





Preview text:
Journal of Food Processing and Preservation ISSN 1745-4549
MECHANICAL AND BARRIER PROPERTIES OF HYDROXY
PROPYL METHYL CELLULOSE EDIBLE POLYMER FILMS WITH PLASTICIZER COMBINATIONS
MAHADEVAIAH1, LACHAKAL RUDRAPPA SHIVAKUMARA1, THIPPAIA DEMAPPA1,3 and VASUDEV SINGH2
1Department of Polymer Science, University of Mysore, Sir M. Visvesvaraya Post-Graduate Center, Tubinakere, Mandya 571402, Karnataka, India
2Department of Grain Science and Technology, CFTRI, Mysore, Karnataka, India 3Corresponding author. ABSTRACT
TEL: 0821 2487382, 08232 291112; FAX: 08232291112;
Edible films and coatings have received considerable attention in recent years
EMAIL: tdemappa2003@yahoo.co.in
because of their advantages including use as edible packaging materials over syn-
thetic films. This could contribute to the reduction of environmental pollution.
Received for Publication November 6, 2015
Edible films of hydroxy propyl methyl cellulose (HPMC) containing mixtures of
Accepted for Publication March 14, 2016
glycerol(Gly) and polyethylene glycol (PEG) as plasticizers were prepared by water
solution caste technique and evaluated mechanical (tensile strength, elongation at doi:10.1111/jfpp.13020
break. burst strength, impact strength and Young’s modulus) and barrier proper-
ties (water vapor transmission rate and oxygen transmission rate), color and haze
properties were measured. Intermolecular interactions between HPMC-glycerol
and HPMC-PEG were measured using FT-IR technique. Thermal properties were
investigated by differential scanning calorimetry analysis. All these properties
were found to be improved with variation of plasticizers concentrations. PRACTICAL APPLICATIONS
This work is on food packaging. Polymer films are edible films. These films are eco-
friendly and bio-plastics and have good mechanical, barrier and optical properties. INTRODUCTION
oxygen, carbon dioxide and lipid movement in food sys-
tems, provide potential solutions to such concerns (Habig
For a long time, polymers have supplied most of common
McHugh et al. 1993). Polysaccharide-based edible films are
packaging materials because they present several desired fea-
hydrophilic and provide strong hydrogen bonding that can
tures like softness, lightness and transparency (Siracusa et al.
be used to bind with functional additives such as flavors,
2008). Generally plastics are relatively permeable to small
colors and micronutrients (Miller and Krochta 1997;
molecules like gases, water vapor, liquids and organic
Saucedo-Pompa et al. 2009). Polysaccharides films have
vapors; they provide a broad range of heat and mass transfer
characteristics ranging from excellent to low barrier value,
poor water-vapor barrier properties because of their hydro-
which are necessary in the case of food products (Siracusa
philic characteristics, but these hydrophilic polymers can
et al. 2008). However, increased use of synthetic packaging
form strong chain-to-chain interactions that provide a good
films has led to serious ecological problems due to their total
barrier to O2 and CO2 (Ayranci and Cetin 1997; Ayranci
nonbiodegradability. Although their complete replacement
et al. 1997). Glycerol (Gly) is the most widely used plasti-
with eco-friendly packaging films is just impossible to
cizer for carbohydrate films because of its small size, low
achieve, at least for specific applications like food packaging
molecular weight and hydrophilic nature, which make it
the use of bio-plastics should be the future. Consumers
compatible with HPMC films. Glycerol and polyol (ethylene
demand higher quality and longer shelf-life in foods while
glycol)s have been shown the most effective plasticizers for
reducing disposable packaging materials and increasing
cellulose-based films and are efficient oxygen and aroma
recyclability. Such concerns have caused increased interest
barriers, but they are ineffective against water-vapor because
in edible film research. Edible films, by regulating water,
they are hydrophilic (Donhowe and Fennema 1993; Park
Journal of Food Processing and Preservation 00 (2016) 00–00 V C 2016 Wiley Periodicals, Inc. 1
CHARACTERIZATION AND APPLICATION OF HPMC EDIBLE POLYMER FILMS MAHADEVAIAH ET AL.
et al. 1993). It is a high boiling point plasticizer, water-
0.05% and PEG 0–0.05%) concentrations were optimized
soluble, polar, nonvolatile and protein and carbohydrate
for preparation of pure and blends of HPMC-Gly and
miscible. These properties make glycerol the most suitable HPMC-PEG films.
plasticizer for use with a compatible water-soluble polymer
(Banker et al. 1966). Gly is also harmless as plasticizers for
films in contact with food stuff and is frequently used as Film Thickness Measurement
sweetener in food stuff. The addition of a plasticizing agent
Thicknesses of films were measured using a hand-held
is necessary in order to reduce brittleness and to increase
micrometer and the units were expressed in mm. The dried
flexibility through a reduction in film cohesion, although
HPMC films were placed between the Anvil and Spindle,
plasticizers can also increase film permeability (Gennadios
and thickness was measured at different (at least 5–6) places
and Weller 1990; Gontard et al. 1993). HPMC films are
of the film. The mean of all the measurements were taken to
resistant to fats and oils, and are therefore likely to be good
obtain an average value. Thickness is increased with increase
aroma barriers (Nisperos-Carriedo 1994). The most com-
concentration of HPMC and gradually decreased with
mon plasticizers used are mono-, di-, polyols and oligosac-
increase of plasticizers (ASTM 1980b). The film thickness
charides. Aydinli et al. reported the effect of the amount and
measurement was carried out after the permeability test to
the molecular weight of poly ethylene glycol (PEG) on
avoid the effect of mechanical damage that could have
water-vapor permeability (WVP) properties of edible films
occurred on study representative surface structure of the
(Gaudin et al. 1999). They found that the WVP values of
films. The plasticizer of various compositions was added to
edible films increase with both the quantity and molecular
HPMC solution and stirred for 10–15 min followed by weight of PEG up to PEG 600.
degassing, and films were dried as mentioned above
The aim of this study is to prepare edible films based on
method, and various properties were studied.
