







Preview text:
International University, Vietnam National University - HCMIU Date: 23/10/2024
Course: Chemistry Laboratory_S1_2024_Group20 Team: 1 Report
CHEMISTRY LABORATORY REPORT EXPERIMENT 4
-.-.-. Group members and topic .-.-.- ́ ̀ ̃ lOMoARcPSD|47231818 ̃ Score: _________/100 Table of content Page I. Introduction 2 1.1. Prologue 1.2. Safety guideline 1.3. Chemicals/Equipments 1.4. Formula II. Experiment 34 2.1. ACID/BASE EQUILIBRIA
2.2 EQUILIBRIA OF ACID/BASE INDICATORS 6 2.3 COMPLEX ION FORMATION
2.4 EQUILIBRIA OF PRECIPITATION REACTIONS III. Conclusion 7 I. Introduction 1.1. Prologue
A chemical reaction is said to be reversible if the reactants can both move backward and generate a product. For instance,
C+D can be turned into A+B, while A+B can become C+D. In a chemical equation, two arrows pointing in opposite
directions are written. The equilibrium may be upset if the reaction is subjected to stress. Changes in temperature, focus,
or pressure can all cause stress. The Le Ch atelier's principle states that the reaction mixture's composition should change
until equilibrium is restored.
We do various reversible chemical reactions in today's experiment, such as adding more chemicals or altering the
temperature. The additional chemical drop may cause a little color change. 1.2. Safety guideline
The lab will supply you gloves and glassware. We need to purchase lab coats separately due to differences in size and
comfort preferences among members. It is also important to wear a mask to minimize risk and avoid malodorous smells
from chemicals. Wearing long trousers or any clothing that covers your legs, and ensure that your shoes are fully enclosed.
There will be assistants who will support and supervise you. Always ask them for chemicals if it’s dangerous. Follow the
instructions to drop the chemical waste safely. Of course, no vape/smoke, drink, eat. Be careful with the glass equipment, it
can be sharp and can cause harm/bleed. In case you break one, you have to buy it your own, some can be cheap and not so
