



Preview text:
lOMoAR cPSD| 23136115
Homework 3 Chemical equilibria
1) The solubility products for a series of iodides are
List these compounds in order of decreasing molar solubility in (a) water and (b) 0.20 M NaI. CuI(s) ↔Cu+ + I–
Ksp = [Cu+][I–] = 1 × 10–12 AgI(s) ↔Ag+ + I–
Ksp = [Ag+][I–] = 8.3 × 10–17
PbI2(s) ↔Pb2+ + 2I–
Ksp = [Pb2+][I–]2 = 7.1 × 10–9 = S(2S)2 = 4S3
BiI3(s) ↔Bi3+ + 3I–
Ksp = [Bi3+][I–]3 = 8.1 × 10–19 = S(3S)3 = 27S4 (a) For CuI, S = [Cu+] = [I–] = M For AgI, S = [Ag+] = [I–] = M For PbI2, S = M For BiI M
So, solubilities are in the order PbI2> BiI3> CuI > AgI (b)
For CuI, S = 1 × 10–12/0.20 = 5 × 10–12 M For
AgI, S = 8.3 × 10–17/0.20 = 4.2 × 10–16 M For
PbI2, S = 7.1 × 10–9/(0.20)2 = 1.8 × 10–7 M
For BiI3, S = 8.1 × 10–19/(0.20)3 = 1.0 × 10–16 M
So, solubilities are in the order PbI2 > CuI > AgI >BiI3
2) At 25°C, what are the molar H3O+ concentration in 0.0300 M C6H5COOH? For benzoic acid, Ka = 6.28 × 10–5.
Call benzoic acid HBz and the benzoate anion Bz–
HBz + H2O ↔Bz– + H3O+ Ka=¿¿
Mass balance cHBz = [HBz] + [Bz–] = 0.0300 [Bz–] = [H3O+]
Thus, [HBz] = 0.0300 – [Bz–] = 0.0300 – [H3O+] ¿¿¿
Solving the quadratic or solving by iterations gives,
[H3O+] = 1.34 × 10–3 M so [OH–] = 1.00 × 10–14/ 1.34 × 10–3 = 7.5 × 10–12 M
3) Calculate pH of the solution prepared by dissolving 8.00 mmol of sodium acetate in 200 mL of lOMoAR cPSD| 23136115 0.100 M acetic acid.
− (sodium acetate): 0.008mol=0.040 M Concentration of A 0.200 L
Concentration of HA (acetic acid): Given as 0.100M.
pKa of acetic acid ≈ 4.74. Henderson-Hasselbalch formula: 0.040 pH = 4.74 + log( )=4.342 0.100
The pH of the solution prepared by dissolving 8.00 mmol of sodium acetate in 200 mL of 0.100 M
acetic acid is approximately 4.34.
4) What is the pH of a buffer solution prepared by mixing 200 ml of 0.5M sodium acetate and 800 ml
of 0.1M acetic acid which is 1.3% ionized in the solution?
[Acetate] = 200 mL X 0.5 M / 1000 mL = 0.10 M
[acetic acid] = 800 mL X 0.1 M / 1000 mL = 0.08 M
pH = pKa + log [acetate]/[acetic acid]
pH = 4.75 + log (0.10/0.08) = 4.85
The initial % ionization of the acetic acid is irrelevant once you add sodium acetate to the solution to form a buffer.