HPMC and blends of glycerol (Gly) and poly (ethylene gly-
col) (PEG) as plasticizers, in order to determine the
mechanical properties and barrier properties of these films. Tensile Strength
The purpose of packaging of this HPMC edible film is to
preserve freshness of the content inside the barrier to main-
A universal testing machine (UTM) (Lloyd, UK) was used
tain and prolong the quality throughout the product’s shelf-
to measure tensile strength (TS) and percent elongation (%
life and thus increase the shelf-life. Importantly to reduce
E) at break point as per standard method D-882 using 10
the use of synthetic, non-biodegradable packaging materials
samples were cut from each film of 1.5/8 cm. The films pre-
for reduce polymer solid waste, and to make the environ-
pared after storing in Desiccators at 51% RH for about 5 ment clean and friendliness.
days were removed and cut into standard sizes of 1.5 cm/8
cm, minimum five pieces were prepared. The film was fixing
both ends and strength was measured. Various parameters EXPERIMENTAL
like Young’s modulus, maximum loads, extension at break
and tensile strength were calculated. TS were calculated for Materials using the formula:
HPMC (E 15 LV Premium, Loba Chemie Pvt., Ltd., Pub Maximum area
Chem CD 57503849 or E464) samples supplied from Loba- TS 5 NM2: (1) Original cross section area
Chem Mumbai, India; Glycerol AR grade, SD fine-Chem,
Mumbai, India; pub chem CD 753, Polyethylene glycol 200-
This testing was carried out at around 25–30C temperature
AR grade SD fine Chem, Mumbai, India; pubchem CD 174
for each test. Around 5 min were required (starting from fix-
and cleaned and leveled local own made glass plate (24 3
ing the film till end of the testing). The clamp distance was
19 cm2) was used to preparation of the films.
around 10 inches. The thickness of the film varied from 0.3
to 0.6 mm. The blends of HPMC films were prepared with
different concentration of plasticizers ranges between 0.01– Methods
0.04% glycerol and 0.01–0.05% PEG. Table 1 shows
Plasticizer(s) with different ratios and 5 g of HPMC were
mechanical, barrier and haze properties.
mixed thoroughly with 100 cm3 of distilled water on contin-
The changes in mechanical properties characterized by
uous stirring. After complete dissolution, the solution was
the plasticizers weakening the intermolecular forces between
passed through 80 no. sieve followed by degassing and films
the chain of adjacent macromolecules, increasing the free
were prepared by solution casting method (Srinivasa et al.
volume and causing a reduction of mechanical resistant
2002). The HPMC (5%, w/v) and plasticizer(s) (Gly 0–
(Sobral et al. 2001). Thus the increase in the plasticizer 2
Journal of Food Processing and Preservation 00 (2016) 00–00 V C 2016 Wiley Periodicals, Inc. MAHADEVAIAH ET AL.
CHARACTERIZATION AND APPLICATION OF HPMC EDIBLE POLYMER FILMS
TABLE 1. MECHANICAL AND BARRIER PROPERTIES OF HPMC FILMS WITH DIFFERENT CONCENTRATIONS OF PLASTICIZERS GLYCEROL AND POLYETHYLENE GLYCOL Water-vapor transmission Tensile strength Strain at Modulus Burst strength Impact rate (WVTR) g/m2 day at OTR cc/m2 Con % TS (Mpa) break (%E) (Mpa) kg/cm2 strength (Psi) 92%RH at 38.7C day atm Haze 0 38.35 9.39 1654.75 1.25 8.6534 4410 25.001 15.60 (38.35) (9.40) (1655.76) (1.25) (8.6535) (4412) (25.02) (15.61) 0.01 29.33 8.69 859.55 1.46 1,55,573 3846 28.52 8.90 (28.75) (65.50) (18.42) (2.45) (2,00,593) (1470) (32.00) (7.30) 0.02 24.77 25.67 830.60 1.58 168763 3745 33.04 6.75 (20.42) (50.55) (12.18) (2.08) (2,12,496) (2536) (38.13) (8.65) 0.03 19.60 39.97 50.32 1.80 1,98, 852 2877 42.01 4.02 (19.60) (40.49) (5.16) (1.80) (1,92,226) (2853) (43.42) (9.45) 0.04 10.01 23.02 20.46 3,12,500 1856 46.05 2.06 (8.63) (38.95) (3.02) (1.36) (1, 32,421) (3500) (48.63) (10.45) 0.05 (0.80) (1,10, 852) (3850) (50.26) (12.63)
The values in the parenthesis are for poly (ethylene glycol).
concentration causes a reduction of the TS due to the
test dishes were weighed at 1 h interval till a constant rate of
decrease in the intermolecular interactions.
