

Preview text:
International University, Vietnam National University - HCMC 1
GENERAL CHEMISTRY LABORATORY REPORT2
EXPERIMENT 2: pH AND BUFFERS
Group: 04 Submission date: 10/14/2023 Score: _________ No. Full name Student ID Contribution (%) 1 Le Thi Thu Ha IELSIU21194 100% 2 Le Thanh Thai IELSIU20412 100% 3 Le Huu Phat IELSIU20386 100% 4 Le Phuong Thao IELSIU20418 100% 5 Dinh Nam Dan BEBEIU21199 100%
International University, Vietnam National University - HCMC 2
GENERAL CHEMISTRY LABORATORY I. Introduction Theories
According to the Arrhenius theory, an acid is a substance that dissociates in water to form
hydronium ion (H3O+), and a base is a substance that dissociates in water to form hydroxide
(OH-) ions. For the Lewis-Brłnsted theory, an acid is a proton donor, and a base is a proton
acceptor. In an aqueous solution, the H+ from an acid is associated with water to form H3O+ (a
hydronium ion), while a base accepts a proton from water to form OH- (a hydroxide ion).
Strong acid/strong base is completely dissociated in water to produce hydronium ion/hydroxide
ion, respectively. Weak acid/base dissociates only partially in an aqueous solution and forms
little or very little H3O+ /OH-.
Acid: HA (aq) + H2O ⇌ H3O+(aq) + A-(aq) Ka Base: A-(aq) + H2O
⇌ HA (aq) + OH– (aq) Kb
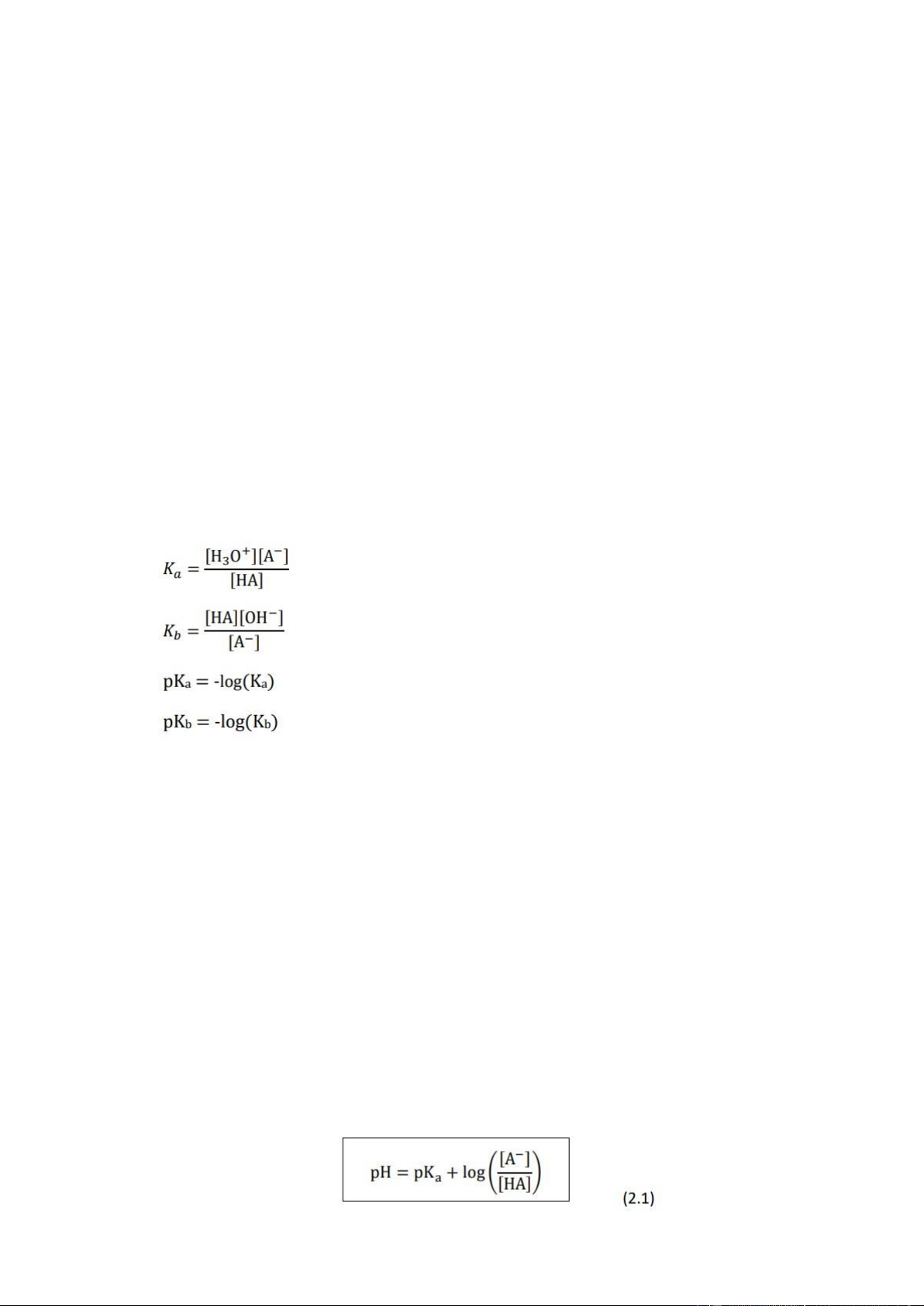
We have: Ka x Kb = Kwater = 1.0 x 10-14 at 25oC
The pH scale is a compact way to specify the acidity of a solution: pH = - log[H3O+] Therefore:
• Acidic solution: pH < 7 or [H3O+] > [OH-]
• Basic solution: pH > 7 or [H3O+] < [OH-]
• Neutral solution: pH = 7 or [H3O+] = [OH-]
Strong acids and strong bases are completely dissociated in water to produce hydrogen ions or
hydroxide ions, respectively. Weak acids dissociate only partially and form little or very little
H+ . A buffer is a solution of a weak acid or weak base and its conjugate weak base or weak
acid, respectively. Buffers have the function that resists a large change in pH on the addition of
H+ or OH- . This is because the weak base, A-, will react with added H+ and the weak acid, HA,
will react with added OH- . Changes in pH of buffer solutions can be determined using the
Henderson-Hasselbach equation:
International University, Vietnam National University - HCMC 3
GENERAL CHEMISTRY LABORATORY
A pH meter can be used to measure the pH of prepared solutions. Different classes of chemicals
behave differently when dissolved in water. By doing this experiment, you will gain a better
understanding of strong acids and strong bases, weak acids and weak bases, salts and buffers.