5) What mass of sodium formate (HCOONa) must be added to 500.0 mL of 1.00 M formic acid to produce
a buffer solution that has a pH of 3.50? pH=3.50=pKa+log¿¿¿ 3.50=3.74+log¿¿¿ ¿¿ mmolHCOOH 500mL×1.00 =500mmol mL
So, amount of HCOO– needed = 0.575 × 500 mmol = 287.5 mmol
287.5 mmol × 10–3 mol/mmol = 0.2875 mol HCOO–
Mass HCOONa = 0.2875 mol × 67.997 g/mol = 19.6 g
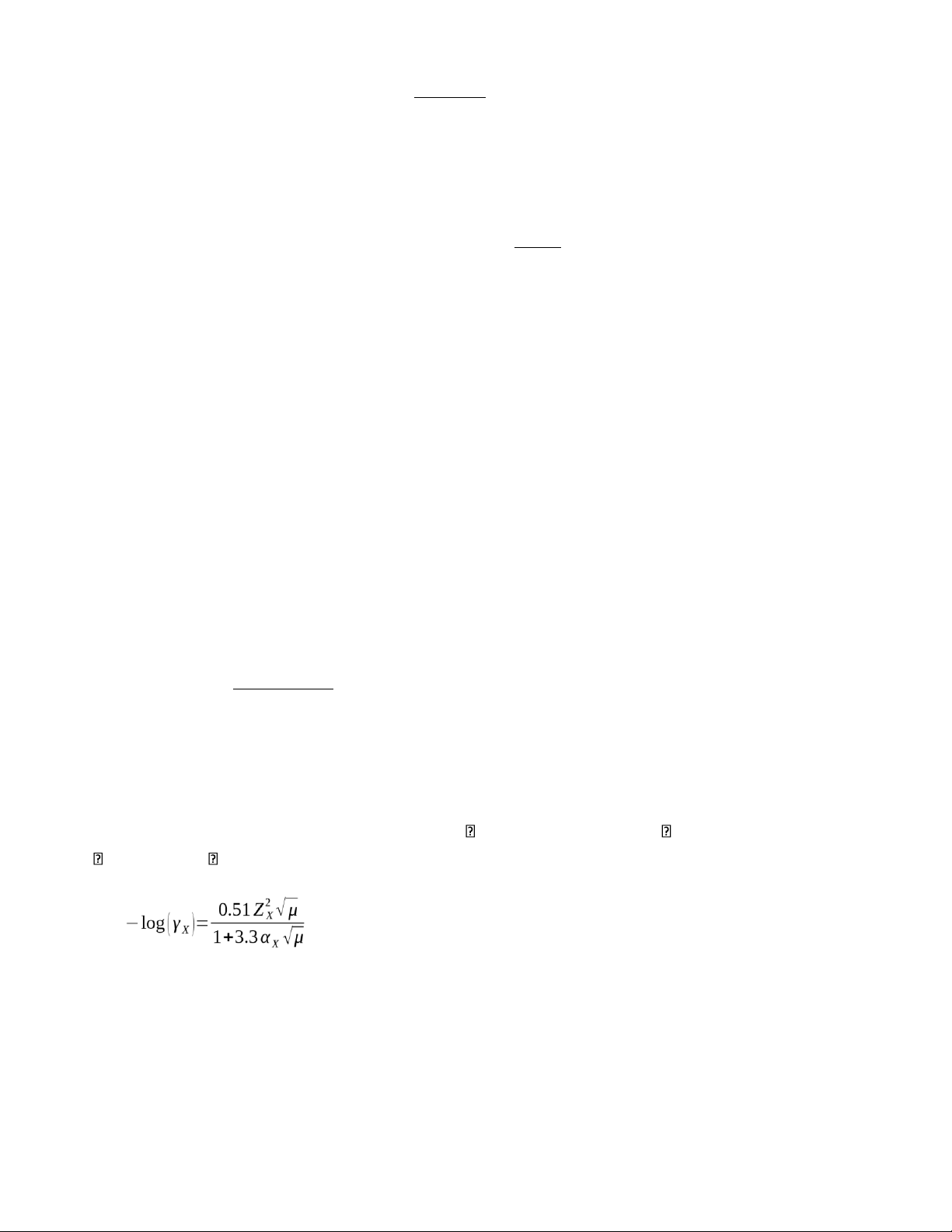
6) Calculate the activity coefficient of (a) Fe3+ at = 0.062 and (b) Ce4+ at = 0.070.
X of Fe3+: 0.9, X of Ce4+: 1.1 Debye- Hückel equation: (a) −log¿¿ −log¿¿
γ Fe3+¿=0.2203 ≈ 0.22¿ (b) −log¿¿ −log¿¿
γC e4+¿=0. 0792≈0. 08¿ lOMoAR cPSD| 23136115
7) For a solution in which = 8.0 x 10-2, calculate K’sp for AgSCN. For Ag+, Ag+ = 0.25. For SCN–, SCN– = 0.35. We must use For Ag+, α + – –
Ag+ = 0.25. At μ = 0.08, γAg = 0.7639; For SCN–, αSCN = 0.35 and γSCN = 0.7785 retaining
insignificant figures for later calculations. ' K sp Ksp = − = ¿ SCN (0.7639)(0.7785) ¿
8) Use activities to calculate the molar solubility of Zn(OH)2 in (a) 0.0200 M KCl and (b) 0.0300 M K2SO4. =
Zn(OH)2(s) ↔ Zn2+ + 2OH– Ksp 3.0 × 10–16
(a) μ = ½[0.02 × 12 + 0.02 × 12] = 0.02 Using Debye-Hückel equation, γZn2+ = 0.5951 γOH- = 0.867
K’sp = αZn2- α2OH- = γZn2+[Zn2+] × γ2OH- [OH-]2 3.0×10−16 [Zn2+][OH-]2 = 3.0×10 ¿= 2 ¿ OH (0.5951) (0.867 )
Solubility = S = [Zn2+] = ½ [OH-] S(2S)2 = 6.706 × 10-16 S = ( 6.706×10 ) −16
31=5.5×10−6 M 4 (b)
μ = ½[2 × 0.03× 12 + 0.03 × 22] = 0.18 Using Debye-Hückel equation, γ 2+ - Zn = 0.3386 γOH = 0.7158
K’sp = αZn2- α2OH- = γZn2+[Zn2+] × γ2OH- [OH-]2 3.0×10−16 [Zn2+][OH-]2 = 3.0× 10 ¿= 2 ¿ OH (0.3386) (0.7158)
Solubility = S = [Zn2+] = ½ [OH-] S(2S)2 = 1.729 × 10-15 lOMoAR cPSD| 23136115 S = ( 1.729×10 ) −15
13=7.6×10−6 M 4
9) Calculate the molar solubility of BaSO4 in a solution in which [H3O+] is
(a) 3.5 M. (b) 0.080 M.