gain was achieved (tc), WVTR was calculated using follow-
ing equation (Park and Chinnan 1995): Burst Strength WVTR 5 slope=film area; (2)
This test measures the ability of the film to withstand pneu- or
matic load. This test gives a sort of combined tear and tensile WVTR
properties. For the present study PNEUMATIC BURST
5 weight gain in 1 h 3 200 3 24 g/m2/day, WVTR
strength tester was used. The samples free from creases were
5 slope/film area, where slope 5 weight loss/gain
placed in position and clamped firmly. The tester was con- versus time,
nected to the compressed air pipeline fitted with the two
Water vapor permeability (WVTR) was determined (Park
gauges and open valve to suit pressure requirement. A steady
and Chinnan 1995) by using Eq. (3)
pressure at the rate of 70–80 kg/cm2 was allowed to pass inside WVP 5 ðWVTR=P22P1Þ 3L; (3)
until specimen ruptures and burst pressure was recorded.
where P1 a partial pressure (kPa) inside the cup and P2 the Impact Strength
water vapor partial pressure(kPa) at the film outer surface
in the film system. L is the average film thickness (mm),
Impact strength is the strength of the films using “SPENSER
weight loss graphs were plotted with respect to time and lin-
IMPACT” tester and specimen size is 8 cm 3 8 cm for the
ear least square method used to calculate water vapor trans-
(ASTM D-265). The specimen is to be clamped vertically as
mission rate (WVTR) and then divided by the area of the
a cantilever beam. The specimen to strike by a swing of a film exposed.
pendulum released from or fixed distance from the speci- men clamp. Oxygen Transmission Rate (OTR)
Water Vapor Transmission Rate (WVTR) (ASTM D-1436-66)
WVTR is measured as the quality of water-vapor in grams
Oxygen transmission rate is normally determined by meas-
that will permeate one side to the other side of the film of an
uring the change in volume at constant pressure of gas flow-
area of 1 m2 in 24 h. The test was carried out according to
ing across the film compiled as volume at NTP. The test
ASTM E-96-97 method. In this method, anhydrous CaCl
procedure was as ASTM E 96-66 standard methods. 2
was sealed with crease free specimen (8 cm diameter using
an admixture of 60:40 microcrystalline and paraffin wax). Color and Opacity
The assembly was then placed in a humidity chamber (Lab-
oratory thermal equipment, Glasgow, UK), which is main-
Color values of the films were measured with a C.M.3500 d,
tained at 92% RH and 37.8C (Rockland 1960). Aluminum
MINOLTA (Minolta Camera, Co., Ltd., Japan). The films
Journal of Food Processing and Preservation 00 (2016) 00–00 V C 2016 Wiley Periodicals, Inc. 3
CHARACTERIZATION AND APPLICATION OF HPMC EDIBLE POLYMER FILMS MAHADEVAIAH ET AL.
were placed on a standard plate and hunter Lab color scale RESULTS AND DISCUSSION
was used to measure the color. Five measurements were taken on each type of film. Effect of Tensile Strength
Both Gly and PEG have plasticizing effect on HPMC films. Haze
Indeed, incorporation of Gly and PEG caused a decrease in
TS and Young’s Modulus. This implied that PEG could make
The percentage of transmittance that light passed through
HPMC film flexible. Park et al. (1993) and Cao et al. (2009)
the film was recorded using “Recording spectrometer” hav-
also found that the addition of PEG led to a decrease in TS
ing incident light at 660 nm wavelength. Before the test, the
and elastic modulus. The tensile strength of the films
instrument was calibrated with distilled water as standard
decreases with an increase of plasticizers concentrations. In (0% haze).
case of Gly, the tensile strength decreases drastically with
increasing Gly concentration compared to PEG (Table 1).
Tensile strength of the HPMC films decreases with increase of
Differential Scanning Calorimetry
glycerol concentration. The maximum tensile strength of the (DSC) Analysis
films is found at 29.33 and 28.75 Mpa at 0.01% glycerol and
DSC analysis was recorded as temperature versus heat flow
PEG, respectively. At 0.03% glycerol and PEG concentrations
until 500C at 10C/min heating rate under a nitrogen atmos-
the tensile strength of the films are found to be the same
phere. DSC Model, DSC 2010 with a thermal analyst 2100
(19.60 Mpa). The results in Table 1 indicated that the addi-
system (TA Instruments) was used. About 10 mg polymer
tion of plasticizers have statistical significant effect on tensile
samples were sealed in aluminum pans and used for experi-
strength, elongation percentage and Young’s modulus.
ments. All experiments were carried out with a sealed empty
Increasing the level of Gly in the films led to decrease in ten- pan as the reference with a N
sile strength (TS) and Young’s modulus. Gly plasticized films
2 gas flushing. The sealed pans
with samples were first cooled to 230C held isothermally
were weaker and more stretchable, flexible and durable than
for 1 min and then ramped (10C/min) to 300C to obtain the
PEG plasticized films. The tensile tests showed that the func- heat flow curves.
tion of glycerol by enhancing the fracture properties of the
emulsion films, decrease intermolecular attractions between
adjacent polymeric chains, decreasing tensile strength and Fourier Transforms Infrared
Young’s modulus and increasing elongation break or strain at Spectroscopy (FT-IR)
break. This is because of the small size and large number of
glycerol molecule in the same moles of PEG and GLY. The
FT-IR spectra of the blends were measured on FTIR-Perkin
films were analyzed in a climate room at 51% RH and 25C.