cheap. Do not do any UNAUTHORIZED experiments, joking and using personal devices (except phones for photos). Clean
your hands with soap after the experiment, and change gloves if needed. When something unexpected happens, take a
deep breath, and don't overreact, it is crucial to know the call for emergency help, location, and equipment.
1.3. Chemicals/Equipments Equipments Test tube : 10 Beaker 250ml : 1 Beaker 100ml : 2 Cylinder : 1 Graduated pipette: 1 Pump : 1 Stirring rod : 1 Test tube holder : 1 Rack : 1 Plastic pipette : 1 Chemicals lOMoARcPSD|47231818 1.4. Formula
The equilibrium constant (K) expresses the relationship between products and reactants of a reaction at equilibrium with
respect to a specific unit. For the hypothetical reaction above, the equilibrium constant expression is written as: K = [C]^c×[D]^d [A]^a×[B]^b
In reversible reaction, if K>>1 then equilibrium favors products, or the reaction lies to right; if K<<1 then equilibrium favors
the reactants, or the reaction lies to left. II. Experiment 2.1. Acid/Base equilibria A. Procedures
Step 1: Prepare 3 test tubes, all having 10 drops of K₂CrO₄
Step 2: Observe the color of the tubes
Step 3: add 5 drops of concentrated HCl to tubes #2 and #3
Step 4: Observe and compare the color to tube#1
Step 5: add 10 drops of 6M NaOH to tube #3
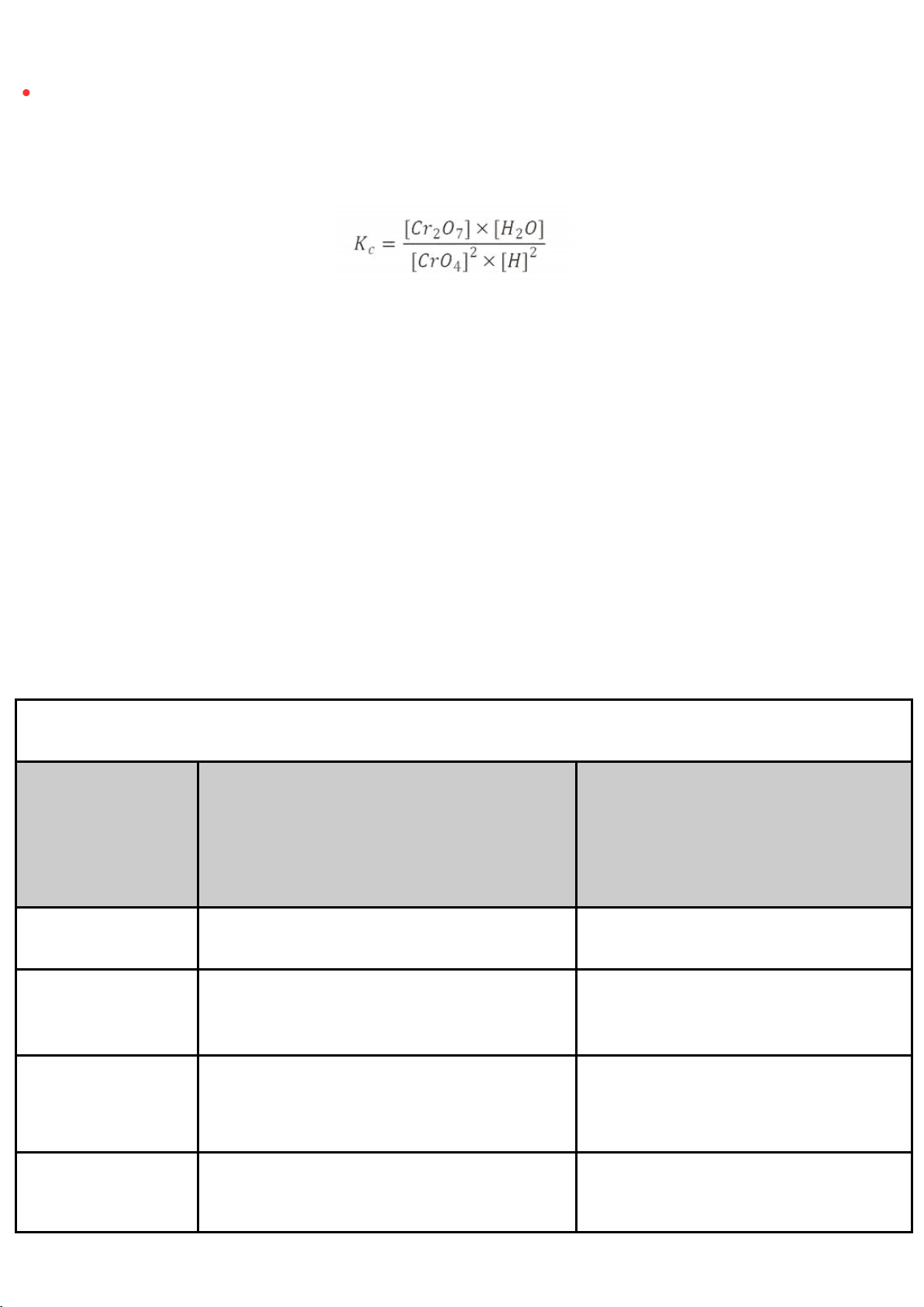
Step 6: Observe and compare the color of tube #3 to tube #2 B. Observations 2CrO₄²⁻ + 2H⁺
Cr₂O₇²⁻ + H ₂O observation initial solution yellow yellow yellow orange orange + conc HCl +6 N NaOH Yellow yellow + heat C. Explainations
Yellow solution due to the CrO₄²⁻ ion in K₂CrO₄ lOMoARcPSD|47231818
The solution turns orange due to the formation of more Cr₂O₇²⁻ when the reaction proceeds forward upon adding H⁺ ions
The solution turns back to yellow-orange because H⁺ reacts with OH⁻, shifting the equilibrium, increasing the CrO₄²⁻ ion. D. Comments
The equilibrium constant expression for this reaction is:
We observed a clear change in the color of the solution through several additions of a catalyst, which altered the color.
