


Preview text:
International University, Vietnam National University - HCMC1 CHEMISTRY LABORATORY REPORT
EXPERIMENT 1: CHEMICAL REACTIONS Semester: 2 Year: 2023 - 2024 Group: 3 Session: Date: Group members: Full name Student ID Signature Score No % Contribution . (Total = 100%) 1
Nguyễn Ngọc Trúc An IEIEIU23043 20% 20
2 Nguyễn Thị Lan Hương IELSIU23114 20% 20 3 Vũ Thị Thùy Linh IELSIU23049 20% 20 4 Lê Kha Thy IELSIU23090 20% 20 5
Trần Ngọc Đan Trâm IELSIU23092 20% 20 Total score: 100/100 I. Introduction (10 pts)
Along with the knowledge written in books, chemical experiments are the source of experience.
When we observe a chemical reaction such as the color change of the solution, the formation of
precipitate, the generation of air bubbles, the heat generation..., we can comment and draw
conclusions about the lesson we are studying, which is forming logical thinking from practical
observations. Based on the results recorded from this experiment including reactions of Cu2+,
reactions of Silver halides, reactions of H2O2, reactions of KMnO4, reactions of Fe2+ and Fe3+,
reactions of Al3+, flame tests, we can classify chemical reactions into the following 5 common types
The report must be typed and handed in together with the signed data sheet by the deadline
International University, Vietnam National University - HCMC2 CHEMISTRY LABORATORY
which consist of synthesis, decomposition, single displacement, double displacement, and
combustion. Finally, this experiment will provide interesting phenomena that occur between different
substances, followed by the chemical equations, commentary on the results, and illustrating images of those reactions. II. Experimental (15 pts) 1. Reactions of Cu 2+
Step 1: Prepare two test tubes called tube 1 and tube 2.
Step 2: Add 10 drops of 0.5M CuSO4 to each tube.
Step 3: Add 10 drops of 2M NaOH to tube 1 and 10 drops of 2M NH4OH to tube 2.
Step 4: Mix tubes gently and observe.
Step 5: Add 10 drops of 2M NaOH to tube 1 and 10 drops of 2M NH4OH to tube 2 again.
Step 6: Mix tubes gently and observe all chemical phenomena.
2. Reactions of Silver halides
Section 1: Reactions of Potassium Chloride (KCl)
Step 1: Prepare two test tubes called tube 1 and tube 2.
Step 2: Add 10 drops of 0.5M KCl to each tube.
Step 3: Add 10 drops of 0.1M AgNO3 to each tube.
Step 4: Add 10 drops of 2M NH4OH to tube 2.
Step 5: Mix tubes gently, wait 2 minutes and observe all chemical phenomena.
Section 2: Reactions of Potassium Bromide (KBr)
Step 1: Prepare two test tubes called tube 1 and tube 2.
Step 2: Add 10 drops of 0.5M KBr to each tube.
Step 3: Add 10 drops of 0.1M AgNO3 to each tube.
Step 4: Add 10 drops of 2M NH4OH to tube 2.
Step 5: Mix tubes gently, wait 2 minutes and observe all chemical phenomena. 3. Reactions of H 2O2
Step 1: Prepare three test tubes called tube 1, tube 2 and tube 3.
The report must be typed and handed in together with the signed data sheet by the deadline
International University, Vietnam National University - HCMC3 CHEMISTRY LABORATORY
Step 2: Add 1 drop of 0.1M KMnO4 to tube 1, 5 drops of 0.1M KI to tube 2 and 10 drops of 3% MnO2 to tube 3.
Step 3: Add 5 drops of 2M H2SO4 to tube 1, tube 2 and add a pinch of MnO2 to tube 3.
Step 4: Add 5 drops of 3% H2O2 to tube 1 and tube 2.
Step 5: Mix tubes gently, wait 2 minutes and observe all chemical phenomena.
4. Reactions of KMnO 4
Step 1: Prepare three test tubes called tube 1, tube 2 and tube 3.
Step 2: Add 10 drops of 0.5M Na2SO3 to each tube.
Step 3: Add 5 drops of 2M H2SO4 to tube 1, 5 drops of 6M NaOH to tube 2 and 5 drops of distilled water to tube 3.
Step 4: Add 5 drops of 0.1M KMnO4 to each tube.
Step 5: Mix tubes gently and observe all chemical phenomena.
5. Reactions of Fe 2+ and Fe3+
Section 1: Ferric ion (Fe3+)
Step 1: Prepare two test tubes called tube 1 and tube 2.
Step 2: Add 10 drops of 0.5M FeCl3 to each tube.
Step 3: Add 5 drops of 2M KOH to tube 1 and 5 drops of 2M NH4OH to tube 2.
Step 4: Mix tubes gently and observe all chemical phenomena.
Section 2: Ferric ion (Fe2+)
Step 1: Prepare two test tubes called tube 1 and tube 2.
Step 2: Add 10 drops of 0.5M FeSO4 to each tube.
Step 3: Add 5 drops of 2M KOH to tube 1 and 5 drops of 2M NH4OH to tube 2.
Step 4: Mix tubes gently and observe all chemical phenomena. 6. Reactions of Al 3+
Step 1: Prepare two test tubes called tube 1 and tube 2.
Step 2: Add 10 drops of 0.5M Al2(SO)4 to each tube.
The report must be typed and handed in together with the signed data sheet by the deadline
International University, Vietnam National University - HCMC4 CHEMISTRY LABORATORY
Step 3: Mix tubes gently and observe.
Step 4: Add 20 drops of 2M HCl to tube 1 and 20 drops of 2M NaOH to tube 2.
