


Preview text:
lOMoAR cPSD| 58504431
PRACTICAL 5: BACTERIAL POPULATION COUNTS
INTRODUCTION TO BACTERIAL
IDENTIFICATION PROCESS CULTURING
UNKNOWN BACTERIUM FOR SUBSEQUENT ANALYSIS AIMS OF THE PRACTICAL
Introduce students different methods used to estimating size of a bacterial population.
Familiarize students with the procedure used to characterize an unknown bacterial microorganism.
Culturing unknown sample for practical assessment. 1. INTRODUCTION 1.1.
Bacterial population counts
Many bacteriological studies require that we be able to determine the number of organisms that
are present in a given unit of volume. Several different methods are available to us for such
population counts. The method one uses is determined by the purpose of the study. We learn
the principals of quantitative plating (Standard Plate Count, or SPC) and turbidity
measurements to determine the number of bacteria in a culture sample. Although the two
methods are somewhat parallel in the results they yield, there are distinct differences. For one
thing, the SPC reveals information only as related to viable organisms; that is, colonies that are
seen on the plates after incubation represent only living organisms, not dead ones. Turbidimetry
results, on the other hand, reflect the presence of all organisms in a culture, dead and living. In
this exercise we do SPC method, turbidity measurement method is referred in appendix 4.
Quantitative plating method (Standard Plate Count)
In determining the number of organisms present in water, milk, and food, the standard plate
count (SPC) is universally used. It is relatively easy to perform and gives excellent results. We
can also use this basic technique to calculate the number of organisms in a bacterial culture. It
is in this respect that this assignment is set up. One example of diluting the organisms with a
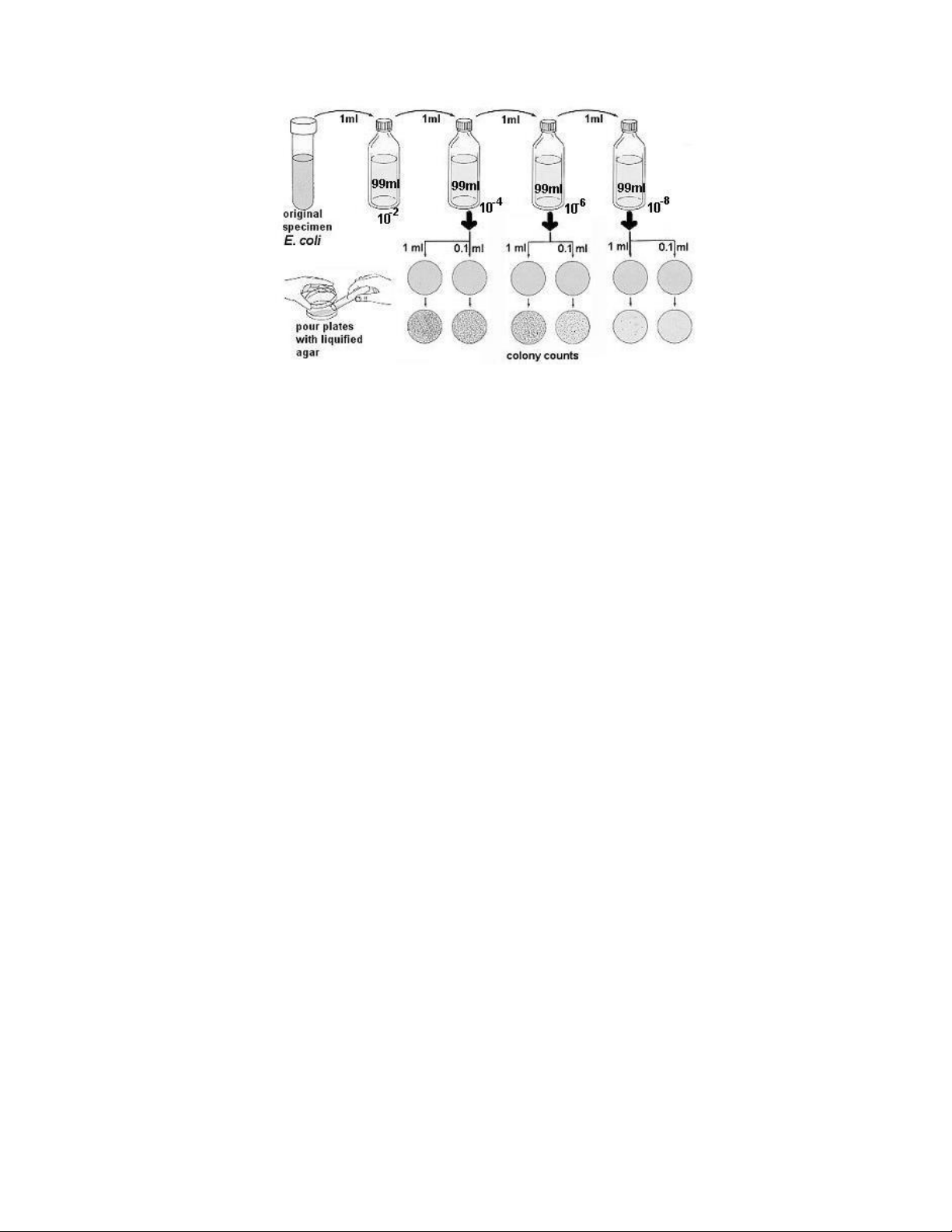
series of sterile water blanks is illustrated in figure 5.1. lOMoAR cPSD| 58504431
Figure 5.1. Quantitative plating procedure.