The original color of the K₂CrO₄ solution is yellow. The solution turns orange due to the equilibrium shift in the forward
reaction when H⁺ ions from HCl are added. II. Experiment
2.2. equilibria of acid/base indicators A. Procedures
Step 1: add 2 drops of methyl violet along with 20ml of distilled water
Step 2: divide equally into two separated tubes
Step 3: observe the color of tube#1
Step 4: add in 6M of HCL until no significant changes, and record the color change
Step 5: add in 6M of NaOH until no significant changes, and record the color change
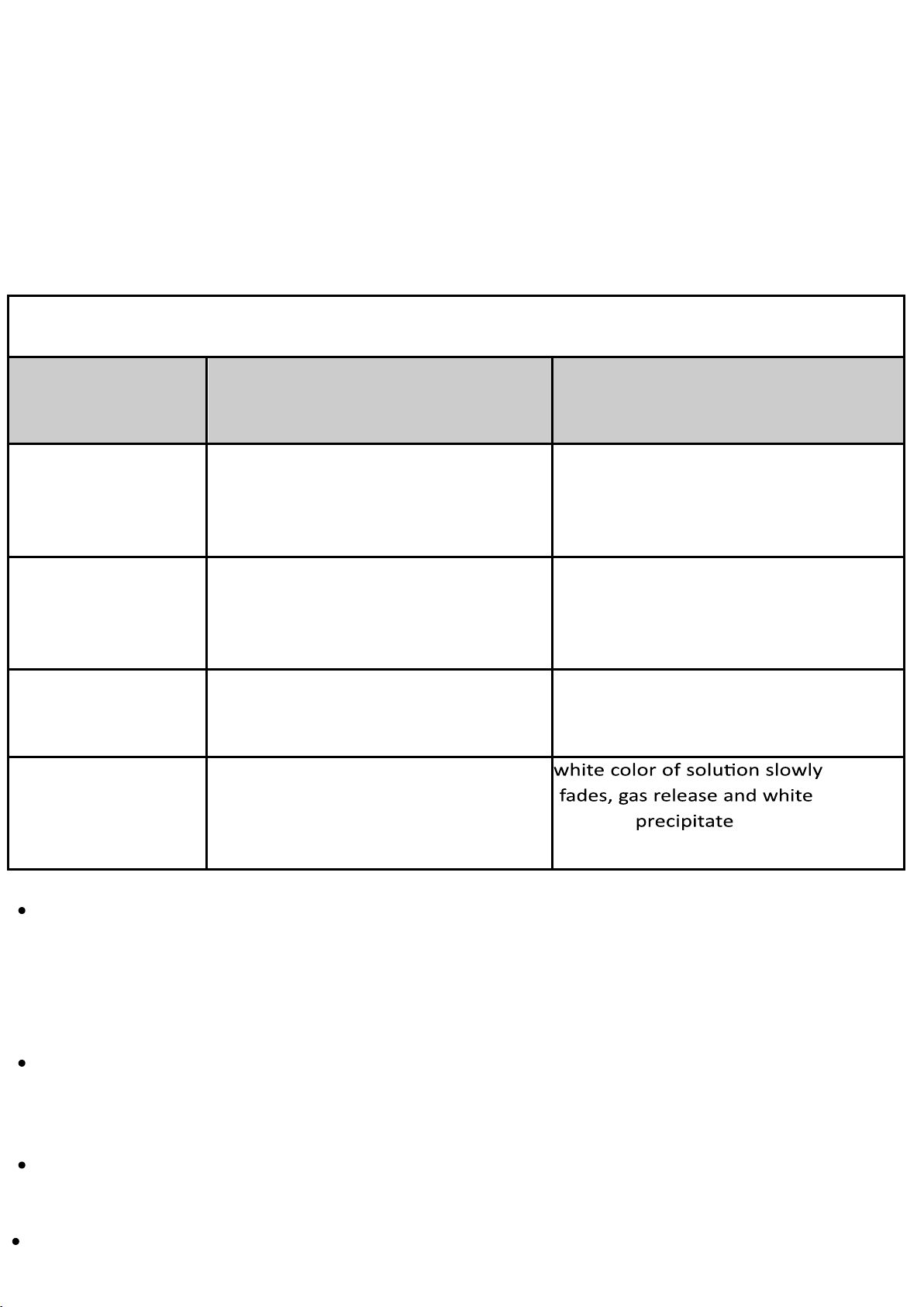
Step 6: add in 6M of HCL until no significant changes, and record the color change B. Observations H(MV)(aq) + H₂O(l) H₃O⁺(aq) + MV⁻(aq) addition predicted observation violet none (c vio on let trol) viole t violet violet Blue 6M HCl blue blue light violet (transparent) 6M NaOH violet 6M HCl blue light blue (transparent) C.Explanation
When distilled water is added, the solution remains violet, which is the original color of MV When HCl is added,
excess H⁺ ions are introduced. This shifts the equilibrium in the reverse direction, reducing H₃O⁺ concentration
and thus increasing the pH, causing the solution to turn blue.
When NaOH is added, it increases the OH⁻ ions. This shifts the equilibrium in the forward
direction, reducing OH⁻ concentration, thus decreasing the pH, causing the solution to turn violet "Similar to the
color change in the first reaction, when H⁺ ions are added, the pH increases, causing the solution to turn blue again D. Comments
the equilibrium constant expression for this reaction is:
We observed a clear change in the color of the solution through several additions of a catalyst, which altered the
color. The original color of the MV solution is violet. The solution turns blue due to the equilibrium shift in the reverse
reaction when H⁺ ions from HCl are added.