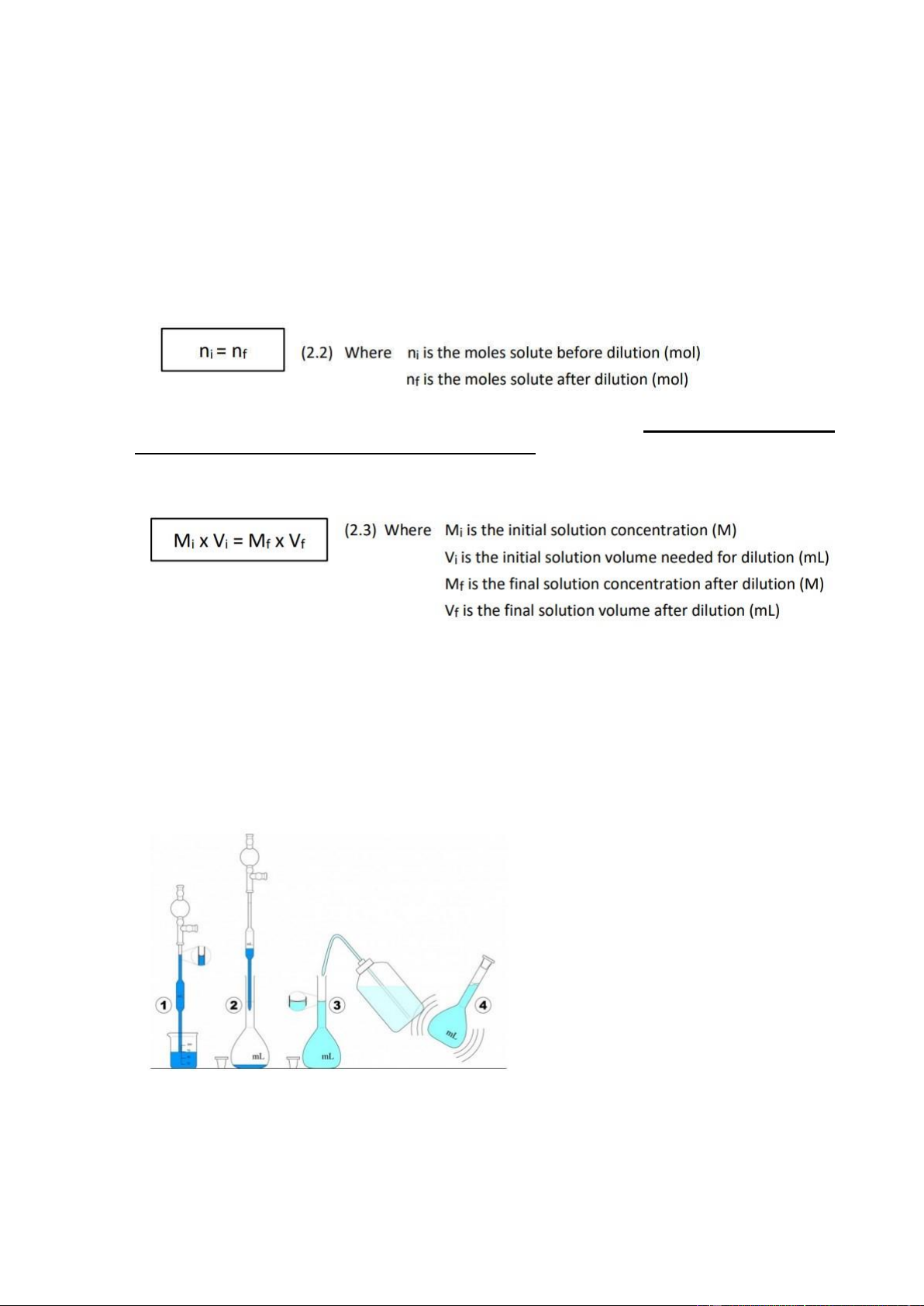
Dilution is the process of reducing the concentration of a solution by adding solvent into that
solution. In fact, the moles of solute after being diluted in solution are equal to the moles of
solute in the initial solution.
Furthermore, based on the concentration formula, we can know that the moles of solute = the
solution volume x the concentration of the solution. Therefore:
The protocol to make a standard solution-solution dilution:
a) Calculation: First, determine the volume of initial solvent needed for dilution by
substituting the given values into the formula Mi x Vi =Mf x Vf. Finally, Vi can be obtained. b) Equipment:
+ 1 Volumetric Flask (The value of Volumetric Flask must be equal to Vf.
+ 1 Pipette (The volumetric pipette is highly recommended due to its high accuracy measurement).
+ A container containing the amount of known concentration.
+ Solvent (must be the same as the solvent of the initial solution).
