> Ca2+ + 2NO3 lOMoAR cPSD| 23136115 nCa(NO3)2 = 2nNO3-
Molar mass of Ca(NO3)2: MCa(NO3)2 = 40 + 2 × (14 + 3 × 16) = 164 g/mol
Molar mass of NO₃⁻: MNO₃⁻ = 14 + 3 × 16 = 62 g/mol We
have the mass concentration of Ca(NO3)2:
CCa(NO3)2 = 12 ppm = 12 mg/L = 12 × 10-3 g/L
The concentration of NO₃⁻ C ×62
NO₃⁻ = 12×10−3× 2164 =9.52 ppm
The concentration of NO₃⁻ in the solution is approximately 9.52 ppm.
3) A 48.0 wt% solution of HBr in water has a density of 1.50 g/mL.
(a) Find the formal concentration of HBr.
(b) How much solution is required to prepare 0.250 L of 0.160 M HBr? a. Molar
mass of HBr: MHBr = 1.00 + 79.91 = 80.91 g/mol Assume: A solution volume of
V = 1.000 L = 1000 mL msolution = d × V = 1.50 × 1000 = 1500 g mHBr = 48% × 1500 = 720g mHBr 720 nHBr=
MHBr=80.91=8.90mol nHBr 8.90 CHBr = = =8.90 M V 1.000
The formal concentration of HBr is approximately 8.90 M.
b. Using the dilution equation: C1V1 = C2V2 C2V2
0.160M ×0.250 L V1= C1 = 8.90 M =0.004495 L
Thus, we will need approximately 4.50 mL of the 48.0 wt% HBr solution to prepare 0.250 L of 0.160 M HBr. lOMoAR cPSD| 23136115
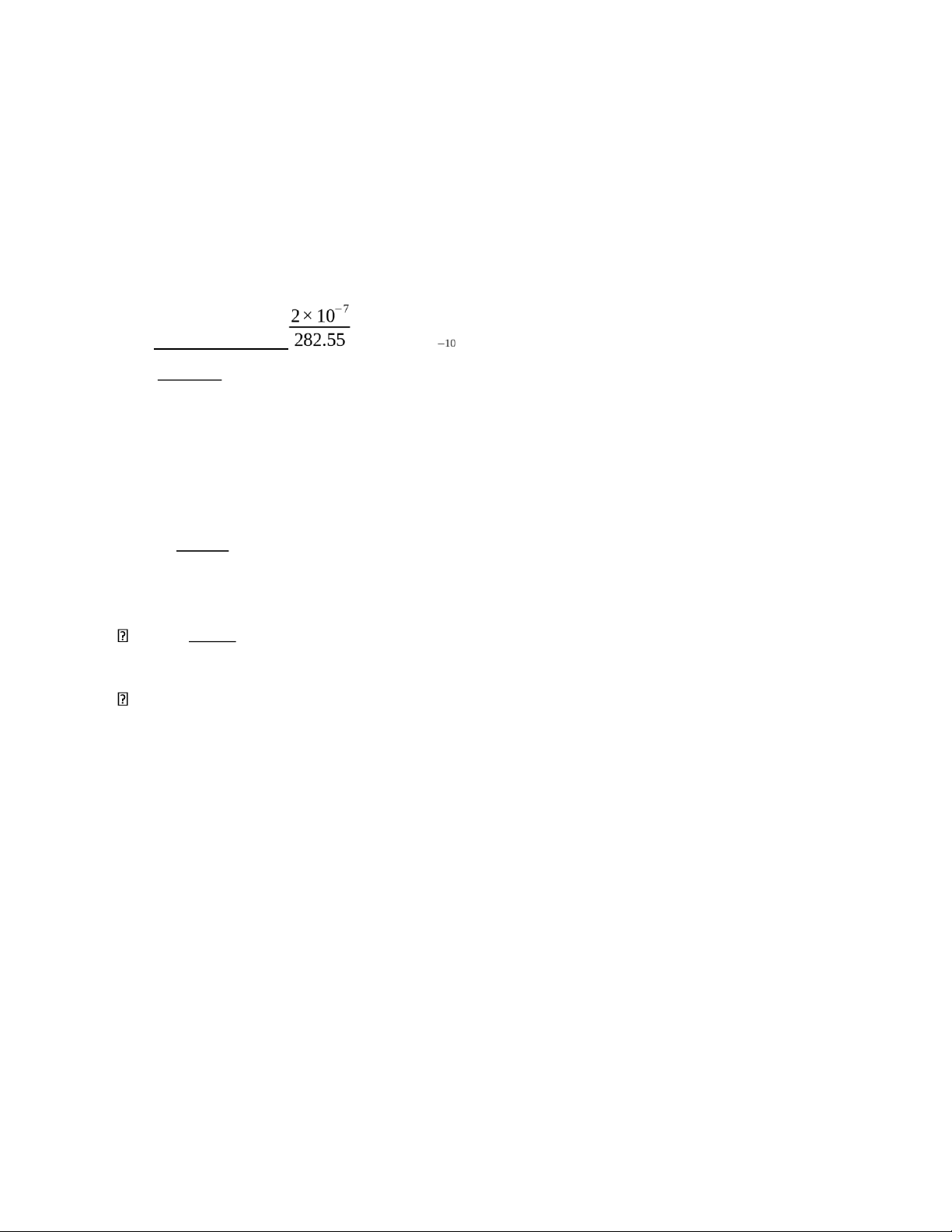
4) The concentration of the alkane C20H42 (FM 282.55) in a particular sample of rainwater is
0.2 ppb. Assume that the density of rainwater is close to 1.00 g/mL and find the molar
concentration of C20H42
The concentration of C₂₀ ₄₂H : Cppb = 0.2 ppb = 0.2×10-3 mg/L = 2×10-4 mg/L
Mass of C₂₀ ₄₂H is 2 × 10-4 mg per 1L of the solution:
mC H ₂₀ ₄₂ = 2×10-7 g/L
The molar concentration of C₂₀ ₄₂H : numbersof moles M= =
=7.08×10 M volume∈L 1.00
Thus, the molar concentration of C₂₀ ₄₂H in the rainwater is approximately 7.08 ×10-10 M
5) How many grams of perchloric acid, HClO4, are contained in 37.6 g of 70.5 wt% aqueous
perchloric acid? How many grams of water are in the same solution? mHClO 4 wt HClO4= msolution ×100% mHClO 4 70.5%= ×100% 37.6
mHClO =70.5%×37.6=26.508 g 4
The solution contains 26.508 g of perchloric acid (HClO
).₄ mwater = msolution – mHClO4 = 37.6 – 26.508 = 11.092 (g)
The solution contains 11.092 g of water.