The color change of the solution continues when NaOH is added, reducing the concentration of H⁺ ions as H⁺ reacts
with OH⁻. This causes the equilibrium to shift in the reverse direction, turning the solution back to violet due to the
decrease in H⁺ concentration. H⁺ + OH⁻ → H₂O lOMoARcPSD|47231818 II. Experiment
2.3. equilibria of precipitation reactions A. Procedures
Step 1: add in 5ml of 0.1M CaCl2 to both tubes
Step 2: add in 1ml of 0.1 Na2C2O4 to tube#1 and observe the color change
Step 3: add in 1ml of 0.1 H2C2O4 to tube#2 and observe the color change
Step 4: add in 10 drops of 6M HCl to tube#2 and observe the color change Step 5: add in 10
drops of 6M NH4OH and observe color change B. Observations
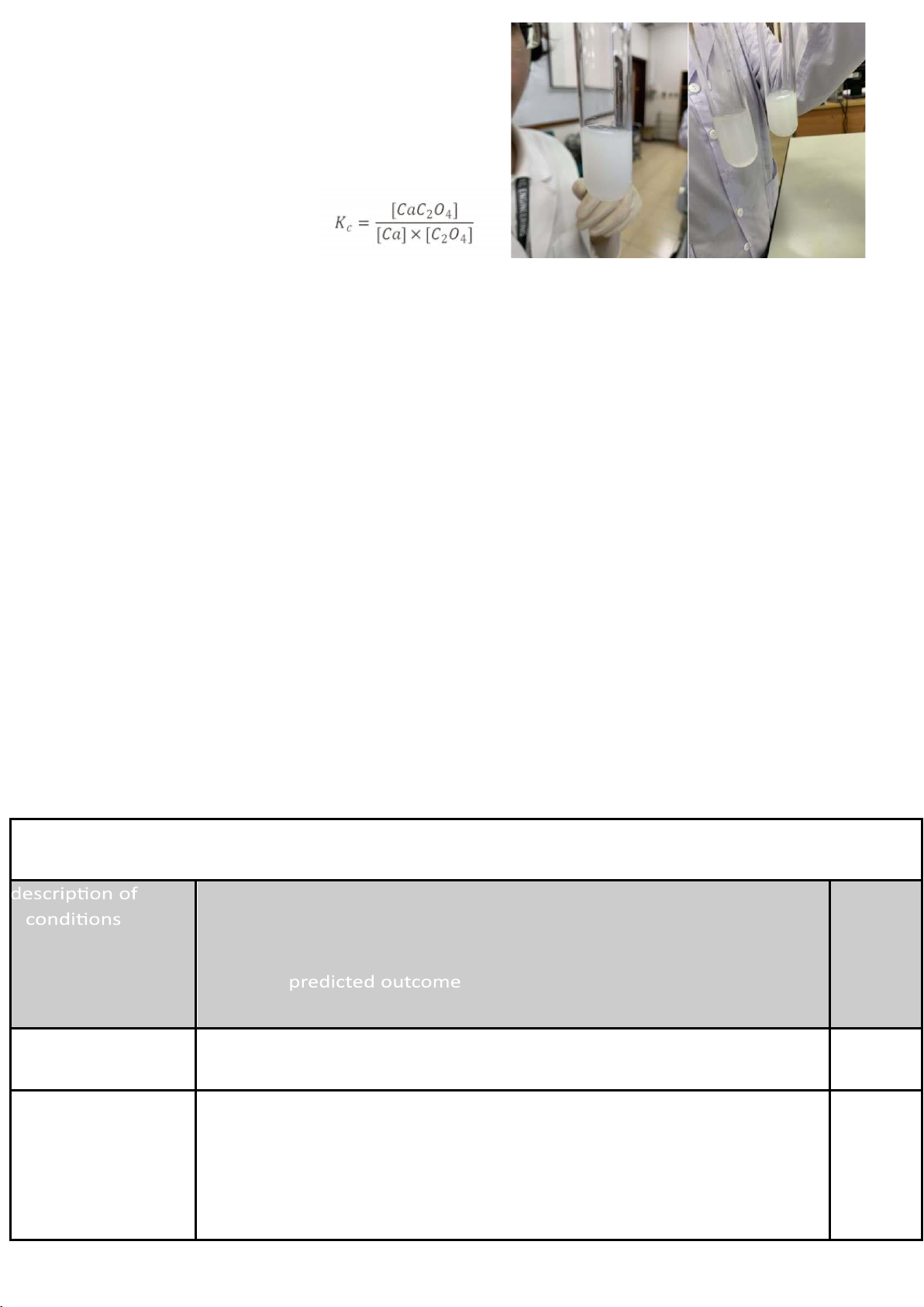
Ca²⁺(aq) + C₂O₄²⁻(aq) CaC₂O₄(s) predicted outcome addition observation tes wht ittu e b p e r e#1 ci pitate white precipitation
white precipitation initially, then
slowly dissolves partially into the solution and form white color
white precipitation initially, then test tube #2 white precipitate
slowly dissolves partially into the solution + 0.1M H2C2O4 and form white color test tube #2 +6M
white precipitation and white color HCl Test tube #2
color slowly returns to color of reactants +6M NH4OH C. Explainations
When Na₂C₂O₄ was added, it reacted with CaCl₂ to form solid CaC₂O₄, which had a white precipitate. The system shifted to the right.