International University, Vietnam National University - HCMC 4
GENERAL CHEMISTRY LABORATORY c) Dilution process
(1) First take the needed volume of initial solution (Vi) by pipette
(2) Transfer the needed volume into volumetric flask
(3) Add solvent into the volumetric flask until the solution reaches the marked level of flask (meniscus)
(4) Close the cap and shake the flask gently
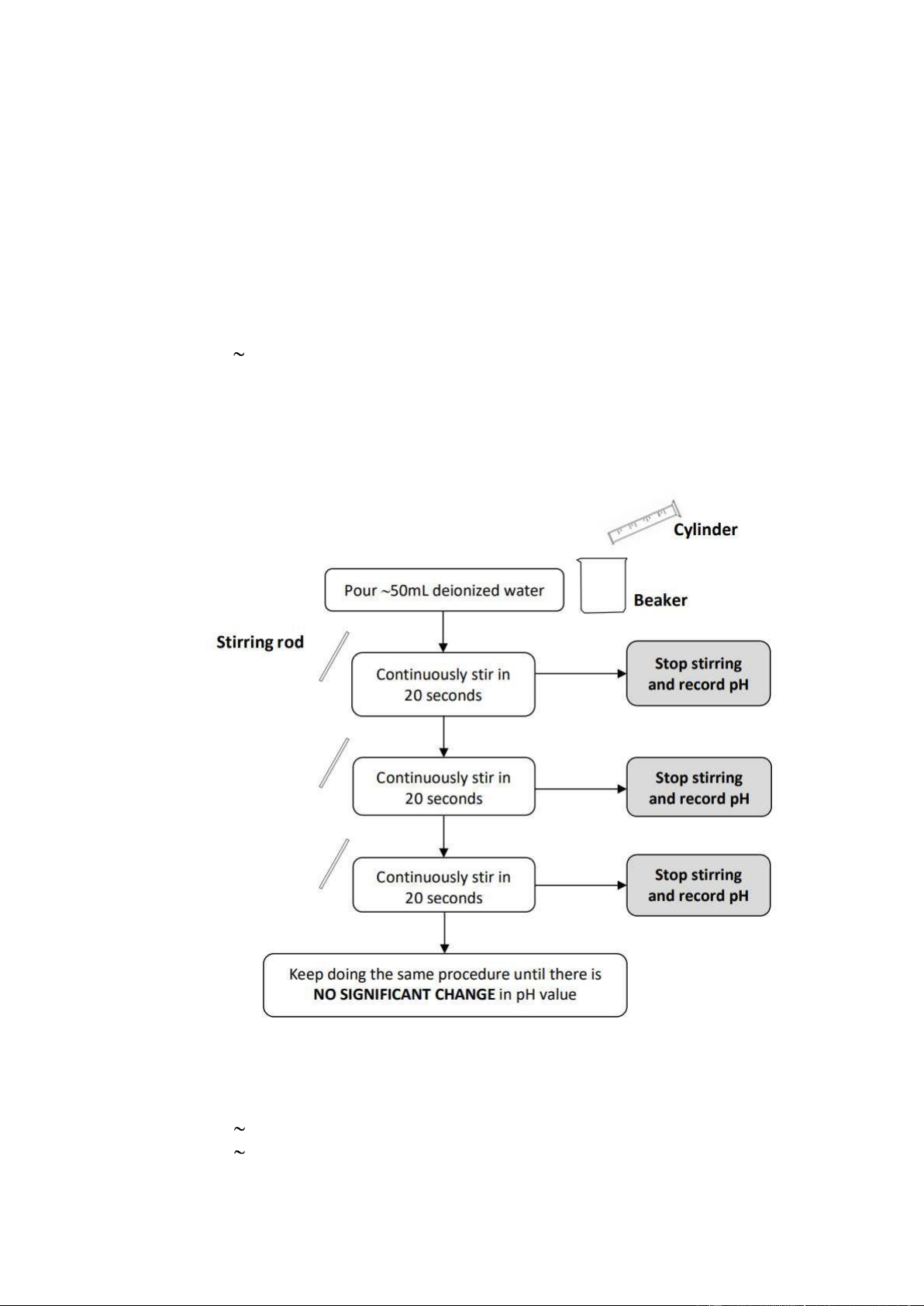
Experimental 1. pH OF DEIONIZED WATER
• Step 1: Pour 50mL deionized water.
• Step 2: Stirring rod. Continuously stir in 20 seconds -> Stop stirring and record pH.
• Step 3: Continuously stir in 20 seconds-> Stop stirring and record pH.
• Step 4: Continuously stir in 20 seconds-> Stop stirring and record pH.
• Step 5: Keep doing the same procedure until there is NO SIGNIFICANT CHANGE in pH value.
2. pH OF STRONG ACID Section 1: Preparation
• Step 1: Take 10mL 0.1M HCl.
• Step 2: Take 20mL 0.1M NaOH.
• Step 3: Prepare 100ml 0.01M NaOH solution (10mL 0.1M NaOH: 90mL H2O).
International University, Vietnam National University - HCMC 5
GENERAL CHEMISTRY LABORATORY
Section 2: pH measurement
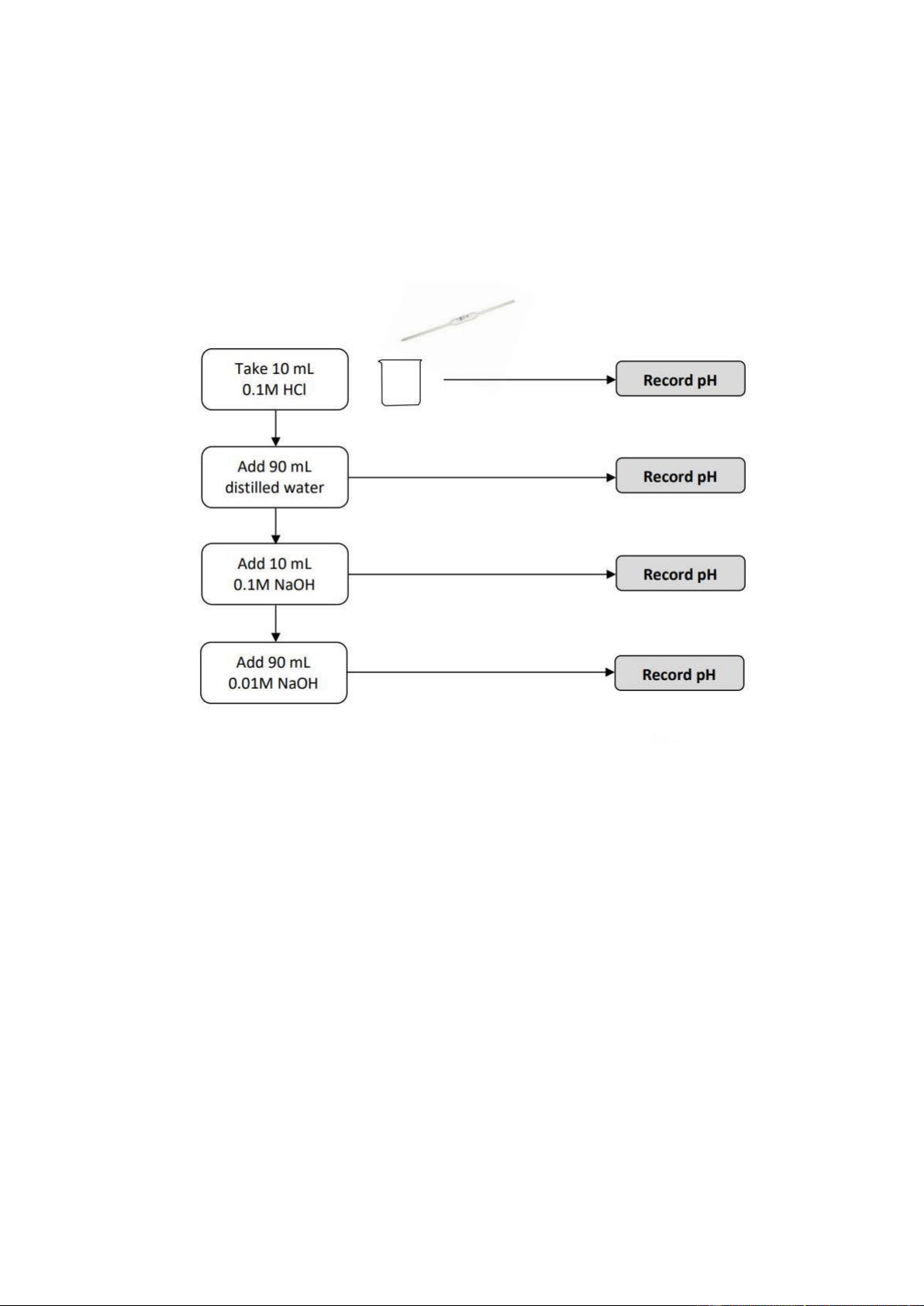
• Step 1: Take 10 mL 0.1M HCl –> Record pH.