Elmer-spectrometer. The samples were prepared by making
Initial grip separation was set at 4 cm. Tensile strength was
KBr (potassium bromide) pellets containing 3 wt % of
calculated by dividing the maximum load by the cross-
materials. Fourier transform infrared (FT-IR) spectroscopy
section area of the film. Physicochemical properties of plasti-
of blend films was carried out in order to detect any peak
cizer such as chemical structure, shape, polarity, chain length,
shift that could be attributed to weak interactions between
physical state and number of active functional groups deter- the two polymers, such as hydrogen bonding or
mine its ability to plasticize a polymer network. The differen- complexations.
ces in plasticizing effect between plasticizers were possibly
due to the different availability of oxygen atoms for hydrogen Statistical Analysis
bonding. The spacing of O atoms in PEG 200 may have
allowed more room for the formation of hydrogen bonding
The effect of Gly and PEG on physical properties and the
with biopolymer chains (Sothornvit and Krochta 2000).
effects of different film formulations were evaluated. Meas-
urements of each property were triplicate for thickness, ten-
Elongation (Strain) at Break (%E)
sile strength, elongation at break, Young’s modulus, burst
strength, impact strength, water vapor transmission rate
Elongation at break is found to be increases with increase of
(WVTR), oxygen transmission rate (OTR), color, opacity
glycerol concentration whereas decrease in PEG concentra-
and haze properties. Statistics on a completely randomized
tion. The flexibility of the films is found to be increase with
design were determined. When analysis of variance
increase of plasticizers. The maximum flexibility or strain at
(ANOVA) revealed a significant (McClavej and Dietrich
break of the films is found to be (39.97%) at 0.03% of glyc-
1985) effect (P < 0.05) were reported using f(x) 2 82 scien-
erol, and 65.50% at 0.01% PEG. The plasticizers decrease tific calculators.
the intermolecular attractions between the chains and it led 4
Journal of Food Processing and Preservation 00 (2016) 00–00 V C 2016 Wiley Periodicals, Inc. MAHADEVAIAH ET AL.
CHARACTERIZATION AND APPLICATION OF HPMC EDIBLE POLYMER FILMS
to increase film flexibility (Garcia et al. 2000). Plasticizers
TABLE 2. COLOR AND PERCENT OPACITY FOR HPMC-GLYCEROL AND
increase the free volume of polymer structures or the molecu- HPMC-PEG FILMS
lar mobility of polymer molecules. The result of plasticizer Con % L A B % Opacity
addition is a reduction in polymer chain-to-chain interac- Standard 98.42 20.25 20.38 7.45
tions, a lowering the biopolymer transition temperature, and (98.42) (20.25) (20.38) (7.45)
an improvement in film flexibility (lowering of films elastic White 97.12 20.30 20.49 7.50
modulus). Also, elongation of the films (stretchiness or duc- (97.42) (20.40) (20.45) (7.62)
tility) increases, and film strength decreases (Han 2005). In 00 96.50 20.84 20.55 7.60
general, the addition of plasticizers leads to a decrease of ten- (97.01) (20.35) (20.40) (7.55) 0.01 96.10
sile strength in both GLY and PEG, and an increase of elonga- 21.01 20.82 8.01 (96.98) (20.30) (20.46) (7.80)
tion in Gly up to certain extent, whereas decrease in PEG 0.02
concentration level (Wypych 2004).The elongation at break is (96.25) (20.38) (20.50) (8.02)
found to be almost the same (39.97 and 40.49 Gly and PEG, 0.03 97.78 21.22 20.66 8.09
respectively) at 0.03% for both glycerol and PEG. (97.00) (20.29) (20.60) (7.66) 0.04 (97.35) (20.49) (20.75) (7.50) Effect of Burst Strength
The values in parenthesis are for poly(ethylene glycol).
The burst strength gives an indication of tensile strength
and strength of the film. Elongation at break is expressed as
the percentage of change in the original length of film strips
pure HPMC films, it led to decrease the shelf-life, fragrance/
before breaking. McHugh and Krochta (1994) also found
flavor of the food materials. Additions of plasticizers both
significantly decreased tensile strength and increased elon-
Gly and PEG are control the WVTR. Increase the level of
gation of HPMC films with higher glycerol contents. The
glycerol to the films is found to be decreasing the WVTR.
HPMC film gave burst strength of 1.25 kg/cm2, when the
But in PEG blended films the value of WVTR increased with
glycerol concentration is increased, but not much change
increase of PEG plasticizer level mainly due to the high
was observed. However, addition of PEG (0.01%) showed
hydrophilic nature of PEG. It was observed that the WVP
more than twofold increase in burst strength (2.45 kg/cm2)
value is maximum (4410) at pure HPMC concentration.
and decreases as % of PEG increased (0.80 kg/cm2 at 0.05%
This could be explained by the fact that Gly reduces internal
PEG) (ASTM D-774). The burst strength is found to
hydrogen bonding and increases intermolecular spacing,
increase with increase of Gly level, but increased with
thereby decreasing the permeability of films (Kamper and
increase of PEG level up to certain extent (2.45 at 0.01%)
Fennema 1984; McHugh and Krochta 1994; McHugh et al.
and then decreased. The increase of burst strength in Gly
1994; Park et al. 1994; Mahamadou et al. 2007; Lotti et al.
concentration level is due to its effectiveness of plasticizer.
2008). The decrease in WVTR with increasing Gly content is
The burst strength of the films is found to be the same
the results of a well-known hygroscopic of Gly. At 0.05%
(1.80 kg/cm2 ) at 0.03% of both plasticizers Gly and PEG.
