



Preview text:
lOMoAR cPSD| 59085392 BIOCHEMISTRY MIDTERM REVIEW
(The following answers are only suggestions. You are encouraged not to memorize them word for word, but rather getting the main
ideas and then express them with your own words.)
1. Enzyme is widely used in many fields as food processing, environment, agriculture, medicine, …student
chooses one and discuss the application of enzyme.
(The fol owing examples are only suggestions. You are encouraged to find your own examples, or build more into an example that you find interesting.)
Examples of enzyme applications in:
a. Food processing: Fish sauce is a traditional savory in Vietnam and the craft of making it is an income
source for many households in coastal provinces. Traditionally, the process of making fish sauce
takes a very long time (12-24 months) because it relies on the naturally-occurring proteases in fish
guts. By using additional enzymes, such as alcalase and flavourzyme, the production time is cut down
to half, the total nitrogen content is higher, and the production cost is lower.
b. Environment: The production of dairies and textiles discharges a lot of dangerous waste into the
environment. This is a serious health hazard to people working in the factories as well as people living
near the factories. Enzymes, such as catalase, can play a role in degrading toxic wastes, including
formaldehyde, formic acid and alcohols, in a speedy and cost-effective way.
c. Agriculture: Enzymes are used widely in animal feeds production in order to improve the nutritional
values. Hydrolases are the most commonly used for this purpose, including amylase, proteinase,
cellulase and hemicellulase. These enzymes can break down large polymeric molecules in the raw
materials into smaller molecules that can be easily absorbed by even newborn or young animals,
such as calves, chicks, puppies, etc.
d. Medicine: Enzyme diagnostics is a new branch of enzymology and medicine, which uses enzymes to
determine the concentration of components in blood, serum, urine, etc., such as glucose and
cholesterol. Additionally, determination of enzyme activity in biological materials is a useful indicator for damages to vital organs.
2. Coenzyme is helper molecule and it takes an important role in enzyme catalyzed reaction. Discuss about it.
Coenzyme works as an assistant to enzymes. Such helper molecules can be in the form of a small organic
molecules or a metal ion. When a coenzyme is attached tightly to an enzyme by covalent bonds, it is called
a prosthetic group. Coenzymes facilitate the enzymatic reactions in several ways: -
Metal ions can bind different parts of the substrate to warp it into a more stable conformation. They
can also accept electrons from the substrate and stabilize the transition state. Moreover, the positive charges
of the metal ions help stabilize the transition state by shielding negative charges of the substrates. -
Organic molecules, often derived from vitamins, contribute to and determine the type of reaction by
accepting or donating electrons (redox reactions) or other chemical groups. With the help of these
coenzymes, the enzymes themselves are not altered after the reaction even if there is a net transfer to or
from the substrates to form the products.
The important role of coenzymes explains the need for consuming micronutrients from food, such as minerals
and vitamins. A lack of coenzymes lead to a corruption of enzymes function, as most enzymes require
coenzymes, and they cannot perform their catalytic activity without their coenzymes. This manifests into
various diseases, especially metabolic ones.
3. Active site occupies a small part of enzyme protein but it is the place where substrate comes and combines
with. Base your understanding, clarify the structure and property of active site. lOMoAR cPSD| 59085392
Almost all enzymes have a protein nature, a large polymeric molecule with one or several polypeptide chains.
However, where the substrates bind and reaction takes place is only a small part of an enzyme’s total volume.
As a protein is ultimately folded into a 3D form, the active site is also three dimensional, often in the form of
clefts or crevices on the surface of the enzyme.
The amino acids present in the active site are actual y far away in their order in the polypeptide chain, but
brought close together after protein folding.
This explains why enzymes lose their catalytic activity when denatured, or when their active site is damaged.
It also explains why isoenzymes, though arising from different gene, can catalyze the same reaction with the
same substrate, as long as their active sites after protein folding are similar.
The arrangement of residues in the active site also contributes to its specificity.
When a substrate comes into an active site, it is bound by multiple weak attractions with the amino acid
residues, cofactors and coenzymes, and even water molecules present in the active site.
4. Write down six categories of enzyme. Discuss and focus on the first three groups.
Six categories of enzymes: (remember to write them in the correct order) 1. Oxidoreductases 2. Transferases 3. Hydrolases 4. Lyases 5. Isomerases 6. Ligases
a. Oxidoreductases: catalyze the oxidation-reduction (redox) reactions. Their subclasses include
dehydrogenases, oxidases, oxygenases, reductases, peroxidases, and hydroxylases (For more details,
see slides). These enzymes contain coenzymes such as NAD+, NADP+, FAD, FMN, etc. that can accept or donate electrons.
Dehydrogenases: Examples are alcohol dehydrogenase (turning alcohol into aldehyde with
the help of NAD+, seen in physiological degradation of alcohol) and glutamate dehydrogenase
(turning glutamate into ketoglutarate, seen in ammonia fixing microbes and mammals’ amino acid
metabolism). Reductase: Transfer of electrons to oxygen so that it can combine with H+ to form water molecules.