• Step 2: Add 90 mL distilled water –> Record pH.
• Step 3: Add 10 mL 0.1M NaOH –> Record pH.
• Step 4: Add 90 mL 0.01M NaOH –> Record pH.
3. pH OF WEAK ACID Section 1: Preparation • Solution A: 0.1M CH3COOH.
• Solution B: 0.01M CH3COOH (dilute solution A 10 times).
• Solution C: 0.001M CH3COOH (dilute solution A 100 times or dilute solution B 10 times). Section 2: pH measurement
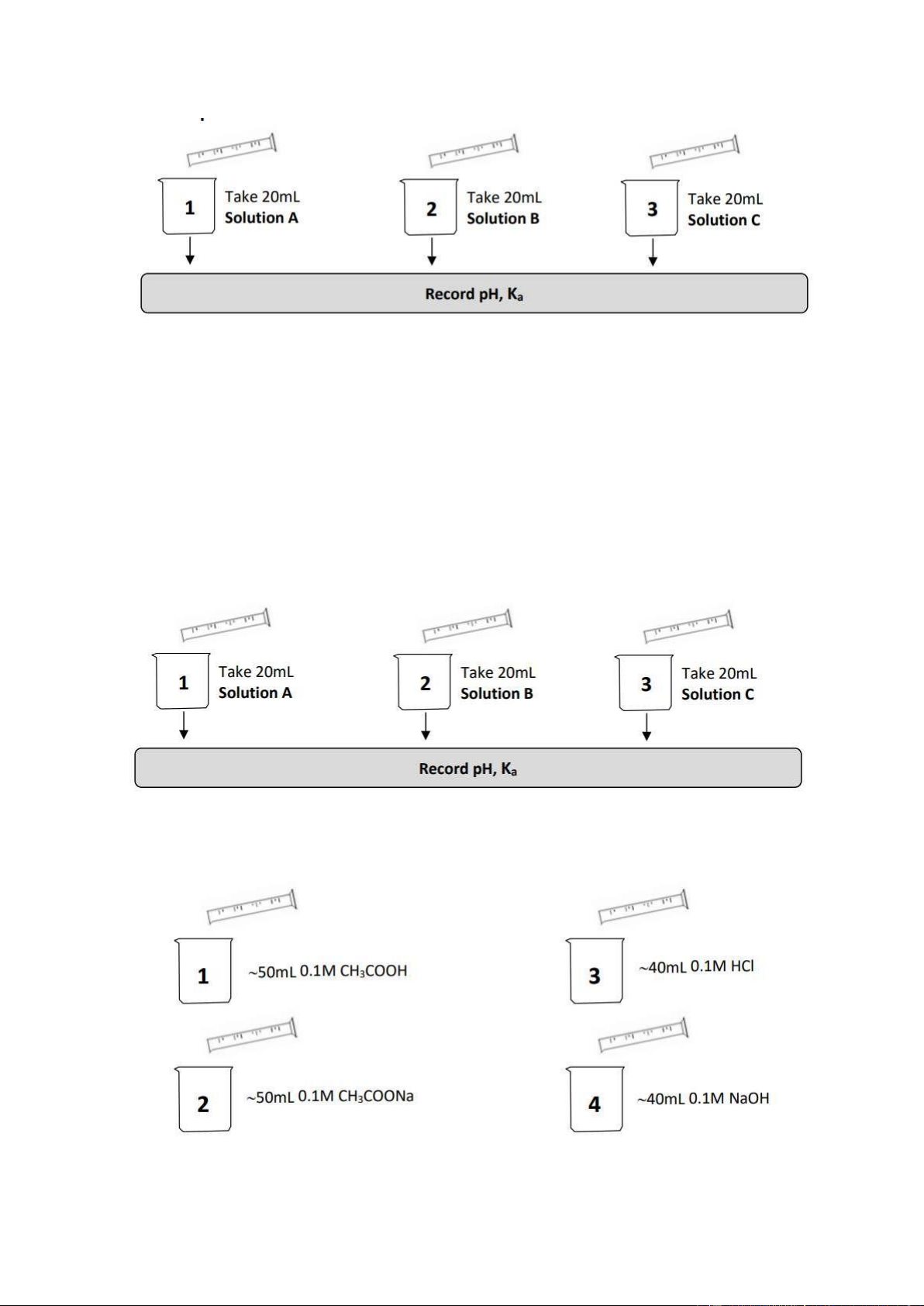
• Step 1: Take 20mL Solution A ->Record pH, Ka.
• Step 2: Take 20mL Solution B ->Record pH, Ka.
• Step 3: Take 20mL Solution C ->Record pH, Ka.
International University, Vietnam National University - HCMC 6
GENERAL CHEMISTRY LABORATORY
4. pH OF SALTS Section 1: Preparation Solution A: 0.1M NaCl Solution B: 0.1M CH3COONa Solution C: 0.1M NH4Cl Section 2: pH measurement
• Step 1: Take 20mL Solution A ->Record pH, Ka.
• Step 2: Take 20mL Solution B ->Record pH, Ka.
• Step 3: Take 20mL Solution C ->Record pH, Ka.