PEG and 0.01% glycerol films show approximately similar
WVTR values (3850 and 3846), shown in Table 1. The glyc- Effect of Impact Strength
erol films showed steep change from 0.02 to 0.04%, WVTR
of 5% HPMC film is found to be 4410 g/m2/day at 92% RH
Impact strength is a measure of toughness. The higher is the at 37.8C.
impact strength of a material, the higher is the toughness and
vice versa. HPMC films with 0.04% glycerol showed good
impact strength (3,12,500 Psi), and with 0.02% PEG concen-
Effect of Oxygen Transmission Rate (OTR)
tration higher impact was observed (2,12,496 Psi). Increasing
the PEG concentration increased impact strength up to
The transfer of oxygen from the environment of food has
0.02% and decreased the impact value, whereas impact
important effect on quality and shelf-life. Oxygen causes
strength increased with increase of Gly. Table 1 shows the val-
food deterioration such as lipid and Vitamins oxidation,
ues of impact strength for glycerol and PEG, respectively.
leading to sensory and nutrient changes. Due to the large
amount of hydrogen bonds biopolymers films are hydro-
philic, which makes them excellent barriers to nonpolar
Effect of Water Vapor Transmission
substances, such as oxygen and some aroma compounds. Rate (WVTR)
Oxygen barrier property of a food packaging material for
The values of WVTR of HPMC and plasticized HPMC films
fresh product (e.g., fruits and salad, ready-to-eat meals)
are shown in Table 1. WVTR values are found higher in
plays an important role in its preservation.
Journal of Food Processing and Preservation 00 (2016) 00–00 V C 2016 Wiley Periodicals, Inc. 5
CHARACTERIZATION AND APPLICATION OF HPMC EDIBLE POLYMER FILMS MAHADEVAIAH ET AL.
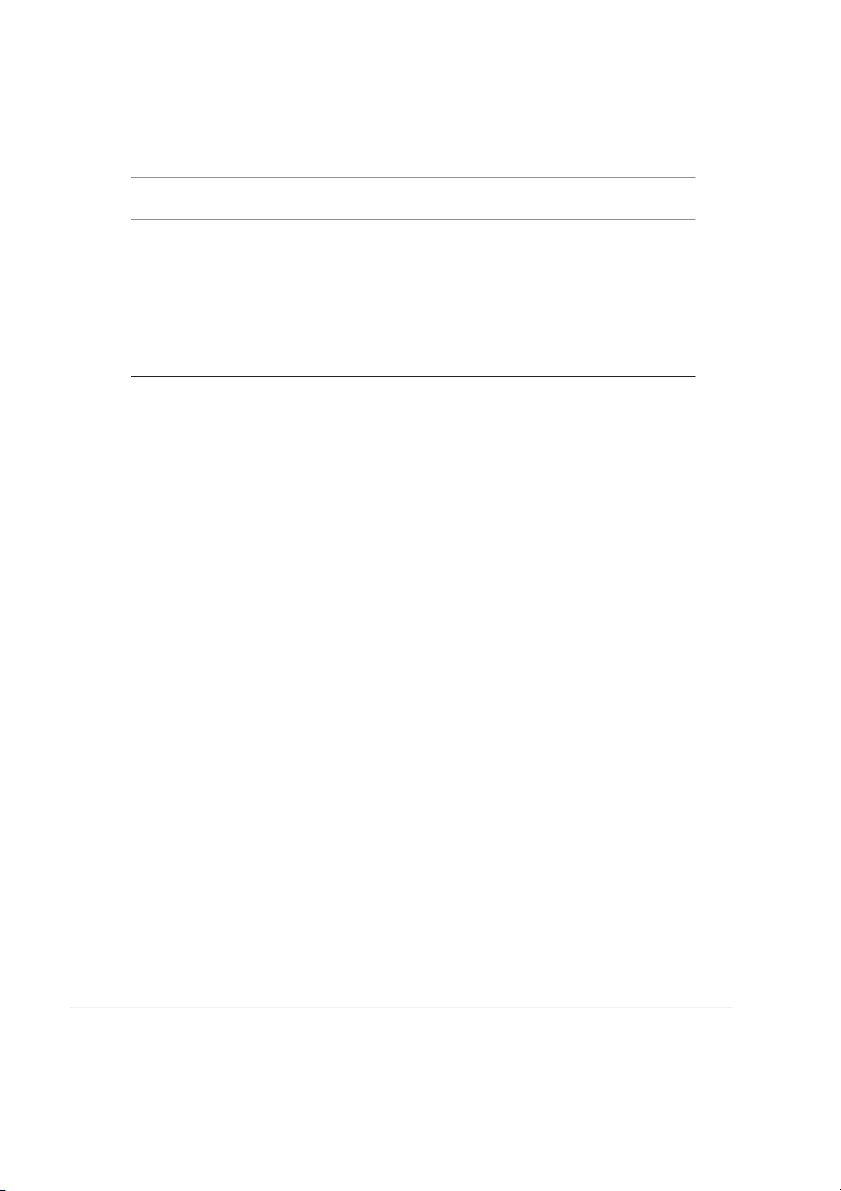
FIG. 1. GLASS TRANSITION TEMPERATURE
(Tg) OF 5% PURE HPMC FILM AND 1–4% GLYCEROL BLENDS, RESPECTIVELY 6
Journal of Food Processing and Preservation 00 (2016) 00–00 V C 2016 Wiley Periodicals, Inc. MAHADEVAIAH ET AL.