CaCl₂ + Na₂C₂O₄ → CaC₂O₄ + 2NaCl
Na₂C₂O₄ → 2Na⁺ + C₂O₄²⁻
C₂O₄²⁻ + Ca²⁺ → CaC₂O₄
When H₂C₂O₄ was added, it reacted with CaCl₂ to form CaC₂O₄ and HCl. Besides, H₂C₂O₄ dissociated partially.
H₂C₂O₄(aq) → H⁺(aq) + C₂O₄²⁻(aq)
Moreover, the concentration of C₂O₄²⁻ increased and the system shifted to the right. Then the solution had a white precipitate.
When HCl was added, H⁺ reacted with C₂O₄²⁻ to form H₂C₂O₄. Thus, the [C₂O₄²⁻] decreased and the system shifted to the left.
CaC₂O₄ + 2HCl → CaCl₂ + H₂C₂O₄
NH₄OH neutralizes H⁺ to form water. The equilibrium will shift to the right, producing more solid CaC₂O₄.
H₂C₂O₄ ↔ H⁺ + C₂O₄²⁻ H⁺ + OH⁻ H₂O Ca²⁺ + C₂O₄²⁻ CaC₂O₄ D. Comments
The equilibrium constant for this reaction:
When more product will be added, the system will be shift to the left. Conversely, when more reactant will be added,
the system will be shift to the right. Thus, the product and reactant are factors which determine the outcome (the amount of precipitate). II. Experiment
2.4. temperature effects on equilibria A. Procedures
Step 1: prepare 3 test tube with 3ml of 0.1M CoCl2
Step 2: add in HCl drop y drop, immediately stop when the solution turns purple-violet
Step 3: set Tube#1 at room temperature, observe the color
Step 4: set Tube#2 in a warm bath, then into an ice bath. Observe the color each time changing the environment
Step 5: set Tube#3 in an ice bath, then into an warm bath. Observe the color each time changing the environment B. Observations
[Co(H₂O)₆]²⁺(aq) + 4Cl⁻(aq)
[CoCl₄]²⁻(aq) + 6H₂O(l) observation none (control) purple violet purple violet
color changes back to initial color dark blue at initial, changes hot water bath to purple when in ice bath lOMoARcPSD|47231818
color might changes slightly in ice Ice water bath
first, then will significantly changes in warm bath explanation
the color is unchanged in room temperature
When the system’s temperature increases, the reaction shifts to counteract the change, forming more [Co(H₂O)₆]²⁺. The
concentration of Co²⁺ decreases, turning the solution dark blue. Conversely, cooling the system shifts the reaction to
form more [CoCl₄]²⁻. The concentration of Co²⁺ increases, returning the solution to its previous color.
When the system's temperature decreases, the reaction shifts to increase the temperature, forming more [CoCl₄]²⁻. The
concentration of Co²⁺ increases, turning the solution purple initially. Conversely, adding the solution to a warm bath
increases the temperature, shifting the reaction towards the reactants and turning the color dark blue. D. Comments
The equilibrium constant expression for this reaction is:
Kc = [CoCl₄²⁻] × [H₂O]⁶
[Co(H₂O)₆²⁺] × [Cl⁻]⁴
When the solution in hot water path (increasing temperature), the equilibrium will shift to the right (endothermic
reaction). Conversely, when the solution in ice water path, the equilibrium will shift to the left (exothermic reaction).
Thus, temperature is one factor that affects the color of solution. III. Conclusion
In this experiment, we learned about the reversible reaction and the effect of applying stresses to a variety of chemical
systems at equilibrium. The chemical reaction needed all those factors to be balanced. An extra drop can change the
difference in the color. Reversible reaction impacted many daily life things such asthe process of photosynthesis in plants,
the formation of lactic acid in muscles, and the baking of bread. During the experiment, we made some mistake such as
recorded the number wrong and made few extra drops, we have to do it again twice but we will improve further more.