Step 5: Mix tubes gently and observe all chemical phenomena. 7. Flame tests
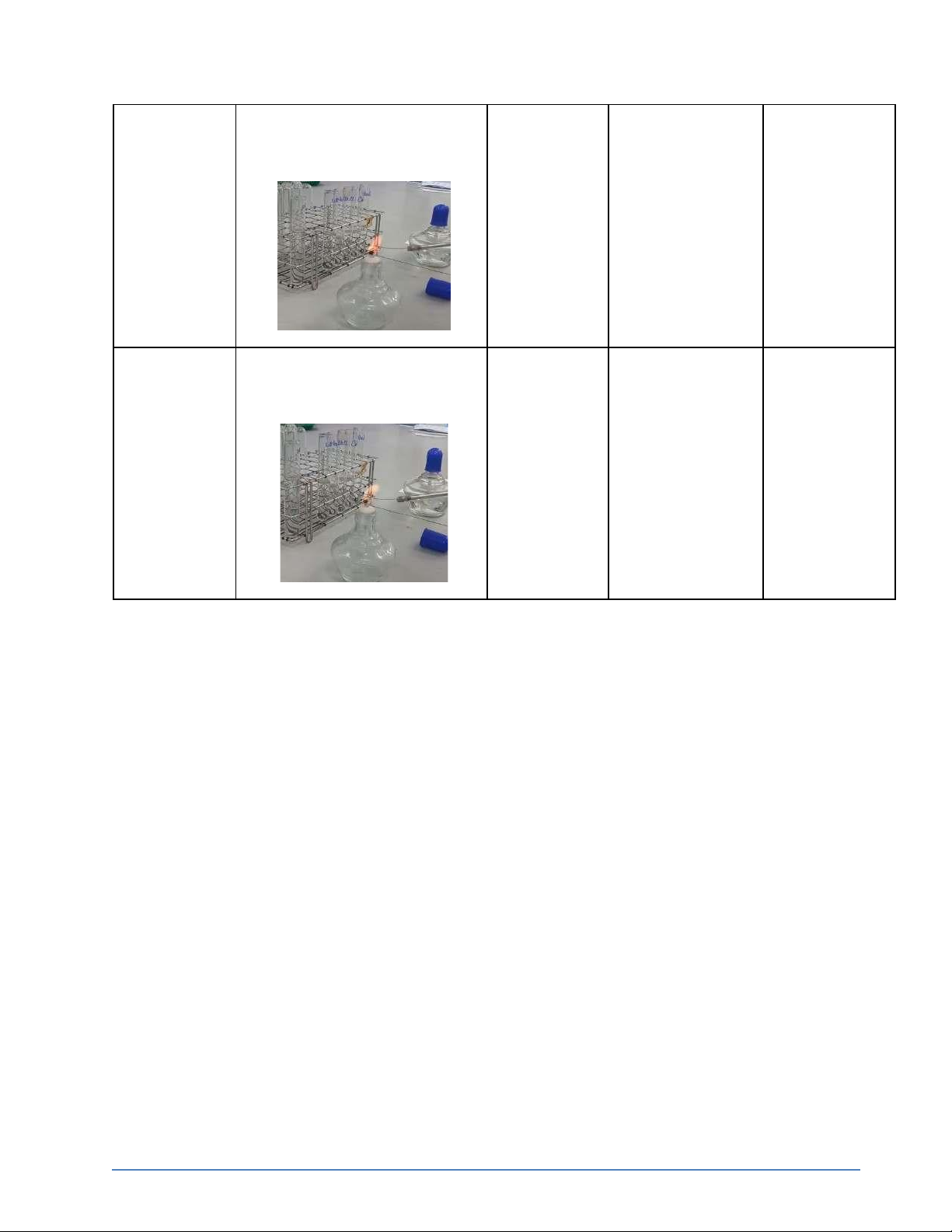
Step 1: Light the Bunsen burner.
Step 2: Clean the loop with distilled water. Step 3: Dry in the flame.
Step 4: Dip the loop into the LiCl
Step 5: Hold it in the flame until it burns out
Step 6: Observe the burning process and record the color of the flame
Step 7: Repeat the same process for solution in the order NaCl, KCl, CaCl2 and BaCl2.
III. Results and Discussions
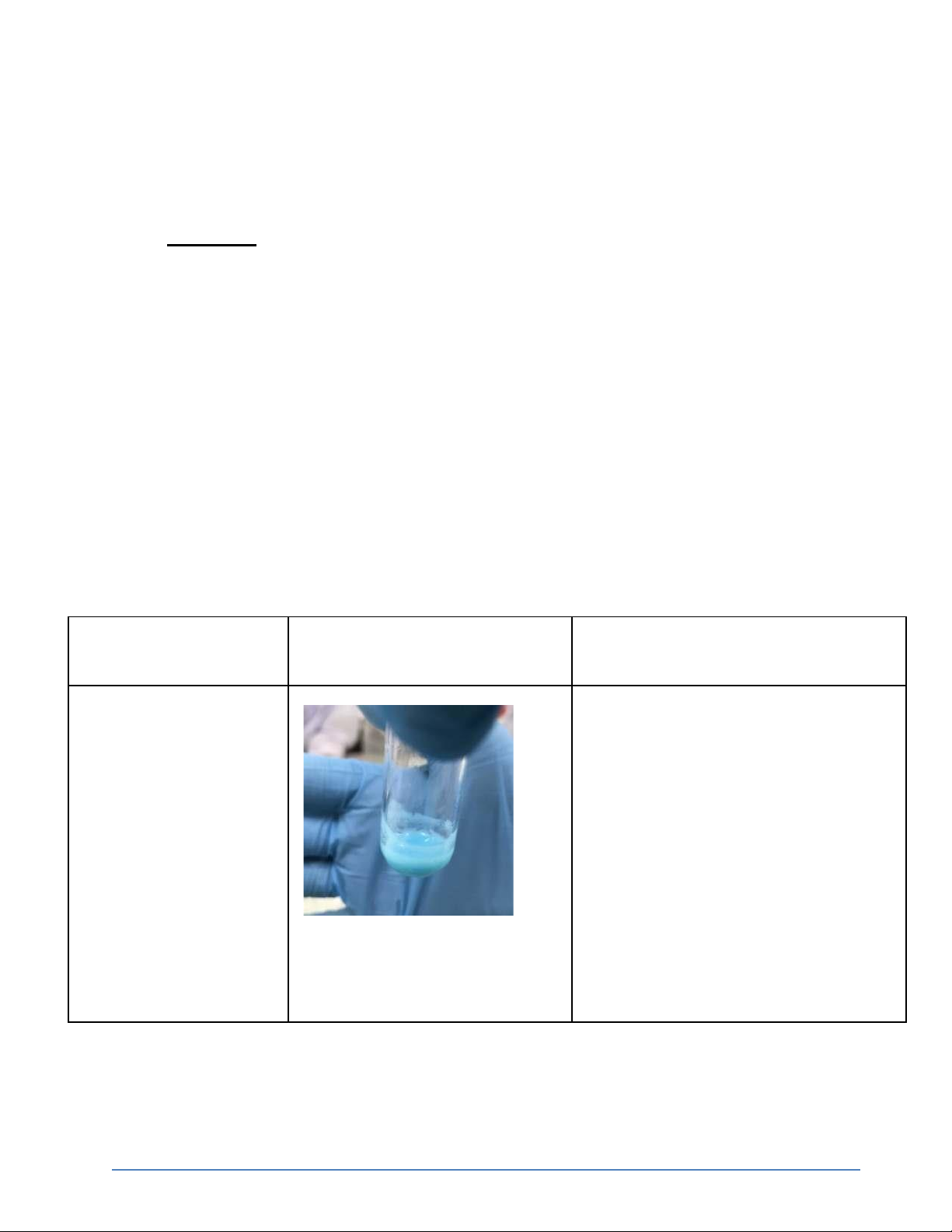
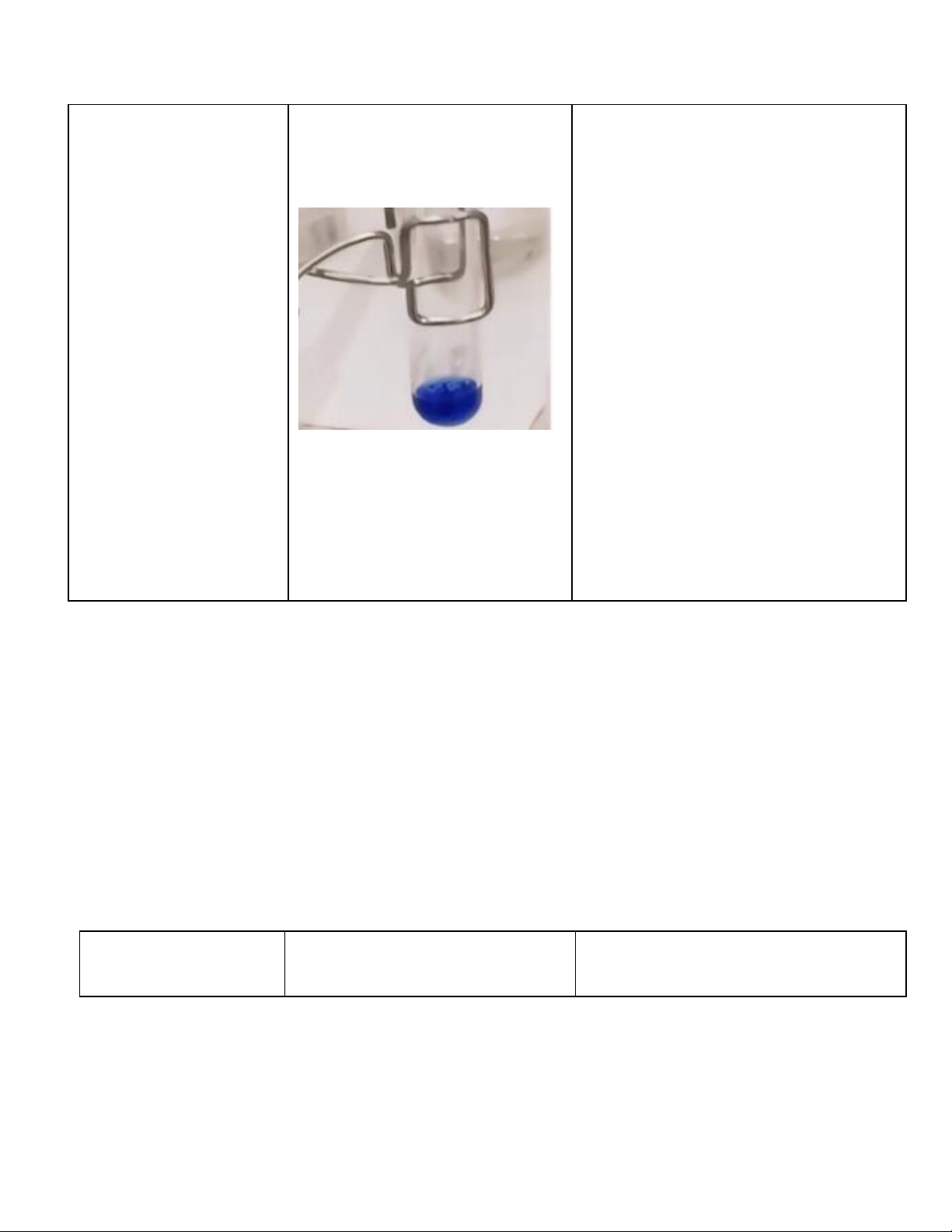
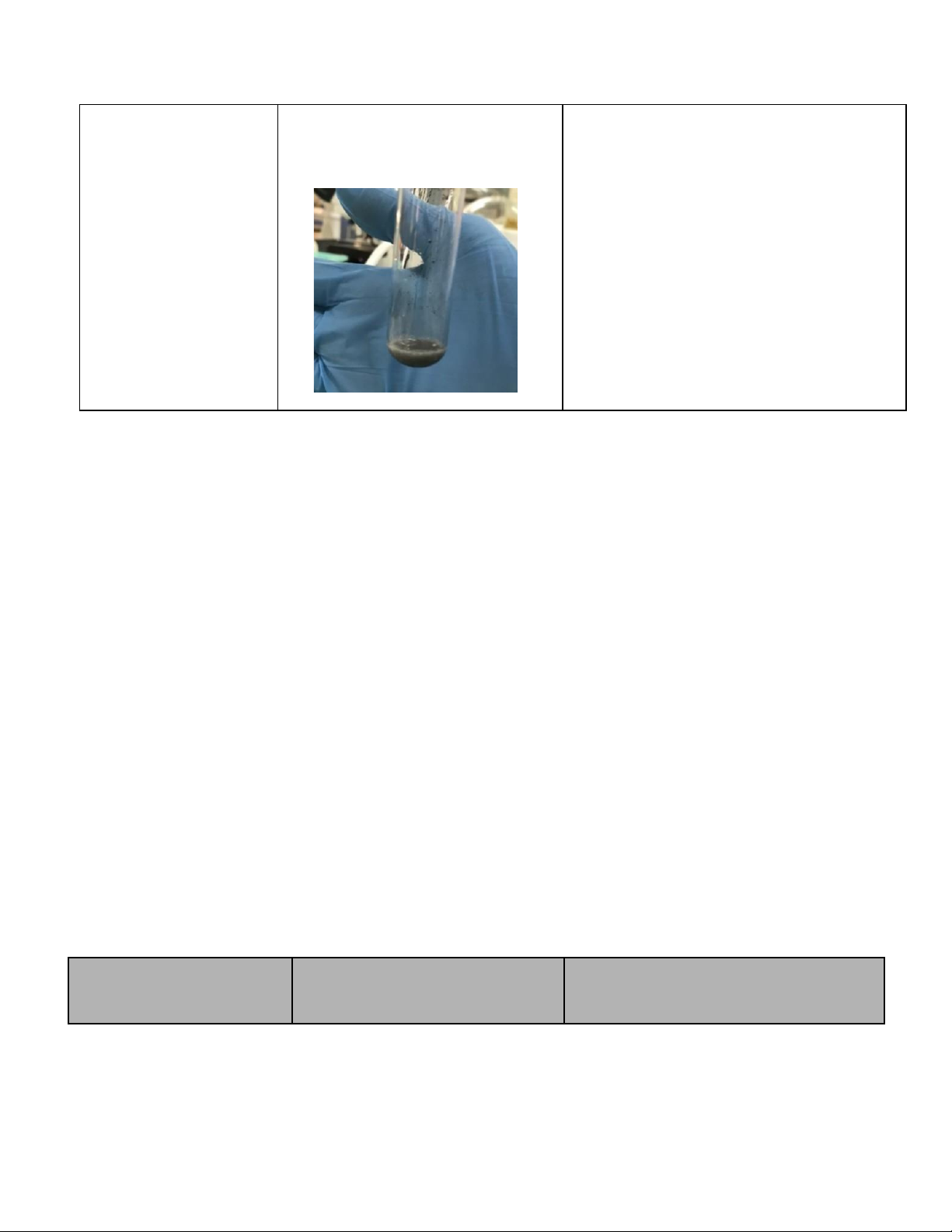
1. Reactions of Cu2+ (5 pt) Reaction Observation Chemical Equation 0.5M CuSO4 + 2M NaOH
CuSO4 + 2NaOH → Cu(OH)2 + Na2SO4
The blue precipitate was formed
The report must be typed and handed in together with the signed data sheet by the deadline
International University, Vietnam National University - HCMC5 CHEMISTRY LABORATORY 0.5M CuSO4 + 2M CuSO4 + 2NH4OH → Cu(OH)2 + NH4OH (NH4)2SO4
Cu(OH)2 + (NH4)2SO4 + 2NH4OH → (Cu(NH3)4)SO4+ 4H2O F
irstly, the pale blue precipitate was
formed then pale blue precipitate
turns into the deep blue color solution when we drip more NH4OH Comments:
- Cu2+ reacts with OH- to form the blue precipitate Cu(OH)2
- The first chemical equation is a type of double displacement reaction.