5. pH OF BUFFERS Section 1: Preparation Section 2: Buffer A
International University, Vietnam National University - HCMC 7
GENERAL CHEMISTRY LABORATORY
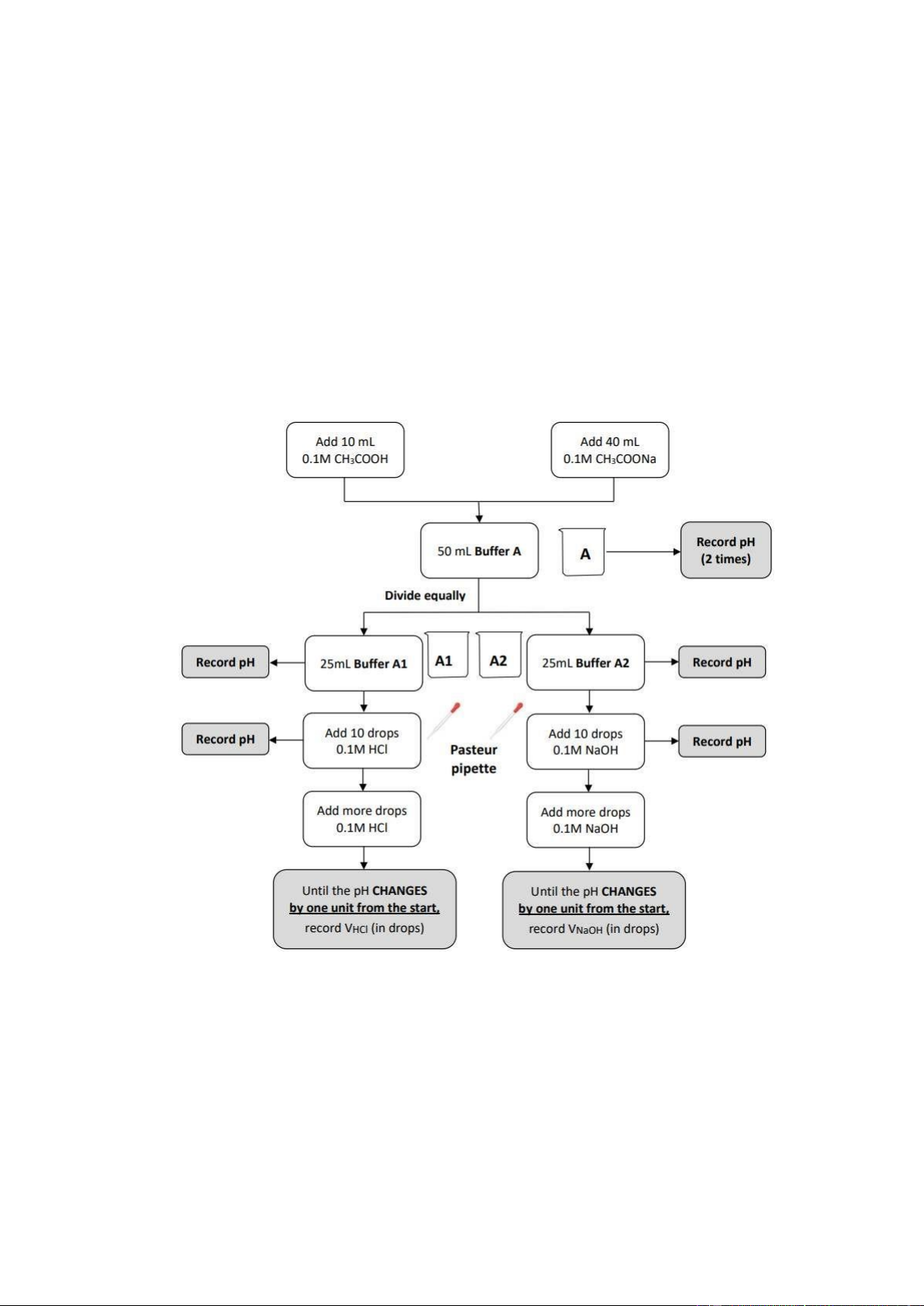
• Step 1: Add 10 mL 0.1M CH3COOH and Add 40 mL 0.1M CH3COONa to have 50 mL Buffer
A-> Record pH (2 times).
• Step 2: Divide equally into 2 parts: - A1
▪ 25mL Buffer A1-> Record pH.
▪ Add 10 drops 0.1M HCl-> Record pH.
▪ Add more drops 0.1M HCl.
▪ Until the pH CHANGES by one unit from the start, record VHCl (in drops). - A2
▪ 25mL Buffer A2-> Record pH.
▪ Add 10 drops 0.1M NaOH-> Record pH.
▪ Add more drops 0.1M NaOH.
▪ Until the pH CHANGES by one unit from the start, record VNaOH (in drops). Section 3: Buffer B
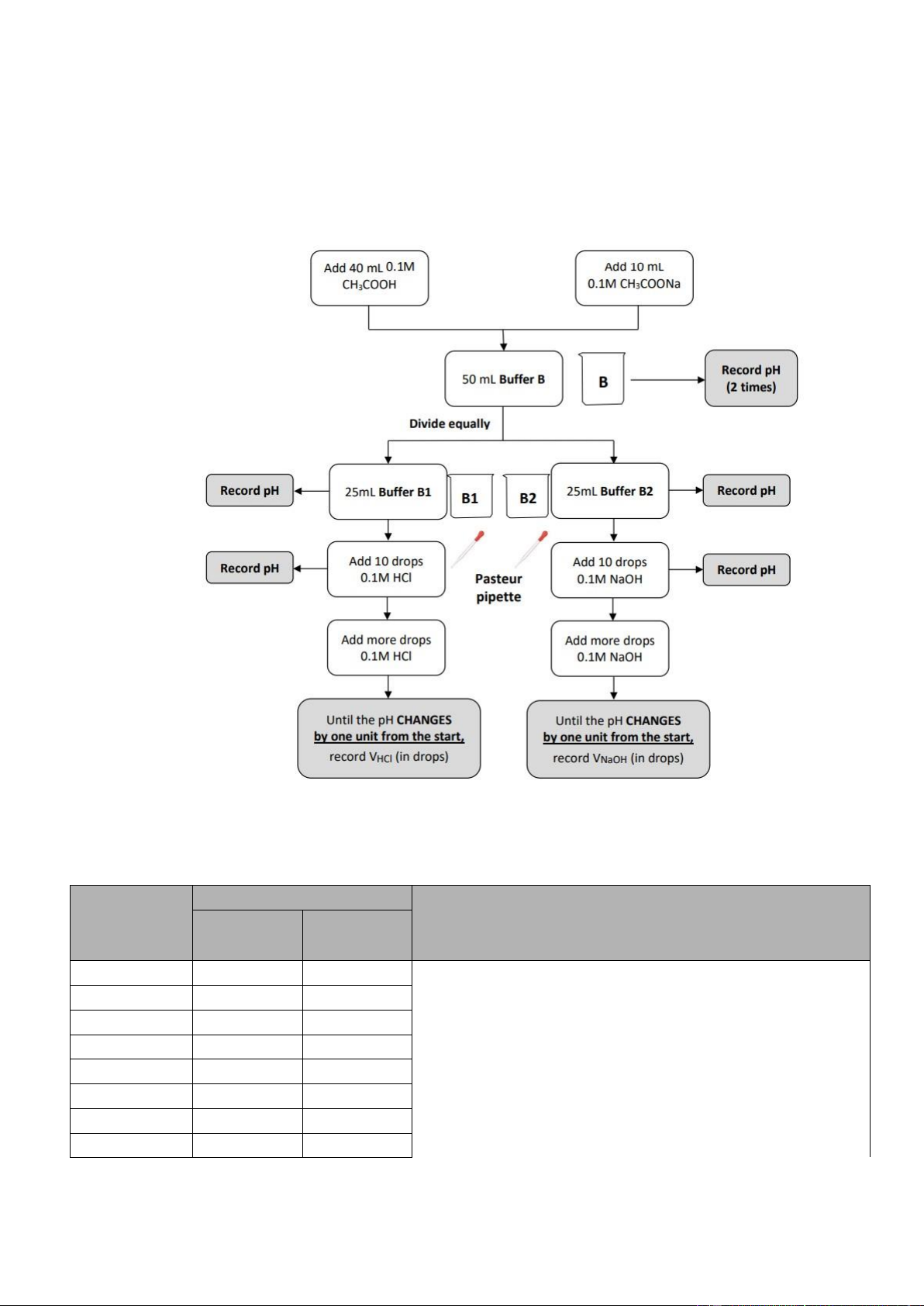
• Step 1: Add 40 mL 0.1M CH3COOH and add 10 mL 0.1M CH3COONa to have 50 mL Buffer
B-> Record pH (2 times).