Therefore, in 37.6 g of 70.5 wt% aqueous perchloric acid:
There are approximately 26.51 g of perchloric acid (HClO ).₄
There are approximately 11.09 g of water.
6) A cell in your adrenal gland has about 2.5 x 104 tiny compartments called vesicles that
contain the hormone epinephrine (also called adrenaline).
(a) An entire cell has about 150 fmol of epinephrine. How many attomoles (amol) of
epinephrine are in each vesicle?
(b) How many molecules of epinephrine are in each vesicle? lOMoAR cPSD| 23136115 1 fmol = 10-15 mol 1 amol = 10-18 mol
a) The moles of epinephrine in cell = 150 × 10-15 mol
Moles of epinephrine in each vesicle
totalmoles∈cell 150×10−15 18
¿ totalvesicle∈cell=
2.5×104 =6×10 mol=6 amol
Thus, there are 6 amol of epinephrine in each vesicle.
b) The numbers of molecules present in 1 mol of epinephrine:
= 6.04 × 10−18mol × 6.023×1023molecules =3,637,892 vescile
1amol 1mol
Thus, each vesicle contains approximately 3.63×106 molecules of epinephrine.
7) The concentration of glucose in human blood ranges from about 80 mg/100 mL before
meals to 120 mg/100 mL after eating. Find the molarity of glucose in blood before and after eating. For 80 mg/dL:
Convert mg to g: 80 mg × (1 g/1000 mg) = 0.08 g
Convert to per liter: 0.08 g/dL = 0.08 g/0.1 L = 0.8 g/L For 120 mg/dL:
Convert mg to g: 120 mg × (1 g/1000 mg) = 0.12 g
Convert to per liter: 0.12 g/dL = 0.12 g/0.1 L = 1.2 g/L
Before meals, the concentration of glucose in human blood is about 80 mg/dL. To find the
molarity, we need to convert the mass of glucose to moles using its molar mass. Glucose has a
molar mass of 180.16 g/mol. Therefore, the molarity before meals is:
Molarity = (80 mg/dL × 1 g/1000 mg) / (180.16 g/mol × 0.1 L) = 0.0444 mol/L
After eating, the concentration of glucose in human blood is about 120 mg/dL. Using the same
calculation, the molarity after eating is:
Molarity = (120 mg/dL × 1 g/1000 mg) / (180.16 g/mol × 0.1 L) = 0.0667 mol/L = 6.667 × 10-3 M
8) How many grams of boric acid, H3BO3 (FM 61.83), should be used to make 2.00 L of lOMoAR cPSD| 23136115 0.050 0 M solution?