- If we add slowly NH4OH into the test tube, it forms blue precipitate Cu(OH)2 then add more
NH4OH, as the pale blue color precipitate of Cu(OH)2 is soluble in excess of NH4OH, so the
ppt has completely disappeared. The deep blue or ink-blue color of the solution is due to the formation of (Cu(NH3)4)SO4
- The second chemical equation is a type of double displacement reaction
→From the above experiment, we can see that there is a similarity between what is observed
and what is reported in the literature so the results can be accepted.
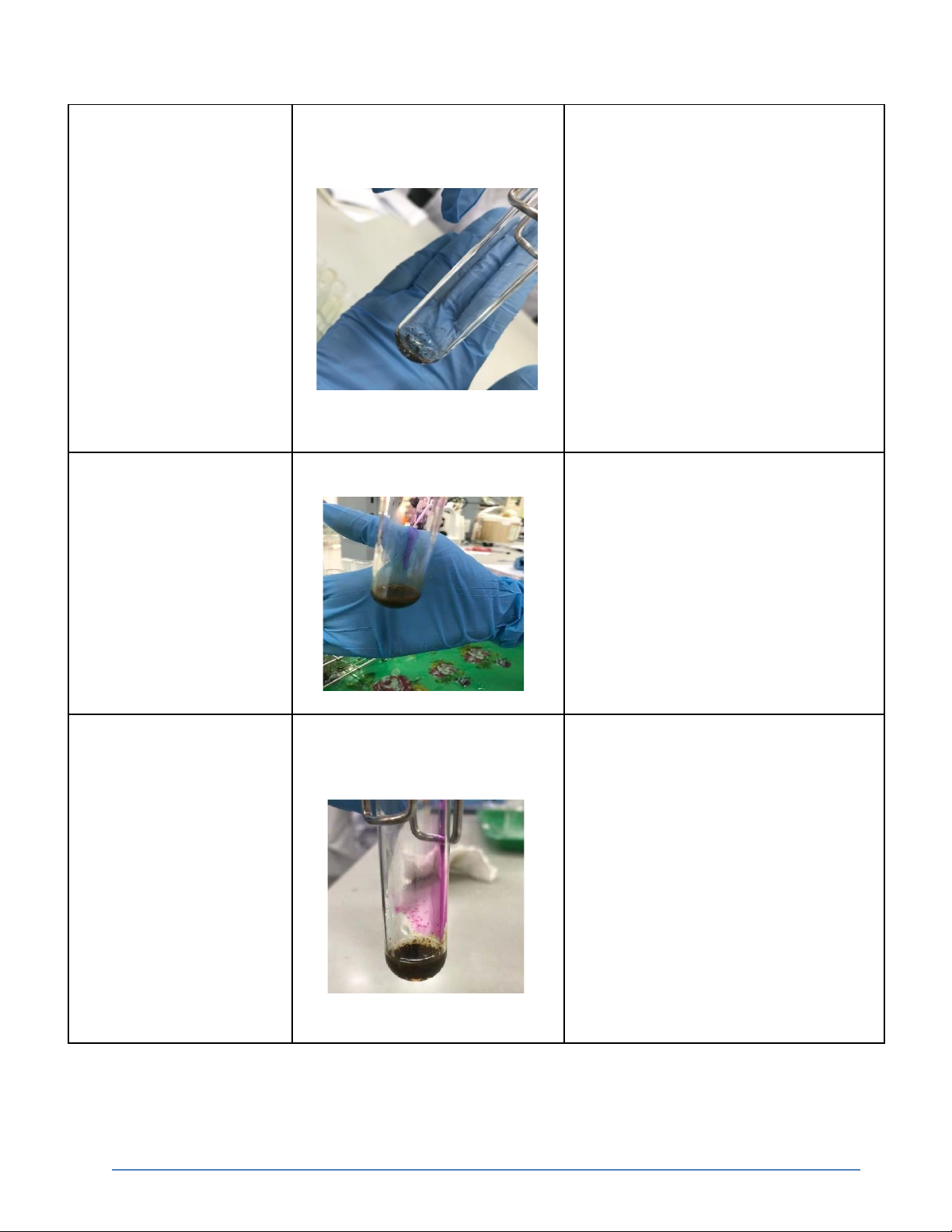
2. Reactions of silver halides (15 pts) Reaction Observation Chemical Equation
The report must be typed and handed in together with the signed data sheet by the deadline
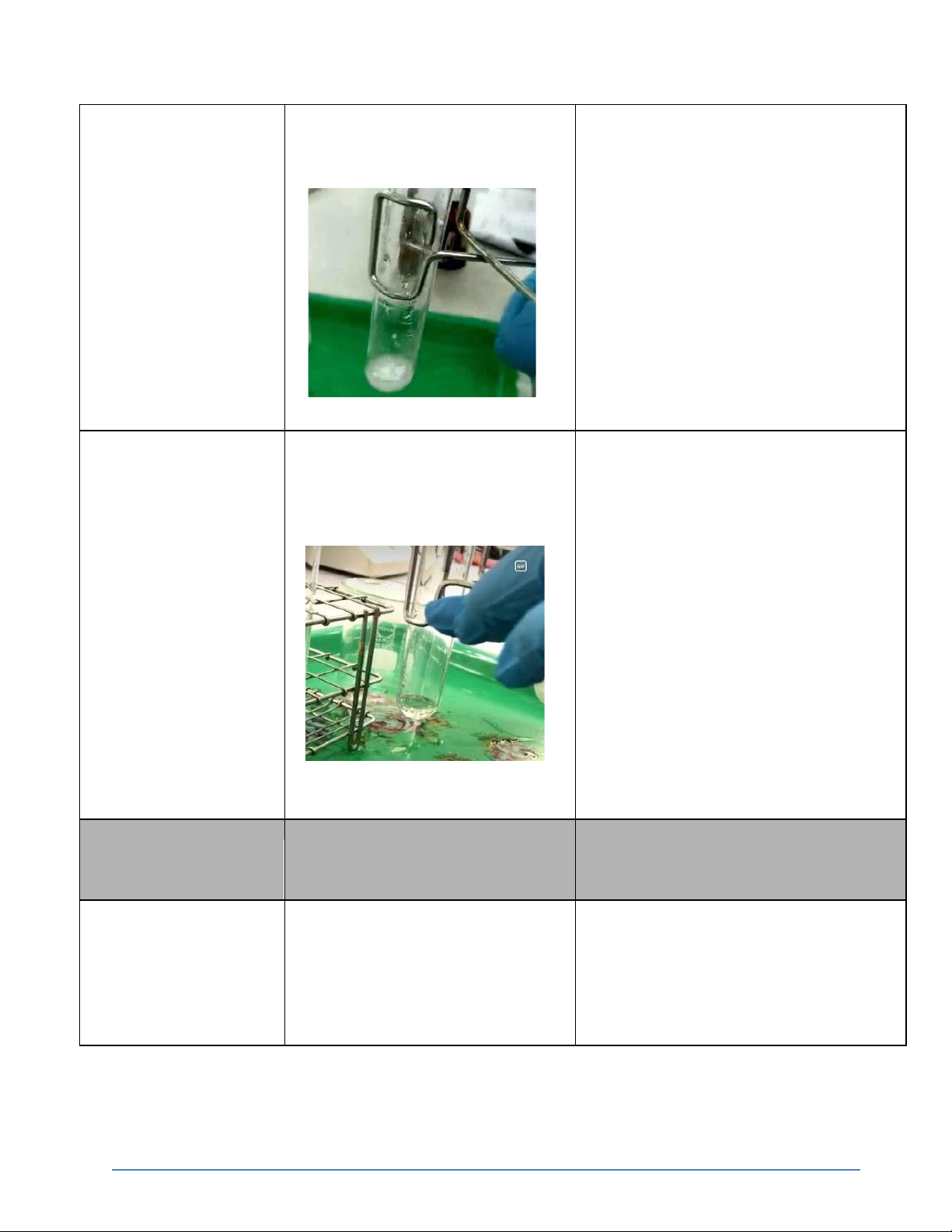
International University, Vietnam National University - HCMC6 CHEMISTRY LABORATORY 0.5M KCl + 0.1M The reaction appear white
KCl + AgNO3 → KNO3 + AgCl ↓ precipitate AgNO3 0.5M KCl + 0.1M
After adding NH4OH, the
KCl + AgNO3 → KNO3 + AgCl↓ AgNO3 + 2M NH4OH
precipitate gradually disappears and the solution becomes clear.
AgCl↓ + 2NH4OH → [Ag(NH3)2]Cl + 2H2O 0.5M KBr + 0.1M The formation of light yellow
KBr + AgNO3 → KNO3 + AgBr↓ precipitate AgNO3
The report must be typed and handed in together with the signed data sheet by the deadline
International University, Vietnam National University - HCMC7 CHEMISTRY LABORATORY 0.5M KBr + 0.1M