Ksp (BaSO4) = 1.1 × 10–10, H2SO4 (Ka1: large, Ka2: 1.2 x 10-2) BaSO 2– – 4 <-> Ba2+ + SO4
Ksp = 1.1 × 10–10 HSO4 + H2O <-> H 2– 3O+ + SO4 K2 = 1.02 × 10–2 S = solubility = [Ba2+] [Ba2+][SO 2– 4 ] = 1.1 × 10–10 ¿¿ Mass balance requires that [Ba2+] = [SO 2– 4 ] + [HSO4]
The unknowns are [Ba2+], [SO 2– 4 ], and [HSO4]
We have 3 equations and 3 unknowns so no approximations are needed.
Substituting eqation (2) into (3) gives ¿
Substituting equation (1) to eliminate [SO 2– 4 ], gives ¿ S=¿ (a) S=¿ (b) S=¿
10) Dilute NaOH is introduced into a solution that is 0.050 M in Cu2+ and 0.040 M in Mn2+.
Ksp of CuOH)2 = 4.8 × 10-20, Ksp of Mn (OH)2 = 2 × 10-13 (a) Which hydroxide precipitates first?
(b) What OH- concentration is needed to initiate precipitation of the first hydroxide?
[Cu2+][OH–]2 = 4.8 × 10–20 [Mn2+][OH–]2 = 2 × 10–13
(a) Cu(OH)2 precipitates first
(b) Cu2+ begins to precipitate when ¿
11) In contrast to many salts, calcium sulfate is only partially dissociated in aqueous solution:
The solubility-product constant for CaSO4 is 2.6 x 10-5. Calculate the solubility of CaSO4 in (a) water
and (b) 0.0100 M Na2SO4. In addition, calculate the percent of undissociated CaSO4 in each solution. (a)
[CaSO s ⇌Ca2+¿+SO24−¿K sp=¿¿¿ 4 ( ) (1)
[CaSO aq ⇌Ca2+¿+SO24−¿Kd=¿¿¿¿ (2) lOMoAR cPSD| 23136115 4 ( ) [CaSO (3)
4 (s) ⇌CaSO4( aq) ] The mass balance gives ¿ (4)
We have 3 equations and 3 unknowns [Ca2+], [SO 2– 4 ], and [CaSO4]aq To
solve we divide (1) by (2) to give Ksp 2.6×10−5 −3 [CaSO4](aq )=
Kd =5.2×10−3=5.0×10
Note that this is the equilibrium constant expression for (3) and indicates that the concentration of
un-ionized CaSO4 is always the same in a saturated solution of CaSO4. Substituting (4) into (1) gives
[Ca2+] = (2.6 × 10–5)1/2 = 5.1 × 10–3 M
and since S = [CaSO4]aq + [Ca2+], we obtain
S = 5.0 × 10–3 + 5.1 × 10–3 = 1.01 × 10–2 M
%CaSO4(aq) = (5.0 × 10–3/1.01 × 10–2) × 100% = 49% (b)
Here [CaSO4]aq is again equal to 5.0 ×10–3 and the mass balance gives [SO 2– 4 ] = 0.0100 + [Ca2+] (5)
Substituting (1) into (5) and rearranging gives 0 = [SO 2– 2–
4 ]2 – 0.0100[SO4 ] – Ksp which may be
solved using the quadratic equation to give [SO 2– 4 ] = 0.0121 [Ca2+] = 2.14 × 10–3
S = 5.0 × 10–3 + 2.14 × 10–3 = 7.14 × 10–3 M
%CaSO4(aq) = (5.0 × 10–3/7.14 × 10–3) × 100% = 70%