Oxigenase: Reactions that involve oxygen in a functional group such as OH, COOH, etc.
Peroxidase: Reactions that involve hydroxy peroxide (H2O2). The common coenzyme for this
subclass is heme. Catalase is a special example of this subclass, as its substrates are H2O2 only.
b. Transferases: Transfer of groups, such as amino, carboxyl, carbonyl, methyl, phosphoryl, acyl, etc.
Their names often include -trans-. The subclasses of these enzymes are very different in nature,
depending on the functional group that they transfer.
c. Hydrolases: Cleavage of bonds by adding water (hydrolysis). Some subclasses are esterases, phosphatases, and peptidases.
(For other categories, see slides)
5. Can you recommend the enzyme source?
Enzyme production can come from 3 main sources: animals, plants and microbes. Enzyme Sources Applications Animal Sources lOMoAR cPSD| 59085392 proteases bovine and porcine digestive enzymes, anti-inflammatory pancreas agents, health food additives pepsin porcine stomach body fortifying agents aldolases liver and muscle fructose digestion catalase liver food industry chymotrypsin pancreas leather industry lipase pancreas food industry ancrod snake venom anti-coagulant urokinase urine thrombolytic agent alkaline phosphatase calf intestine/kidney
diagnostic (indicator in ELISA) Plant Sources bromelain pinapple
anti-inflammatory agents, meat tenderizer papain papaya anti-inflammatory agents urease jack bean
determination of blood urea nitrogen amylase barley brewing industry peroxidase horseradish diagnostic Microbe Sources cellulase Trichoderma
break down of cellulose in detergent and paper industry amylase Bacil us subtilis
break down of starch in industrial raw materials protease Bacil us
degradation of protein content in detergent and food industry alcohol dehydrogenase yeast diagnostic L-asparaginase E. coli leukemia treatment
6. What do you know about enzyme kinetics? Discuss Vmax and KM, equation of Michaelis Menten and
Lineweaver Burk. Advantages and disadvantages of each?
Enzyme kinetics is the study of the rates of enzymatic reactions and how they can be affected by other
conditions. When an enzyme is added to a substrate, the reaction that follows occurs in three stages with distinct kinetics: Phase Concentration of ES Rate of product formation Pre-steady state Rapid burst of
ES Initially slow as ES starts being formed, then complexes formed speeds up Steady state
ES concentration remains Constant rate of formation, faster than constant as it is being
presteady state. This is the rate expressed
formed as quickly as it by Michaelis-Menten kinetics. breaks down Post-steady state
Substrate runs out so ES Slow down as there are fewer ES complexes concentration declines
Michaelis-Menten kinetics is a model of enzyme kinetics that explains the dependence of reaction rate on
the concentration of enzyme and its substrate. There are two important terms in the Michaelis-Menten equation: 𝑉𝑚𝑎𝑥[𝑆] 𝑣 = lOMoAR cPSD| 59085392 𝐾𝑀 +[𝑆] -
Vmax: the maximum reaction rate, when all active sites are saturated with substrate. It is dependent