The formation of light yellow
KBr + AgNO3 → KNO3 + AgBr↓ AgNO3 + 2M NH4OH
precipitate but then dissolved partly
AgBr (↓) + 2NH4OH → [Ag(NH3)2]Br + 2H2O Comments:
- In the first experiment, KCl reacts with AgNO3 to form a white precipitate. After adding
NH4OH, the precipitate gradually disappears and the solution becomes clear.
- In the second experiment, KBr reacts with AgNO3, a light yellow precipitate (AgBr). Then
add NH4OH, a part of the yellow precipitate gradually dissolves due to the displacement reaction of AgBr and NH4OH.
→Both experiences 1 and 2 had similarities between what was observed and what was
reported in the literature, so the results can be accepted.
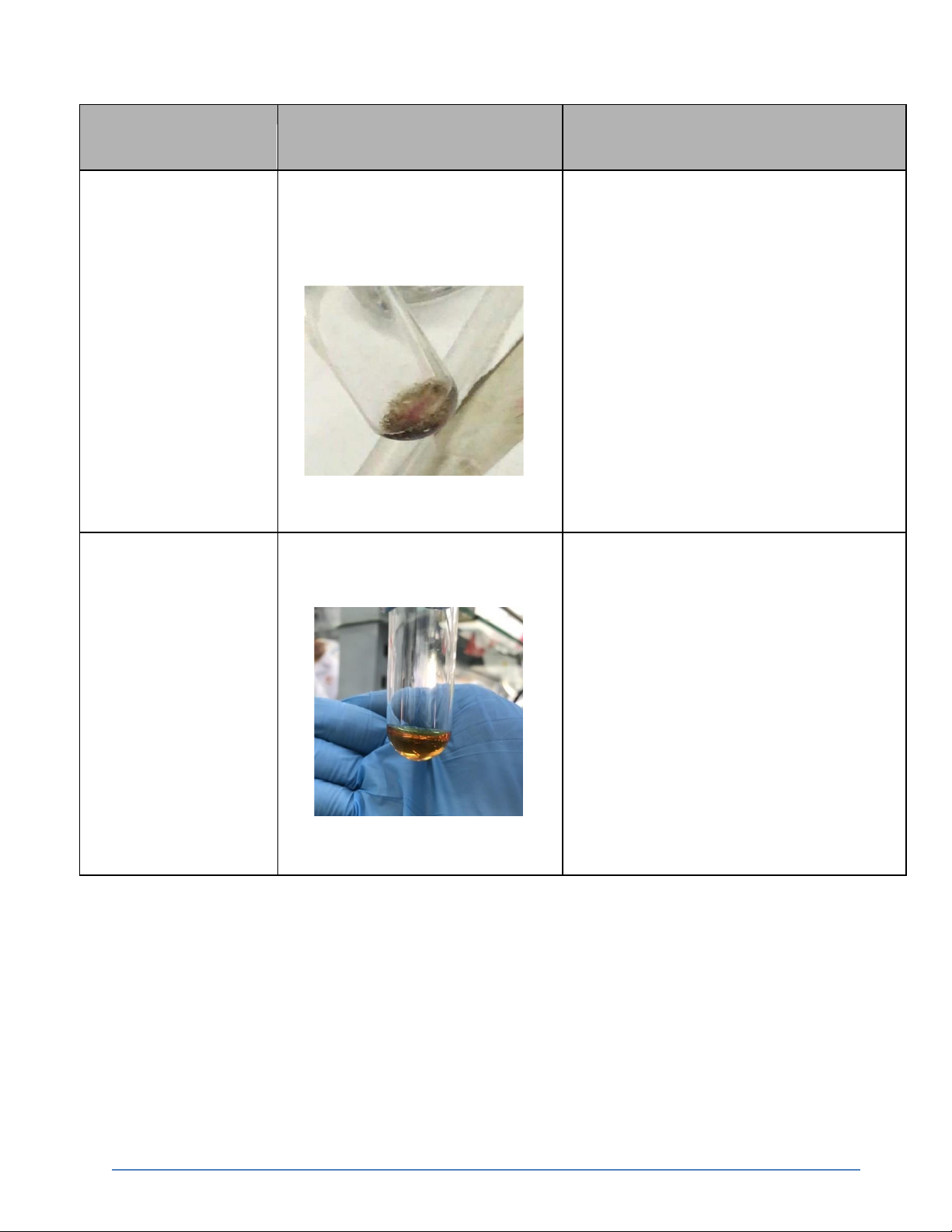
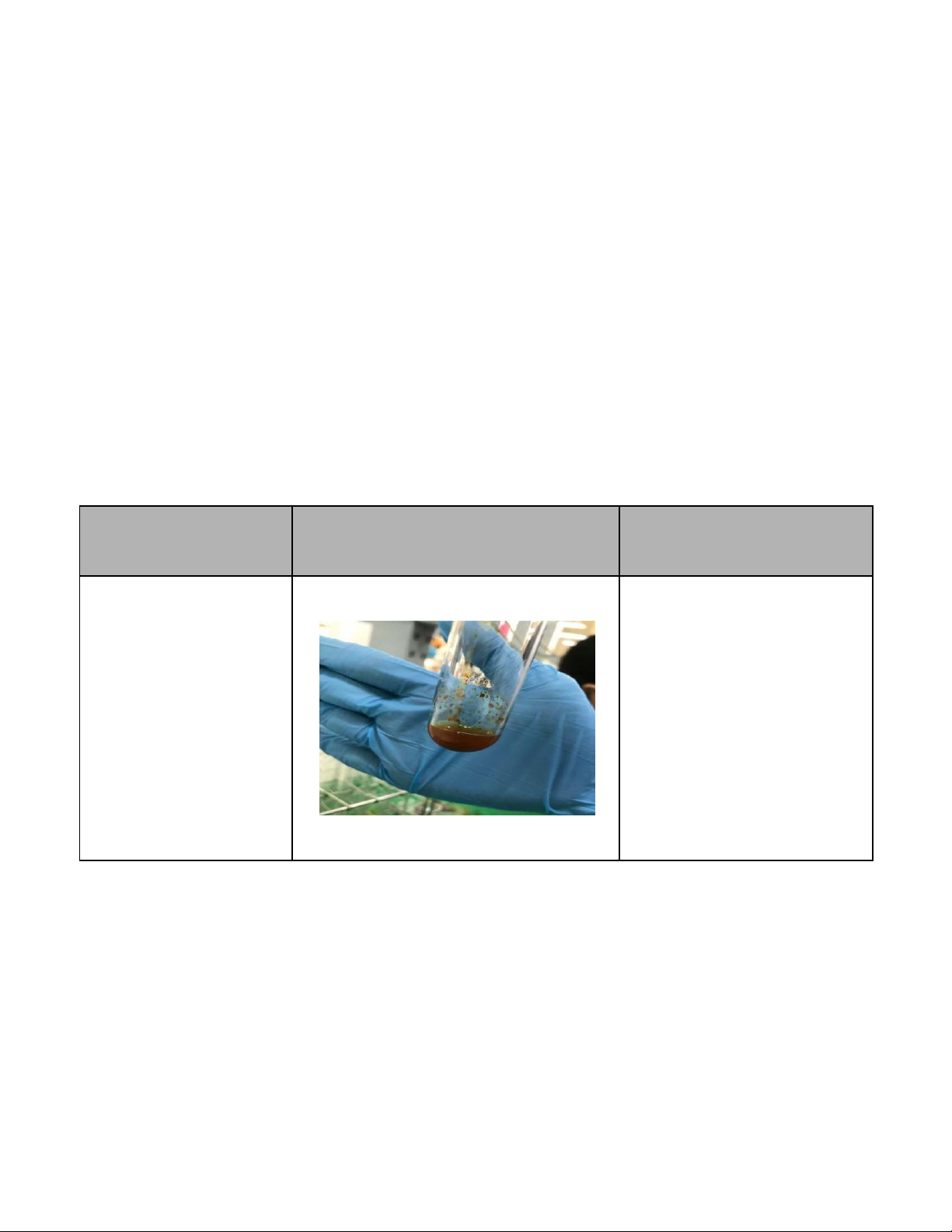
3. Reactions of H2O2 (7.5 pts)
The report must be typed and handed in together with the signed data sheet by the deadline
International University, Vietnam National University - HCMC8 CHEMISTRY LABORATORY Reaction Observation Chemical Equation 0.1M KMnO4 + 2M The solution KMnO4 gradually H2SO4 + H2O2
becomes pale and bubbles due to the production of oxygen gas.
2KMnO4 + 3H2SO4+ 5H2O2 → 2MnSO4 + K2SO4 + 8H2O + 5O2↑ 0.1M KI + 2M H2SO4
The reaction produces a red-brown 2KI + H2O2 + H2SO4 → K2SO4 + 2H2O + I2 precipitate I2 ↓ + H2O2
The report must be typed and handed in together with the signed data sheet by the deadline