CHARACTERIZATION AND APPLICATION OF HPMC EDIBLE POLYMER FILMS
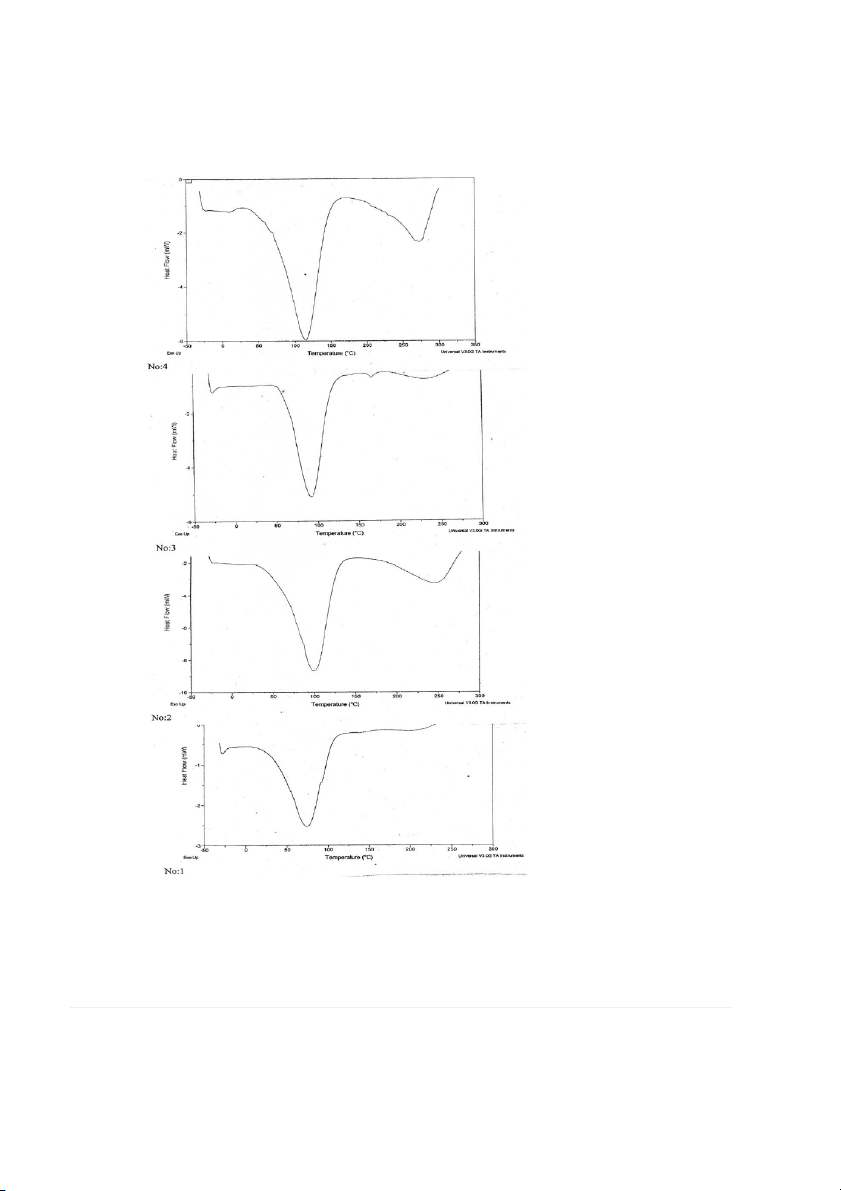
FIG. 2. GLASS TRANSITION TEMPERATURE (Tg)
OF 5% PURE HPMC FILM AND 1–5% POLYETHYLENE GLYCOL BLENDS, RESPECTIVELY
Journal of Food Processing and Preservation 00 (2016) 00–00 V C 2016 Wiley Periodicals, Inc. 7
CHARACTERIZATION AND APPLICATION OF HPMC EDIBLE POLYMER FILMS MAHADEVAIAH ET AL.