Generally, only three bottles are needed, but more could be used if necessary. By using the
dilution procedure indicated here, a final dilution of 1:1,000,000 occurs in blank C. From blanks
B and C, measured amounts of the diluted organisms are transferred into empty Petri plates.
Nutrient agar, cooled to 50° C, is then poured into each plate. After the nutrient agar has
solidified, the plates are incubated for 24 to 48 hours and examined. A plate that has between
30 and 300 colonies is selected for counting. From the count it is a simple matter to calculate
the number of organisms per milliliter of the original culture. It should be pointed out that
greater accuracy can be achieved by pouring two plates for each dilution and averaging the
counts. Duplicate plating, however, has been avoided for obvious economic reasons. Pipette
Handling Success in this experiment depends considerably on proper pipetting techniques (Appendix 5).
Turbidity measurement method
When it is necessary to make bacteriological counts on large numbers of cultures, the
quantitative plate count method becomes a rather cumbersome tool. It not only takes a
considerable amount of glassware and media, but it is also time-consuming. A much faster
method is to measure the turbidity of the culture with a spectrophotometer and translate this
into the number of organisms. To accomplish this, however, the plate count must be used to
establish the count for one culture of known turbidity. You actually used this method to generate
the growth curve of bacteria in practical number 4. Further details about this method are described in Appendix 4. 1.2.
Introduction to bacterial identification process
One of the most interesting experiences in introductory microbiology is to attempt to identify
an unknown microorganism that has been assigned to you as a laboratory problem. The next
exercises pertain to this phase of microbiological work. You will be given one or more cultures lOMoAR cPSD| 58504431