• Step 2: Divide equally into 2 parts: - B1 ▪
25mL Buffer B1-> Record pH. ▪
Add 10 drops 0.1M HCl-> Record pH. ▪
Add more drops 0.1M HCl. ▪
Until the pH CHANGES by one unit from the start, record VHCl (in drops). - B2
International University, Vietnam National University - HCMC 8
GENERAL CHEMISTRY LABORATORY ▪
25mL Buffer B2-> Record pH. ▪
Add 10 drops 0.1M NaOH-> Record pH. ▪
Add more drops 0.1M NaOH. ▪
Until the pH CHANGES by one unit from the start, record VNaOH (in drops).
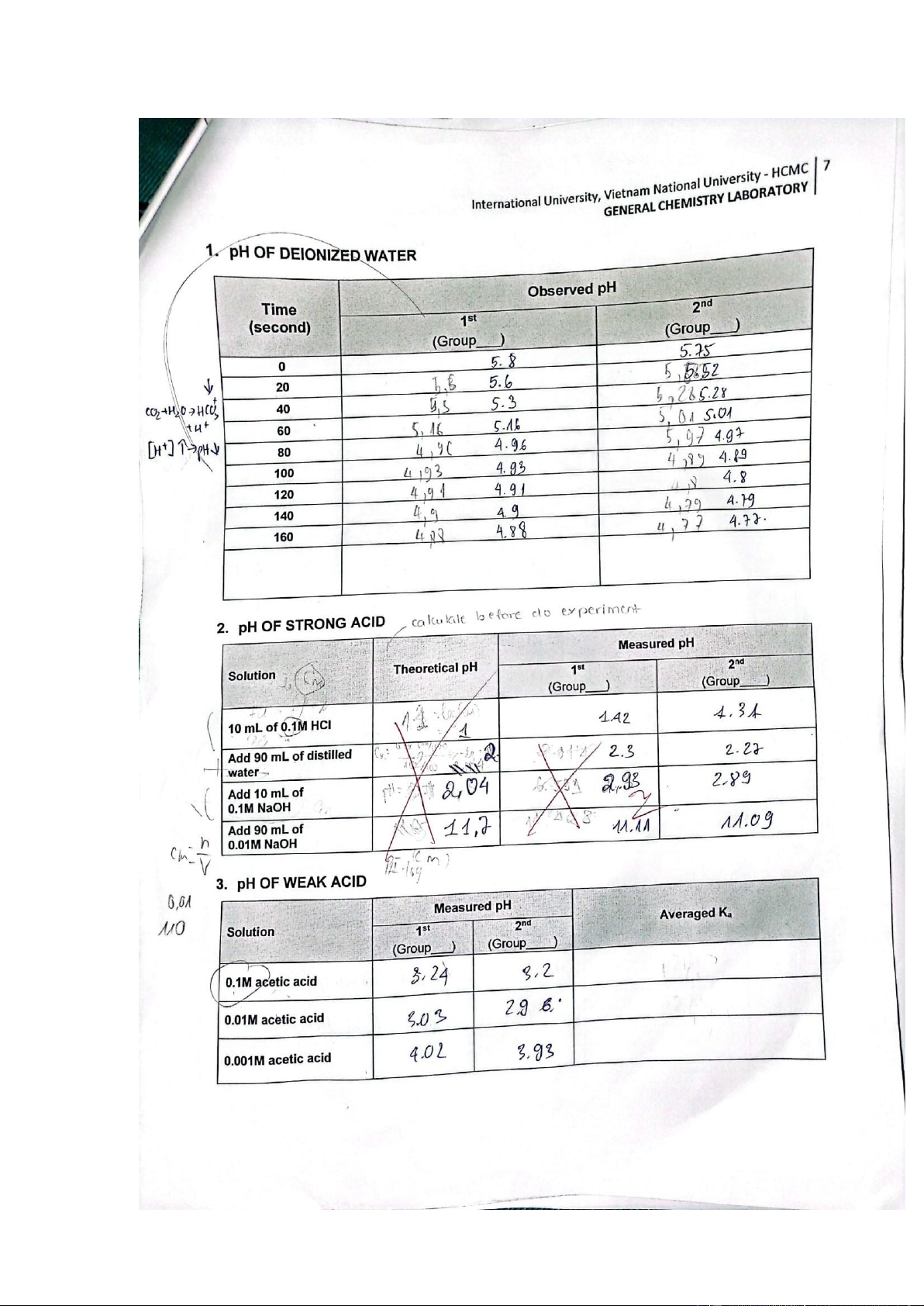
II. Results and discussion 1. pH OF DEIONIZED WATER Observed pH Time 1st 2nd Explanation (second)
(Group___) (Group___) 0 5.80 5.75
According to the theory, the quantity of distilled water is 20
quite limited, resulting in an equal concentration of H₃O⁺ 5.60 5.52
and H⁺ ions. As a result, the pH level of water is almost 7. 40 5.30 5.28 60 5.16 5.01
Nevertheless, following our initial experiment, we (group 80 4.96 4.97
4) discovered that the pH level of distilled water actually 100 4.93 4.89
drops within the range of 5.80 to 4.88. 120 4.91 4.80 140 4.90 4.79
International University, Vietnam National University - HCMC 9
GENERAL CHEMISTRY LABORATORY 160 4.88 4.77
“The possible explanation is that the water container or the
stirring rod might contain a small amount of base, which
consequently causes the pH of the water to undergo a change." Comments:
- According to the theory, the pH of distilled water should be around 7. However, our
experimental results diverged from this expectation. This discrepancy may be attributed to
potential contamination from the water container or the stirring rod used by our group,
which could have introduced a base.