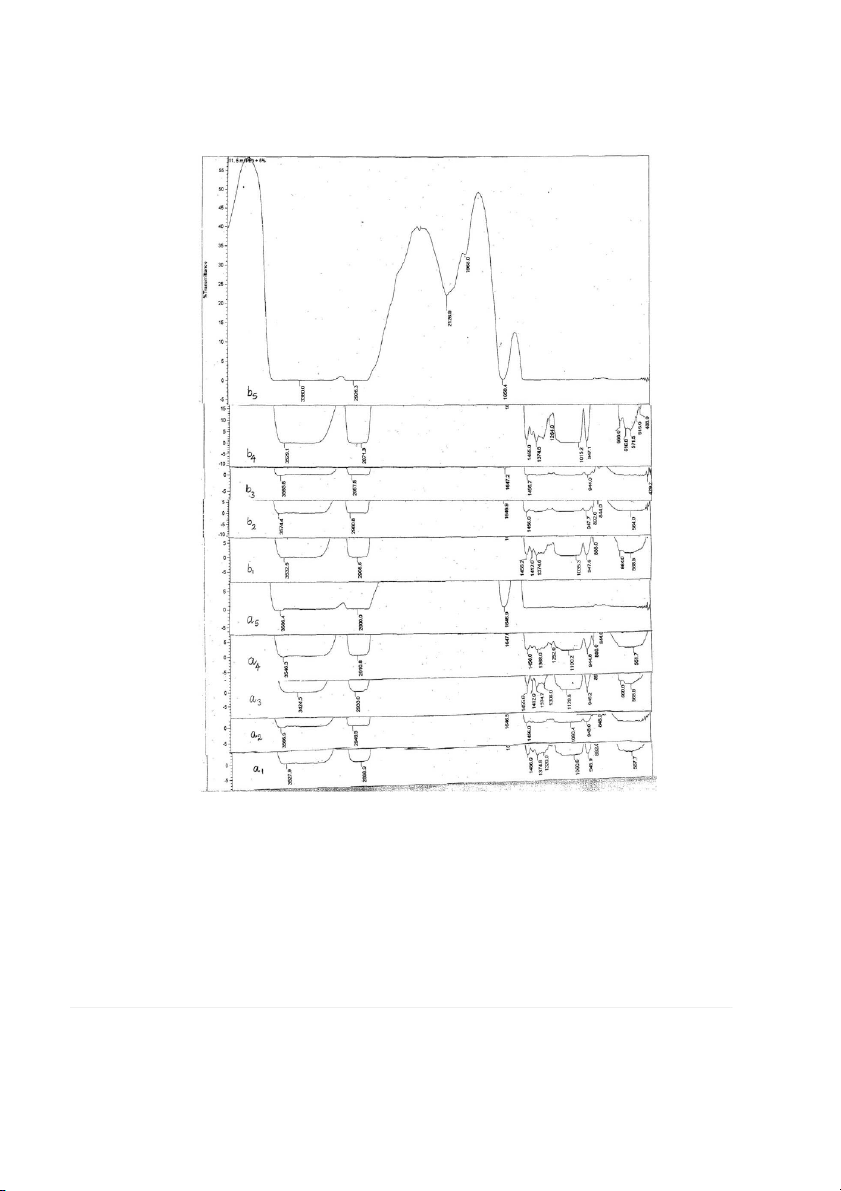
FIG. 3. FTIR SPECTRA OF HPMC FILMS WITH PLASTICIZERS GLYCEROL AND PEG
a1–a5, 5% HPMC and 1–5% glycerol; b1–b5, 5% HPMC and 1–5% PEG.
Commercial data (ANON 1990) provides that HPMC
but two to three orders of magnitude greater than that of
films indicate that they are moderate barriers to oxygen,
poly (vinylidene chloride) (PVDC) and ethylene vinyl alco-
their oxygen permeability is approximately an order of mag-
hol (EVOH) copolymers. The higher oxygen permeability of
nitude lower than that of low density polyethylene (LDPE),
HPMC is probably be attributed to the large HPMC side 8
Journal of Food Processing and Preservation 00 (2016) 00–00 V C 2016 Wiley Periodicals, Inc. MAHADEVAIAH ET AL.
CHARACTERIZATION AND APPLICATION OF HPMC EDIBLE POLYMER FILMS
groups, which results in HPMC having a small cohesive
(Tg 5 75C) and 0.02% polyethylene glycol plasticizers (Tg
energy density, larger free volume and lower crystallinity. The
60C). Hence, Gly is the best plastcizer than PEG.
wide range of values of OTR (Table 1) was observed by effect
of plasticizer concentration. In both plasticizers, the OTR
values increased with increase in concentration of plasticiz- FTIR Analysis
ers. In glycerol films OTR values were better than PEG films.
The band intensity of the hydrogen bond depends on the
alkalinity of the proton acceptor and possibility of their Effect of Color and Opacity
close contacts. As a consequence of hydrogen bonding, the
covalent bonds in the proton acceptor are weaker, while the
Color values were recorded as (0 5 black, 100 5 white),
energy barrier for angle deformation becomes higher .The
a(2a 5 greenness, 1a 5 redness) and b(2b5 blueness,
change in the C-O-C band in the spectrum, suggests that
1b 5 yellowness), where standard white plate had the val-
hydrogen bonding is the underlying mechanism in the inter-
ues A 5 98.42, Q 5 20.25, b 5 20.38 and %opacity 5 7.45.
action. In addition, hydrogen bonding has the strongest
Additions of plasticizers like glycerol and PEG had no much
influence on the donor (in our case the -OH of glycerine/
effect on the color. Color of HPMC films were very good
PEG) plasticizers and the absorption maximum of stretch-
transparent and white in color. Much change in percent
ing vibration shifts toward lower wave numbers compared opacity was not observed in HPMC 1 glycerol or
to that of the pure HPMC. It is also noted that in Fig. 3, the
HPMC 1 PEG films. Table 2 shows the color opacity of the
hydroxyl stretching bands became much broader with
HPMC 1 glycerol and HPMC 1 PEG films, respectively.
increasing plasticizers glycerin and poly (ethylene glycol)
content. This strongly supports the idea that hydrogen bond Effect of Haze
can form between ether oxygen atoms of HPMC and
hydroxyl groups of glycerine and poly (ethylene glycol).
Haze percentage decreased in glycerol added HPMC films,
whereas addition of PEG haze percentage increased with
increased concentration. Data in Table 1 shows haze of the CONCLUSIONS
glycerol plasticized HPMC films are superior than that of
PEG plasticized and pure HPMC films.