International University, Vietnam National University - HCMC9 CHEMISTRY LABORATORY H2O2 + MnO2
There are air bubbles and black
H2O2 + MnO2 → H2O + O2↑ + MnO solid ( MnO) Comments:
1. When adding H2SO4 to KMnO4 (purple), the reaction does not change. When adding H2O2
molecules, it will react with KMnO4 to form MnSO4 (white), so the original KMnO4 solution
will lose its color and gradually fade. The mixture of H2O2 and H2SO4 remaining after the
reaction contains O2 produced. O2 will form effervescent tablets when combined with other
substances and cause the solution to fizz.
This is a redox reaction, H2O2 acts as a reducing agent, while KMnO4 is an oxidizing agent. 2.
When adding H2SO4 to KI solution (colorless), the reaction does not change. Then add H2O2,
KI solution is oxidized to I2, and H2O2 is reduced to water. As a result, I2 is produced in the
reaction, creating a reddish-brown color phenomenon.
This is a redox reaction, KI and H2O2 act as reducing agents, while H2SO4 is an oxidizing agent.
3. When H2O2 reacts with MnO2, oxygen bubbles are released and black residue appears due to MnO2 precipitation.
This is a redox reaction, MnO2 is an oxidizing agent, and H2O2is reducing agent
→From the above experiment, we can see that there is a similarity between what is observed
and what is reported in the literature so the results can be accepted.