- Consequently, our pH value differs from that of the others. In reality, the outcome of the
experiment can be influenced by various factors. To obtain precise results, it is imperative
to exercise caution and ensure that the experimental conditions meet the established criteria. 2. pH OF STRONG ACID Measured pH Theoretical Solution pH Comments/ Explanation 1st 2nd (Group___) (Group____) The variation between two values could be due to the evaporation of the solution,
which results in a reduction in
hydrogen ions. HCl is a potent
acid that fully dissociates in the
solution, releasing a substantial 1.00 amount of H⁺ ions. 1.42 1.31 10 mL of 0.1M HCl Consequently, the concentration of H⁺ ions is
given by: [H⁺] = [HCl] = 0.1M, and the calculated pH of the solution is as follows: pH = log[H⁺] = -log[0.1] = 1.
International University, Vietnam National University - HCMC 10
GENERAL CHEMISTRY LABORATORY The measured pH value closely resembles the theoretical pH.
Upon the addition of distilled
water to the solution, the H⁺ Add 90 mL of distilled 2.00 concentration decreased by 2.30 2.27
0.01 mol/L ([H⁺] = (0.1 mol/L) water x (0.01 L) / (0.1 L) = 0.01 mol/L). Subsequently, the pH value becomes: pH = -log[0.01] = 2.
The measured pH value is close to the theoretical pH. Add 10 mL of 2.04 2.93 2.89 NaOH is a strong base, and 0.1M NaOH strong bases can completely
dissociate to release OH⁻ ions
into the solution. OH⁻ ions react with the H⁺ ions already
present in the solution to form water molecules.
We calculate the concentrations of H⁺ and OH⁻ as follows:
[NaOH] = [HCl] = (0.1 mol/L) x
(0.01 L) / (0.11 L) = 9.1 x 10⁻³. Because [H⁺] = [OH⁻], the
solution is neutral, and the pH is equal to pOH, which is 7.
International University, Vietnam National University - HCMC 11
GENERAL CHEMISTRY LABORATORY
The inadequate stirring of the
solution resulted in the disparity between the theoretical and measured values. When additional NaOH is
introduced, the concentration of
OH⁻ in the solution increases. We Add 90 mL of
have: [OH⁻] = [(0.01 mol/L) x 11.70 11.11 11.09
(0.09 L)] / (0.2 L) = 4.5 x 10⁻³. 0.01M NaOH
Therefore, pOH = -log[OH⁻] = 2.35.
The pH value is then calculated as: pH = 14 - pOH = 14 - 2.35 = 11.65=11.70
Comments: When adding NaOH solution, the H+ ions in the acid are neutralized by OH- ions, and
the excess OH- ultimately results in an increase in pH. 3. pH OF WEAK ACID Measured pH Solution 1st 2nd Ka Comments/ Explanation (Group___ (Group____ ) )
Acetic acid is a weak acid, meaning
it only partially dissociates in the 0.1M acetic acid 3.24 3.20
3.31x10-6 solution. This substance has [H⁺] =
[A⁻] and Ka=(10-3.24)2/0.1=3.31x10- 6
When the solution’s concentration 3.03 2.96
reduced, the dissociation of H⁺ 0.01M acetic acid 8.71x10-5 ions decreased as well Ka=(10- 3.03)2/0.01=8.71x10-5
When the solution’s concentration 9.12x10-6
lowered, there was a reduction in 0.001M acetic acid 4.02 3.93
the dissociation of H⁺ ions Ka=(10- 4.02)2/0.001=9.12x10-6 Comments:
We are provided with an equation to calculate:
Ka=[H+][A−]/[HA].
As indicated by the previous findings, it’s evident that the solution’s concentration affects the
solution’s pH. The higher the pH, the smaller the equilibrium constant Ka and vice versa.
International University, Vietnam National University - HCMC 12
GENERAL CHEMISTRY LABORATORY 4. pH OF SALTS Measured pH Explanation Predicted Solution 1 2 pH st nd (Group____) (Group____) While salt typically has a pH
value around 7, it was measured 7 6.25 6.25 at approximately 5.0 in this 0.1M NaCl experiment. This discrepancy
could be attributed to technical issues with the pH meter.
Sodium acetate (CH₃COONa) is a salt that results from the
combination of a weak acid and >7 7.15 7.18
a strong base. When dissolved in 0.1M CH 3COONa
a solution, it breaks down into
CH₃COO⁻ and Na⁺ ions. The measured pH closely approximates the expected pH.