of bacteria to identify. The only information that might be given to you about your unknowns
will pertain to their sources and habitats. All the information needed for identification will have
to be acquired by you through independent study. Although you will be engrossed in trying to
identify an unknown organism, there is a more fundamental underlying objective of this series
of exercises that goes far beyond simply identifying an unknown. That objective is to gain an
understanding of the cultural and physiological characteristics of bacteria. Physiological
characteristics will be determined with a series of biochemical tests that you will perform on
the organisms. Although correctly identifying the unknowns that are given to you is very
important, it is just as important that you thoroughly understand the chemistry of the tests that
you perform on the organisms. The first step in the identification procedure is to accumulate
information that pertains to the organisms’ morphological, cultural, and physiological
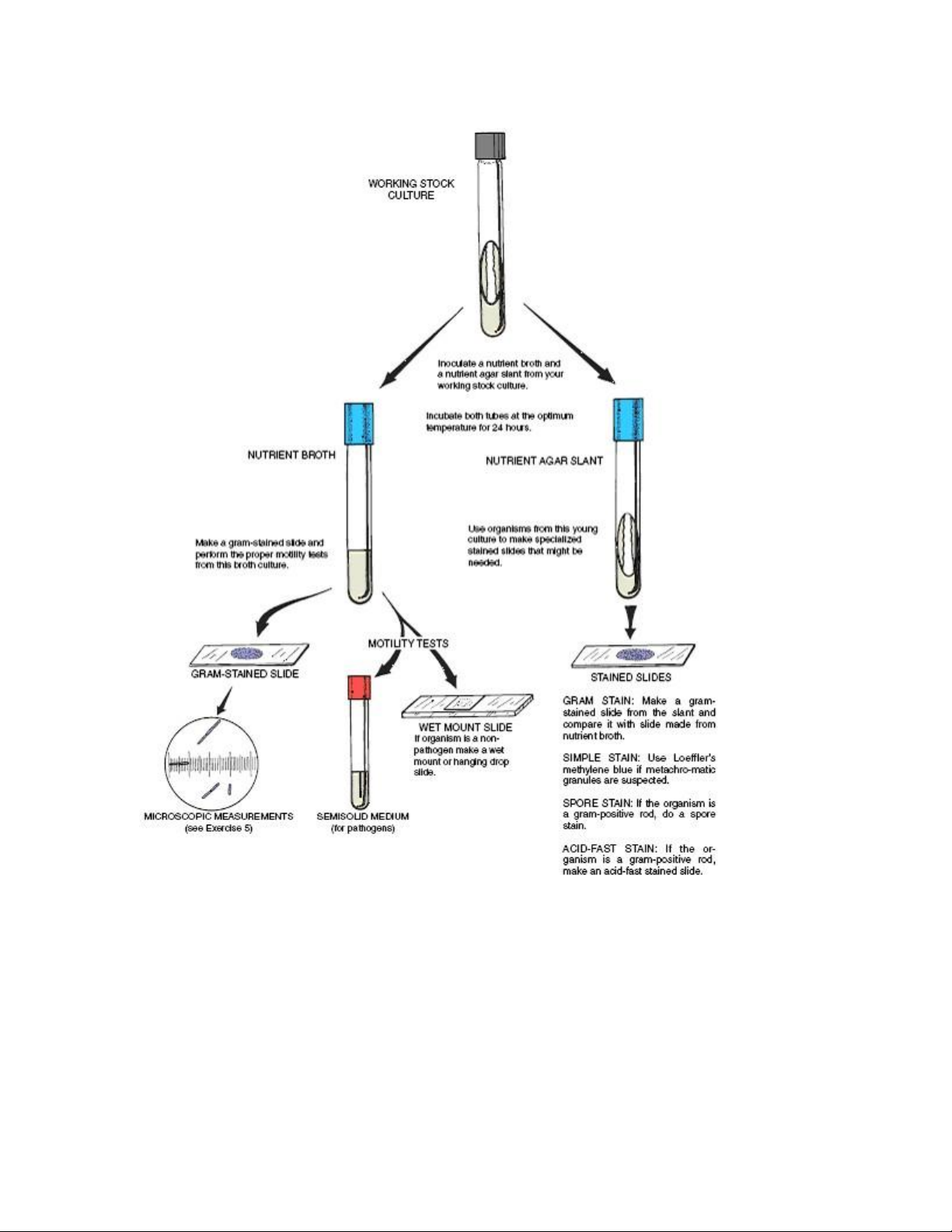
(biochemical) characteristics (figure 5.2 and figure 5.3). This involves making different kinds
of slides for cellular studies and the inoculation of various types of media to note the growth
characteristics and types of enzymes produced. lOMoAR cPSD| 58504431
Figure 5.2. Procedure for morphological study. lOMoAR cPSD| 58504431
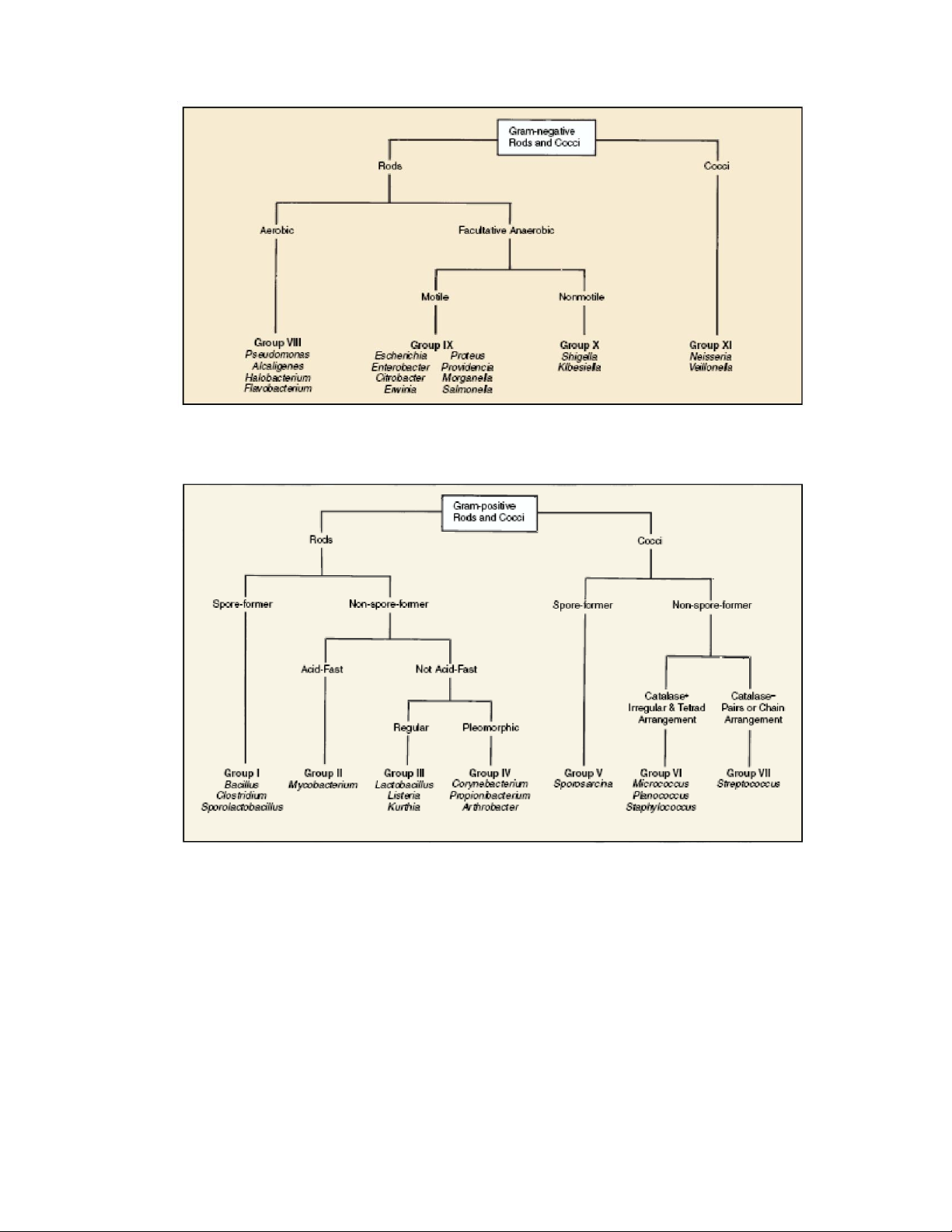
Figure 5.3: Bacterial classification 2. PROCEDURE 2.1.
Diluting and Plating Procedure Materials and tools:
Broth culture of E. coli Sterile LB medium
6 nutrient agar plates or LB plates lOMoAR cPSD| 58504431
P200 and P1000 micropipettors, appropriate sterile micro-tips Sterile tubes Spreader Ethanol Bunsen burner and lighter Tissue paper and marking pen
Cannister for discarded pipettes
1. Shake the culture of E. coli and transfer 0.5 ml of the organisms to the tube of 5 ml