on total enzyme concentration. -
KM (Michaelis constant) – the substrate concentration at which the reaction rate is half of the Vmax.
KM is an important term because it measures the affinity an enzyme has for its substrate. The lower the KM
value, the more efficient the enzyme is at carrying out its function at a lower substrate concentration.
However, Vmax and KM do not depend on each other. Knowing KM does not predict Vmax and vice versa.
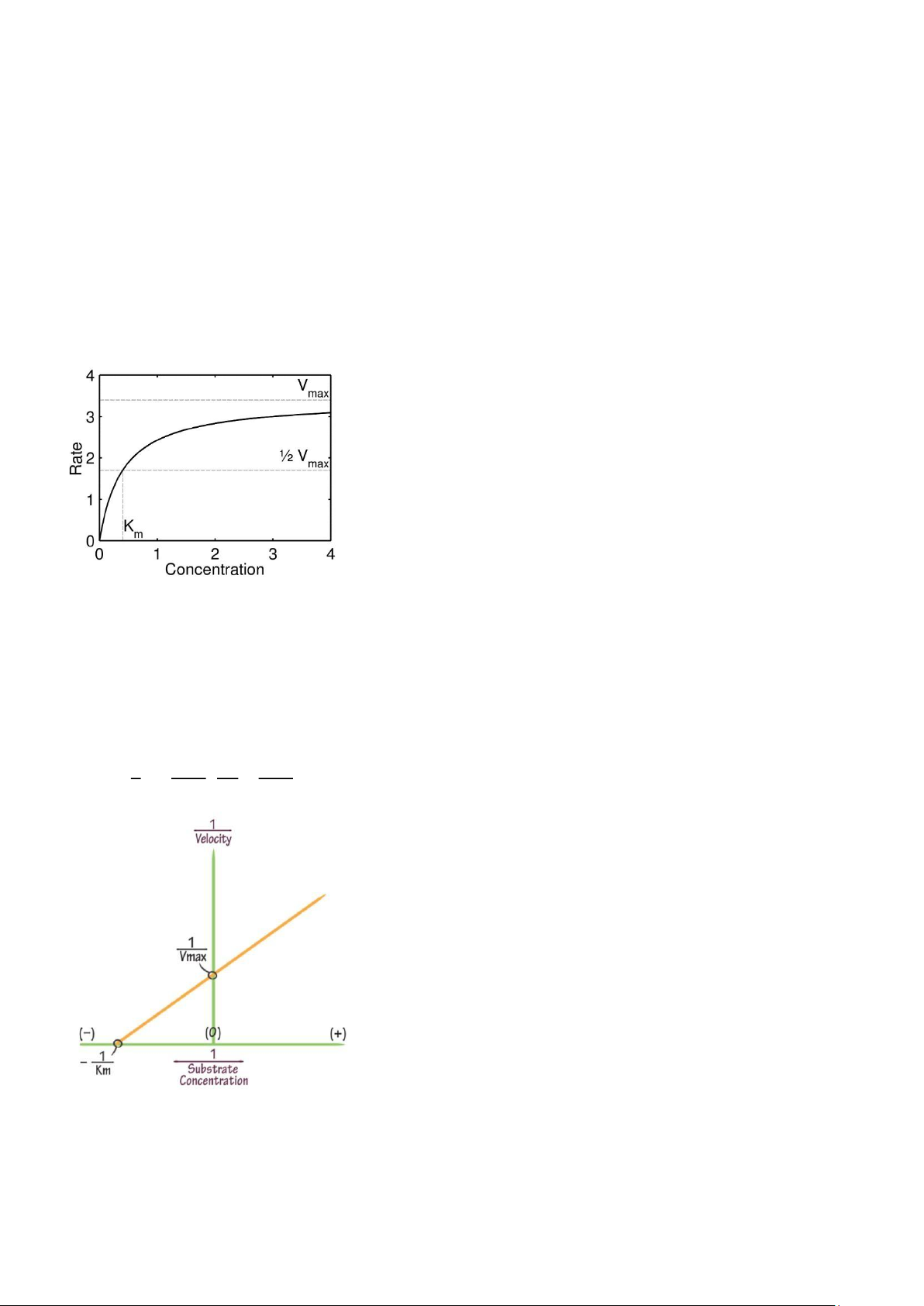
Plotting a graph of substrate concentration against the rate of the reaction gives a rectangular hyperbolic
curve as below. It can be seen how the reaction rate initially increases rapidly in an almost linear fashion as
substrate concentration increases (1st order kinetics). The rate increase then plateaus, and becomes less
sensitive to substrate concentration (0 order kinetics).
Michealis-Menten equation represents exactly the behaviour of reaction rate as substrate concentration
increase. However, it poses difficulties in experimental design, as it is hard to determine KM and Vmax from it.
Lineweaver-Burk equation is the reciprocal of Michaelis-Menten equation. It is more useful in determining KM
and Vmax thanks to its linear nature. By finding the x- and y-intercepts, we can easily work out the Vmax and
KM values. The Lineweaver-Burk equation is as followed. 1 𝐾𝑀 1 1 =( ) 𝑣 𝑉 𝑚𝑎𝑥 [ 𝑆 ] + 𝑉 𝑚𝑎𝑥
However, experimental design needs to be carried out with care, since the Lineweaver-Burk plot poses two
disadvantages: At large [S] (small 1/[S]), data points crowds together, making the resulting curve unreliable.
At small [S] (large 1/[S]), small errors, both human and systematic, can have large effect on the resulting curve.
12. Focus and discuss the energy generating pathways of carbohydrate metabolism. lOMoAR cPSD| 59085392
Include 3 pathways: glycolysis, CAC and oxidative phosphorylation
As the question requires, focus on how much energy is liberated by each pathway, and the total amount of energy yielded.
Glycolysis: After investing 2 ATPs in the first stage (steps 1 and 3), the second stage generates 4 ATPs (steps
7 and 10). So, glycolysis yields a net 2 ATPs for each glucose.
CAC: As the primary energy generated from CAC is contained in the reduced coenzymes, there are only 2
GTPs (equivalent to 2 ATPs) produced in step 5 of the cycle that can be counted as liberated energy.
Oxidative phosphorylation: This is the pathway that generates the most usable energy for the cell. For a total 10
NADHs and 2 FADH2s from glycolysis and CAC, oxidative phosphorylation can generate up to 34 ATPs (3
ATPs for each NADH, 2 ATP for each FADH2 – simplified calculation), or 28 ATPs (2.5 ATPs for each NADH,
1.5 ATPs for each FADH2 – calculated based on numbers of protons pumped).
In total, one molecule of glucose can generate up to 38 ATPs or 32 ATPs after complete oxidation through three pathways.
13. What is Biochemistry and its covering scopes as wel as its applications in life sciences?
Answer based on your understanding.