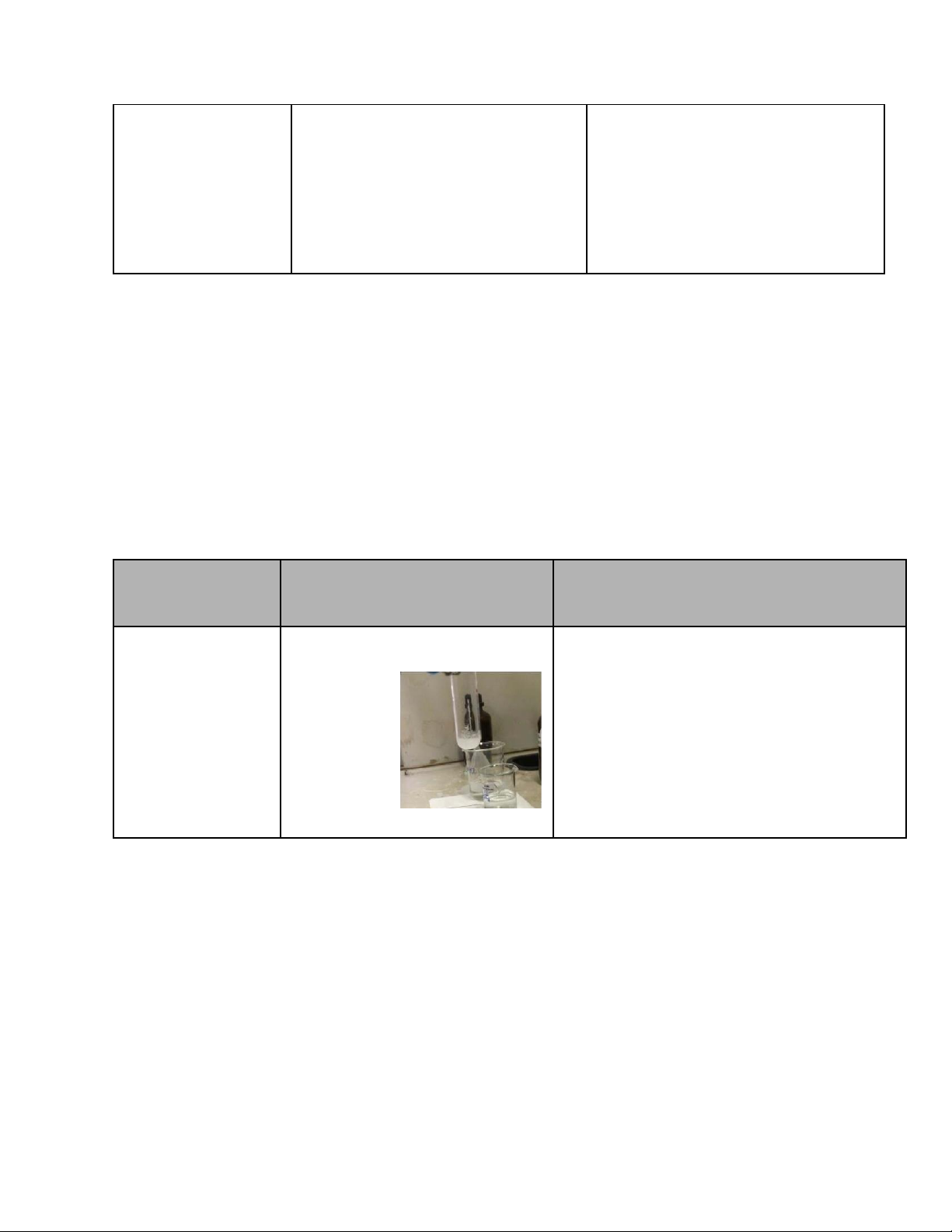
4. Reactions of KMnO4 (7.5 pts) Reaction Observation Chemical Equation
The report must be typed and handed in together with the signed data sheet by the deadline
International University, Vietnam National University - HCMC10 CHEMISTRY LABORATORY
0.5M Na2SO3 + 2M H2SO4 KMnO4 solution loses color and a 5Na2SO3 + 3H2SO4 + 2KMnO4 → + 0.1M KMnO4 black precipitate appears
5Na2SO4 + K2SO4 + 2MnSO4 + 3H2O
0.5M Na2SO3 + 6M NaOH The solution is brown Na2SO3 + 2NaOH + 2KMnO4 → + 0.1M KMnO
K2MnO4 + Na2SO4 + H2O + Na2MnO4 4 0.5M Na2SO3 + H2O The solution has a dark brown 3Na2SO3 + H2O + 2KMnO4 → precipitate + 0.1M KMnO 3Na2SO4 + 2MnO2 + 2KOH 4 Comments:
1. Adding H2SO4 to Na2SO3 solution (colorless), the reaction remains unchanged, then adding
KMnO4 solution (purple), the solution becomes transparent, a black precipitate appears.
The report must be typed and handed in together with the signed data sheet by the deadline
International University, Vietnam National University - HCMC11 CHEMISTRY LABORATORY
This is a redox reaction, Na2SO3 is the reducing agent, H2SO4 and KMnO4 are the oxidizing agents.
2. Adding NaOH to Na2SO3 solution (colorless), the reaction remains unchanged, then add
KMnO4 solution (purple), the solution turns green then turns brown
This is a redox reaction, Na2SO3 is the reducing agent, KMnO4 is the oxidizing agent.
3. Adding H2O to Na2SO3 solution (colorless), reaction remains unchanged, then add KMnO4
solution (purple), dark brown precipitate appears.
This is a redox reaction, Na2SO3 is the reducing agent, KMnO4 is the oxidizing agent.
→From the above experiment, we can see that experiment 1 is different from the literature in
that it produces a black precipitate, not a transparent one. Experiments 2 and 3 had
similarities between what was observed and what was reported in the literature, so the results can be accepted.
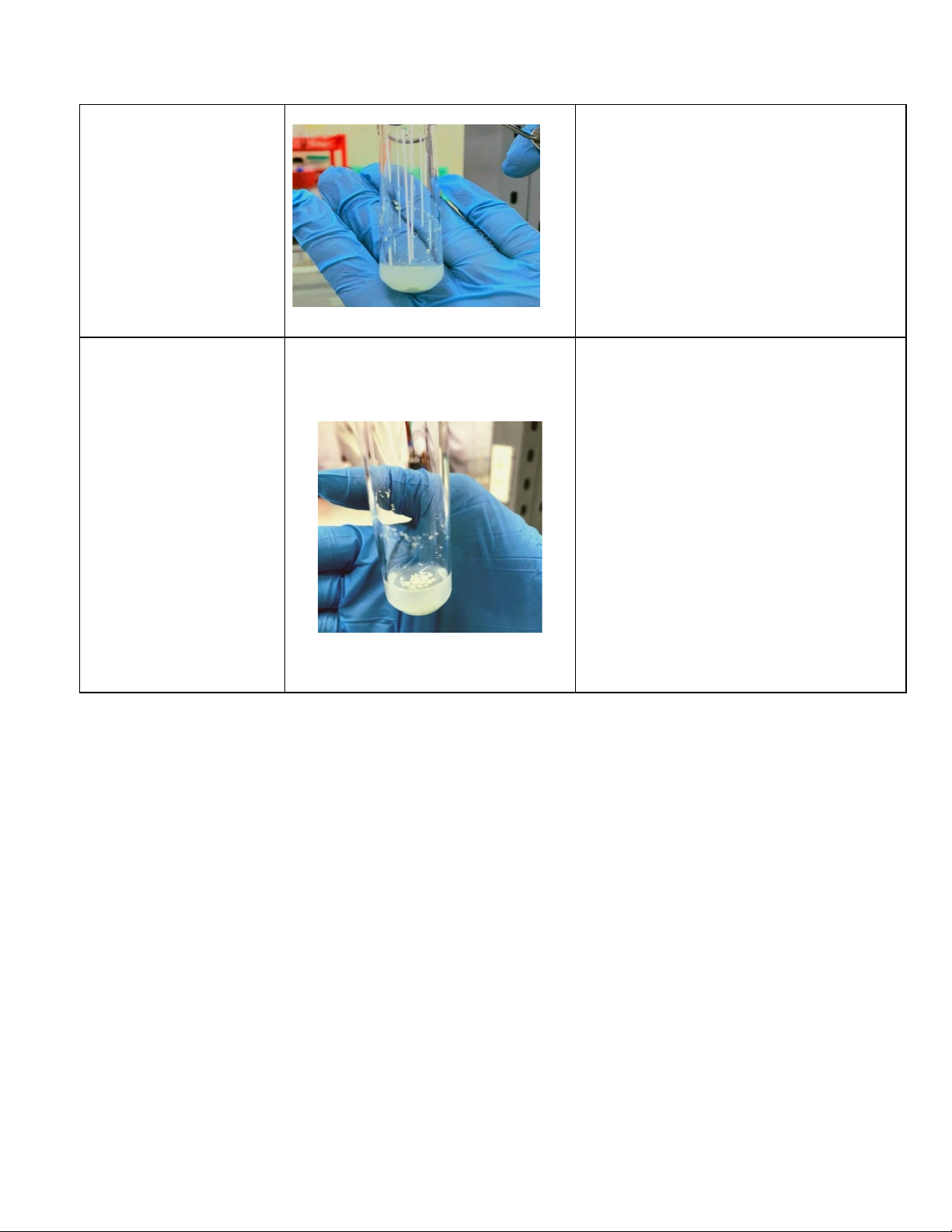
5. A. Reactions of ferric ion Fe3+ (5 pts) Reaction Observation Chemical Equation 0.5M FeCl3 + 2M KOH
Reaction forms a red-brown precipitate FeCl3+ 3KOH→ 3KCl+ Fe(OH)3
The report must be typed and handed in together with the signed data sheet by the deadline
International University, Vietnam National University - HCMC12 CHEMISTRY LABORATORY 0.5M FeCl3 + 2M
Reaction forms a red-brown precipitate FeCl3+ 3NH4OH→ NH4OH 3NH4Cl+Fe(OH)3 Comments:
(1):FeCl3(brown-yellow) reacts with KOH(colorless) to form Fe(OH)3(red-brown)
(2): FeCl3(brown-yellow) reacts with NH4OH(colorless) to form Fe(OH)3(red-brown)
These reactions are double displacement and both reactions form Fe(OH)3 Fe3++3OH- → Fe(OH)3
→The observed results are similar with published literature
5. B. Reactions of ferrous ion Fe2+ (5 pts) Reaction Observation Chemical Equation 0.5M FeSO4 + 2M Reaction forms a white green
2KOH+FeSO4 → K2SO4+ Fe(OH)2 KOH precipitate
The report must be typed and handed in together with the signed data sheet by the deadline
International University, Vietnam National University - HCMC13 CHEMISTRY LABORATORY 0.5M FeSO4 + 2M Reaction forms a white green 2NH4OH+ FeSO4 → (NH4)2SO4 NH precipitate 4OH +Fe(OH)2 Comments:
(1):FeSO4(colorless) reacts with KOH(colorless) to form Fe(OH)2(white green)
(2): FeSO4(colorless) reacts with NH4OH(colorless) to form Fe(OH)2(white green)