Number of moles n = 0.0500 mol/L × 2.00 L = 0.100 mol
mH3BO3 = 0.100 mol × 61.83 g/mol = 6.183g
You should use 6.18 grams of boric acid (H BO ) to make 2.00 L of a 0.0500 M solution.₃ ₃
9) What do the symbols “TD” and “TC” mean on volumetric glassware? Describe how to
prepare 250.0 mL of 0.15 M HCl with a volumetric flask from concentrated HCl 38% (density = 1.19 g/ml)
- In volumetric glassware, the symbol "TD" and "TC" are both used to indicate the accuracy
class of the glassware specify the condition under which the glassware is calibrated.
+ TD: "To deliver " (TD) is used for volumetric glassware such as pipettes and burette, these
instruments are designed to dispense a specific volume accurately.
+ TC: "To contain" (TC) is used for volumetric glassware such as flasks and volumetric
cylinders, these instruments are designed to accurately measure a specific volume of liquid.
- Step 1: Obtain a clean and dry volumetric flask (capacity of 250.0 mL)
- Step 2: To prepare the solution, weigh the solid reagent. Can use the formula M = M×V to determine the mass (m)
- Step 3: Dissolve the solute in a suitable solvent, such as water, in a pa separate container.
Ensure that it is fully dissolved.
- Step 4: Transfer the dissolved solute quantitatively into the volumetric flask using a funnel ora pipette.
- Step 5: Add more solvent, typically water, to the volumetric flask until the solution level is
just below the calibration mark on the neck of the flask.
- Step 6: Then use a dropper or a pipette, add the solvent to the flask drop y drop until the
bottom of the meniscus touches the calibration mark
- Step 7: Stopper the flask and invert it several times to ensure uniform mixing, Steps: Finally,
label the flask with the content identifier, concentration, the solution should be precisely at the marked volume (250.0ml)
=> So, by following these steps, you should be able to prepare 250.0 mL of a 0.1500M
solution using a volumetric flask
10) A 6.42% (w/w) Fe(NO3)3 (241.86 g/mol) solution has a density of 1.059 g/mL. Calculate
(a) the molar analytical concentration of Fe(NO3)3 in this solution. (b) the mass in grams lOMoAR cPSD| 23136115
of Fe(NO3)3 contained in each liter of this solution a. We have: density Fe(NO3)3 = 1.059
g/mL The weight-by-weight volume = 6.42%
So, the molar mass of Fe(NO3)3 = 241.86 g/mol 1.059g 6.42g 1mol Molarity of Fe(NO3)3 ¿
−3× 100 g × 241.86
g/mol=0.281(M) 10 L
b. The mass of Fe(NO3)3 in 1L:
0.281mol 241.86 68.0 g mFe(NO3)3 = 1 L × mol = L of thesolution
11)Calculate the equivalent weights of the following substances as acids or bases: (a)
HCl, (b) Ba(OH)2, (c) HOOC-COOH
(b)Calculate the molarity of a 0.250 eq/L solution of each of the acids or bases above. molecular weight 36.5 a. HCl
chargeoncation/anion= 1 = 36.5 g/eq
Ba(OH)2 = 171.3/2 = 85.65 g/eq HOOC-COOH = 90/2 = 45 g/eq
b. The concentration of 0.25 eq/L of HCl
=> CHCl = 36.5 g/eq × 0.25 eq/L = 9.125 (g/L)
The molarity of HCl: MHCl = C/MW = 0.25 (M)
The concentration of 0.25 eqL of Ba(OH)2
CBa(OH)2 = 85.65 × 0.25 = 21.4125 (g/L)
The molarity of Ba(OH)2 : MBa(OH)2 = 21.4125/171.3 = 0.125 (M)
The concentration of 0.25 eq/L of HOOC-COOH
CHOOC-COOH = 45 ×0.25 = 11.25 (g/L)
The molarity of HOOC-COOH: MHOOC-COOH = 11.25/90 = 0.125 (M)