NH₄Cl is a salt resulting from the
combination of a weak base and a strong acid. When it’s
dissolved in a solution, it breaks
down into NH₄⁺ and Cl⁻ ions. 0.1M NH4Cl <7 4.70 4.69
While the Cl⁻ ion is electrically
neutral, it has acidic properties
that cause the solution to have a pH below 7. The measured pH closely aligns with the anticipated pH. Comments:
The measured pH is in close proximity to the expected pH. In the first experiment, this similarity
might be attributed to potential technical issues with the pH meter or the possibility of the stirring
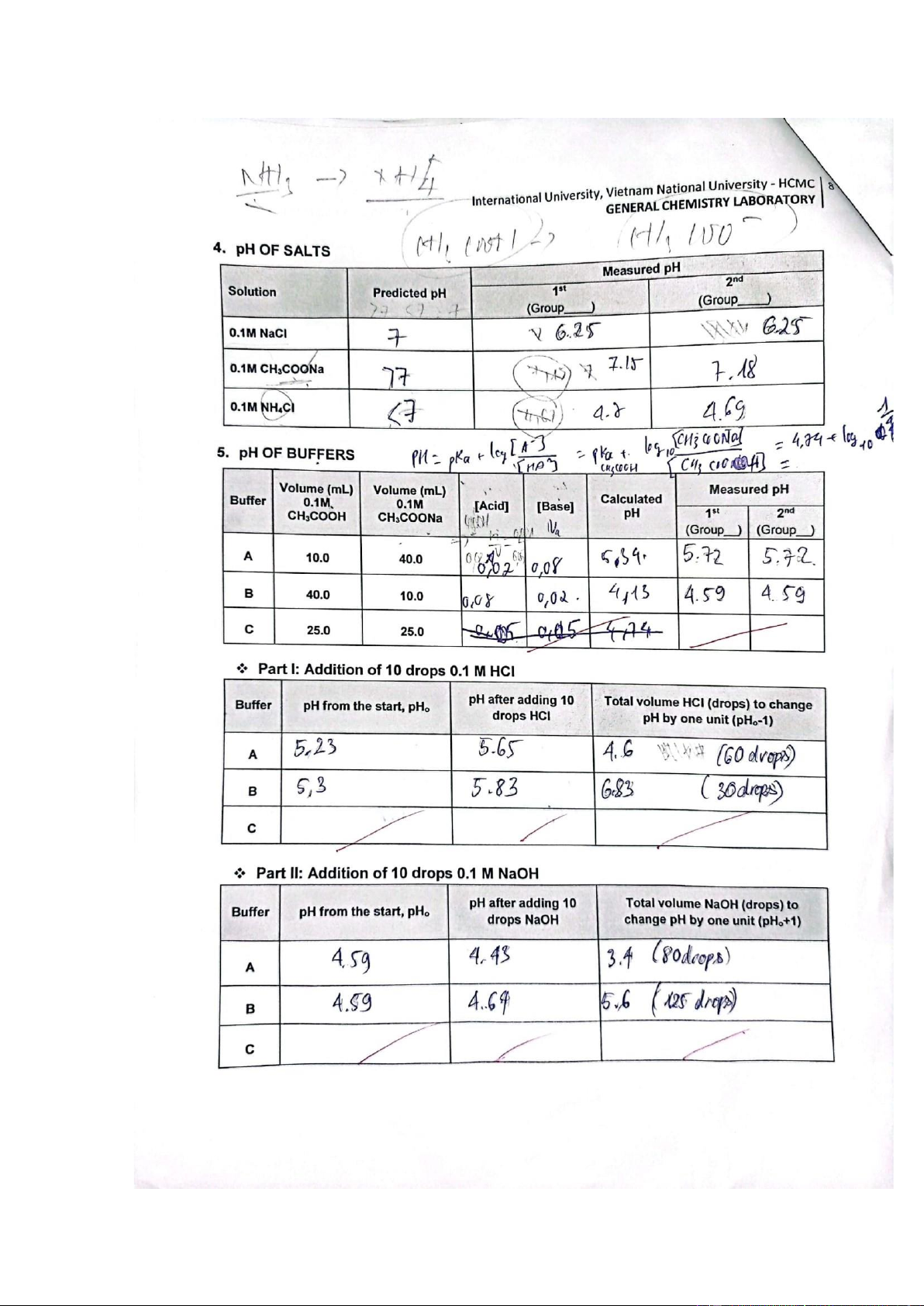
process being affected by some remaining impurities of a base. 5. pH OF BUFFERS Measured pH Volume Volume (mL) Calculated
Buffer (mL) 0.1M 0.1M Acid Base 1st pH 2nd CH 3COOH CH3COONa (Group__ ) (Group__) A 10.0 40.0 0.02 0.08 5.34 5.72 5.72 B 40.0 10.0 0.08 0.02 4.13 4.59 4.59 Comments:
The value pH when calculating and measuring is approximately equal.
International University, Vietnam National University - HCMC 13
GENERAL CHEMISTRY LABORATORY
Applying the same concept for mixture B
Part I: Addition of 10 drops 0.1 M HCl pH from pH after Total volume HCl Buffer the start, adding 10
(drops) to change pH by Comments/ Explanation pHo drops HCl one unit (pHo-1) CH₃COOH is a weak acid
and only partially dissociates in the solution, whereas CH₃COONa completely dissociates. *CH₃COOH ⇌ CH₃COO⁻ + H⁺ *CH₃COONa ⇌ CH₃COO⁻ + Na⁺ * The solution contains CH₃COOH, CH₃COO⁻, H⁺, Na⁺ from CH₃COONa, and CH₃COOH. It is evident that
the acetate ions (CH₃COO⁻) outnumber the H⁺ ions, yet the solution maintains an acidic pH. *When 0.1M HCl A 5.23 5.65 4.6 (60 drops) is added, acetate ions
International University, Vietnam National University - HCMC 14
GENERAL CHEMISTRY LABORATORY combine with H⁺ from HCl, reducing the excess H⁺ and generating more CH₃COOH. Surprisingly, the addition of HCl does not significantly decrease the pH value of the solution. B 5.30 5.83 6.83 (30 drops)
Comments: 0.25 pts Part II: Addition of 10 drops 0.1 M NaOH pH from the pH after Total volume NaOH
Buffer start, pHo adding 10
(drops) to change pH by Comments/ Explanation drops NaOH one unit (pHo+1) A 4.59 4.43 3.4 (80 drops) When NaOH is introduced into the solution, acetic acid (CH₃COOH) reacts with NaOH to produce acetate (CH₃COO⁻) and form B 4.59 4.64 5.6 (125 drops) CH₃COONa. Surprisingly, the addition of NaOH does not
result in a substantial increase
in the pH value of the solution.
Comments: When we add the NaOH base solution to the buffer, the pH value increases
because the concentration of H⁺ ions decreases. The observed volume in the experiment differs
from the theoretical volume due to variations in laboratory conditions and potential errors
during the experimental procedure.
International University, Vietnam National University - HCMC 15
GENERAL CHEMISTRY LABORATORY III. Data sheet
International University, Vietnam National University - HCMC 16
GENERAL CHEMISTRY LABORATORY
International University, Vietnam National University - HCMC 17
GENERAL CHEMISTRY LABORATORY
International University, Vietnam National University - HCMC 18
GENERAL CHEMISTRY LABORATORY IV. Conclusion
When we introduce the NaOH base into the buffer solution, the pH level goes up. This occurs because
the concentration of H⁺ ions decreases in the solution. The deviation in the observed volume compared
to the theoretical volume may stem from differences in laboratory conditions and potential errors
during the experimental process.