These reactions are double displacement and both reactions form Fe(OH)2 Fe2++2OH- → Fe(OH)2
→The observed results are similar with published literature
6. Reactions of Al3+ (7.5 pts) Reaction Observation Chemical Equation 0.5M Al2(SO4)3 Reaction forms a white
6NaOH+ Al2(SO4)3→ 3Na2SO4 +2Al(OH)3 + 2M NaOH precipitate
The report must be typed and handed in together with the signed data sheet by the deadline
International University, Vietnam National University - HCMC14 CHEMISTRY LABORATORY 0.5M Al2(SO4)3 Reaction forms a white
_ 6NaOH + Al2(SO4)3→ 3Na2SO4 +
precipitate and then it dissolves + 2N NaOH 2Al(OH) 3 + 2M HCl
_ 3HCl+ Al(OH)3 → 3H2O + AlCl3 0.5M Al2(SO4)3 Reaction forms a white
_ 6NaOH + Al2(SO4)3→ 3Na2SO4 +
precipitate and then it dissolves + 2M NaOH 2Al(OH) 3 + 2M NaOH
_ NaOH + Al(OH)3 → NaAlO2 + 2H2O Comments:
(1) Al2(SO4)(colorless) reacts with NaOH(colorless) to form Al(OH)3 Al3++3OH- → Al(OH)3
(2) Firstly, Al2(SO4)(colorless) reacts with NaOH(colorless) to form Al(OH)3 and then Al(OH)3
reacts with HCl(colorless) to form colorless solution (NaAlO2 )
(3) Firstly, Al2(SO4)(colorless) reacts with NaOH(colorless) to form Al(OH)3 and then Al(OH)3
continues to react with NaOH to form colorless solution (NaAlO2 )
These reactions are double-displacement
→The observed results are similar with published literature
7. Flame test (12.5 pts)
The report must be typed and handed in together with the signed data sheet by the deadline
International University, Vietnam National University - HCMC15 CHEMISTRY LABORATORY Solution Dominant flame color Wavelength Photon energy (nm) Frequency (J) -1 (s ) LiCl Red 700 nm 4,29.10^14(s-1 ) 2,84.10-19 NaCl Orange-Yellow 610 nm 4,92.10^14(s-1 ) 3,26.10-19 KCl Violet 420 nm 7,14.10^14(s-1 ) 4,73.10-19
The report must be typed and handed in together with the signed data sheet by the deadline
International University, Vietnam National University - HCMC16 CHEMISTRY LABORATORY CaCl2 622 nm 4,82.10^14(s-1 ) 3,20.10-19 Orange-red BaCl2 587nm 5,11.10^14(s-1 ) 3,39.10-19 Orange
Note: Provide detailed calculation of each chemical Comments:
The colors of flames of substances above are: + LiCl: Red + NaCl: Orange-Yellow + KCl: violet + CaCl2: Orange-red + BaCl2: Orange
→From the flame test above we can observe that there is a small difference between those
observed and those reported in the literature so the result can be accepted.
IV. Conclusions (10 pts)
In this report, we conduct 7 experiments: + Reactions of Cu2+ + Reactions of Silver halides + Reactions of H2O2 + Reactions of KMnO4 + Reactions of Fe2+ and Fe3+
The report must be typed and handed in together with the signed data sheet by the deadline
International University, Vietnam National University - HCMC17 CHEMISTRY LABORATORY + Reactions of Al3+ + Flame tests
After performing experiments, observing phenomena and discussing together, we can draw the final
conclusions for this report. Generally, all reactions have changes that can be observed with the eye.
There are reactions that have similar phenomena in literature, whereas there are also a few reactions
that are not similar to the information recorded in official documents, which shows that there were
shortcomings during the process of experiment.
The report must be typed and handed in together with the signed data sheet by the deadline