sterile LB, we have a 1/10 dilution of the original one. After using the pipette, place it in the discard cannister.
2. Perform another 10-fold dilution by transferring 0.5 ml culture from the first dilute
sample to another tube containing 5 ml sterile LB. This tube we will have 1/100 dilution of the original culture.
3. Continue perform serial dilutions until generating final dilution of 10-9.
4. Use the spread-plate technique to transfer a small volume from each diluted sample to
a sterile LB agar plate (figure 5.4). Incubate the plates at 37° C for 16 hours in inverted position.
Figure 5.4. Spread-plate technique.
2.2. Counting and Calculations (SPC method) Materials: lOMoAR cPSD| 58504431 Culture plates Quebec colony counter Mechanical hand counter
1. Lay out the plates on the table in order of dilution
2. Place the plate on the Quebec colony counter. Start counting at the top of the plate, using
the grid lines to prevent counting the same colony twice. Use a mechanical hand counter.
Count every colony, regardless of how small or insignificant.
3. Comparing number of colonies among the plates. Choose the right plate to estimate the
number of bacteria in the original culture.
2.3 Introduction to bacterial identification process – Culture an unknown bacterium Materials and tools: Working unknown stock culture MacConkey agar (MC) Blood agar (BA) Inoculating loop Bunsen burner and lighter Marking pen and plastic wrap
Disinfectant and tissue paper Procedure:
Inoculate the unknown bacteria into BA and MC, incubate 370C in 24 hrs.
You will use these cultures to perform characterization of the unknown in the next practical,
which will be assessed by your tutor. 3. DISCUSSION
1. Why is it necessary to perform a plate count in conjunction with the turbidimetry procedure? 2. What is a CFU?
3. Outline some steps that you used to identify your unknown (to answer this question, also read Practical 6).