This study is used to investigate the two plasticizers such as
glycerol and polyethylene glycol for the purpose of preparing
edible polymer films. It is shown that through the incorpora-
Effect of Plasticizers on the Glass Transition
tion of plasticizers such as Gly and PEG, mechanical proper- Temperature
ties of edible films can be significantly improved without
The glass transition temperature (T
reducing film barrier properties. These findings are expected g) of HPMC varied from 170 to 180C. The T
to have a significant impact on the food industry by enabling
g of HPMC determined by differential
scanning calorimetry was found to be 167.15C. The T
them to manufacture edible films with improved tensile g of
HPMC films incorporated with PEG and glycerol was
strength, while maintaining their elongation and water vapor
detected. This could be attributed to the presence of crystal-
permeability values. The DSC thermo grams showed that
line phase in the blend. Both PEG (T
HPMC polymer, which has broad industrial applications, is g, 161.03C) and glycerol reduced the T
resistant to heat up to 350C and that an exothermic degrada-
g of HPMC films. Plasticization lowered the Tg
values and the incorporation of PEG or glycerol in the
tion with an excess decrease in weight occurs above the tem-
HPMC formation was proposed to increase the segmental
perature. Therefore HPMC polymer can be used safely in
mobility of the polymer. The glass transition temperature has
foods, including bakery products up to 350C. From the
been described as the characteristic temperature at which a
results of the analysis of the mechanical and optical properties
polymer changes from a state of relative molecular or seg-
of HPMC-based edible films it can be concluded that the
mental rigidity (glassy phase) to one of considerable chain
most suitable plasticizer among the PEG plasticizers with dif-
mobility (rubber phase) (Nisperos-Carriedo 1994). Incorpo-
ferent molecular weights is PEG 200 and that the maximum
ration of plasticizers affects the glass transition temperature
level for its use is 0.6 mL/0.7 g in edible film formulations.
of the polymer. And more efficient the plasticizers, the greater
is the lowering of the Tg. Glass transition temperature influ- ACKNOWLEDGMENT
ences the physical properties, such as viscosity of the solution
and the mechanical properties and moisture permeability of
The authors would like to thank Dr. A.D. Semwal and the
the film. Figures 1 and 2 state that the compatibility of the
Department of DFRL Mysore to furnish DSC tests and data
polymer blends films is found to be at a 0.01% of glycerin for this work.
Journal of Food Processing and Preservation 00 (2016) 00–00 V C 2016 Wiley Periodicals, Inc. 9
CHARACTERIZATION AND APPLICATION OF HPMC EDIBLE POLYMER FILMS MAHADEVAIAH ET AL. REFERENCES
MCCLAVEJ, T. and DIETRICH, F.H. 1985. Analysis of variance
comparing more than two means. In Statistics
ANON. 1990. A Food Technologist’s Guide to Methocel Premium
(J.T. Mc CLAVE and F.H. DIETRICH, eds.) pp. 1–407, Dellen,
Food Gums, The Dow Chemical Co., Midland, MI. San Francisco, CA.
AYRANCI, B.S. and CETIN, E. 1997. The effect of molecular
MCHUGH, T.H. and KROCHTA, J.M. 1994. Sorbitol vs
weight of constituents on properties of cellulose-based edible
glycerol-plasticized whey protein edible films: Integrated
films. Lebenson. Wiss. Technol. 30(1), 101–104.
oxygen permeability and tensile property evaluation. J. Agric.
AYRANCI, E., TUNC, S. and ETCI, A. 1997. The measurement Food Chem. 42, 841–845.
of carbon dioxide transmission of edible films by a static.
MCHUGH, T.H., ANJARD, J.F. and KROCHTA, J. M. 1994.
J. Sci. Food Agric. 79, 1033–1037.
Plasticized whey protein edible films: Water vapour
BANKER, G.S., GORE, A.Y. and SWARBRICK, J. 1966. Water
permeability properties. J. Food Sci. 59, 416–423.
vapour transmission properties of free polymer films.
MILLER, K.S. and KROCHTA, J.M. 1997. Oxygen and aroma
J. Pharm. Pharmacol. 18, 457–466.
barrier properties of edible filma: A review. Trends Food Sci.
CAO, N., YANG, X. and FU, Y. 2009. Effect of various Technol. 8, 228–237.
plasticizers on mechanical and water vapor barrier properties
NISPEROS-CARRIEDOM, O. 1994. Edible coatings and films
of gelatin films. Food Hydrocolloid. 23(3), 729–735.
based on polysaccharides. In Edible Films and Coatings to Improve
DONHOWE, I.G. and FENNEMA, O. 1993. The effects of
plasticizers on crystallinity, permeability and mechanical
Food Quality (J.M. Krochta, E.A. Baldwin and
M. Nisperos-Carriedo, eds.) pp. 305–336, Technomic, Lancaster,
properties of methylcellulose films. J. Food Process. Preserv. 17, 247–257. U. K.
PARK, H.J. and CHINNAN, M.S. 1995. Gas and water vapor
GARCIA, M.A., MARTINO, M.N. and ZARIZKY, N.E. 2000.
Microstructural characterization of plasticized starch based
barrier properties of edible coatings for fruits and vegetables.
films. Starch-vta’ke 52(4), 118–124. J. Food Eng. 25, 497–507.
GAUDIN, S., LOURDIN, D., LEBOTLAN, D., FORSELL, P.,
PARK, H.J., WELLER, C.L.,VERGANO, P.J. and TESTIN, R.F.
ILLARI, J. and COLONNA, P. 1999. Effect of
1993. Permeability and mechanical properties of
polymer-plasticizer interactions on the oxygen permeability of
cellulose-based edible films. J. Food. Sci. 58, 1361–1364.
starch-sorbitol-water films. Macromol. Symp. 138, 245–248.
PARK, J.W., TESTIN, R.F., PARK, H.J.,VERGANO, P.J. and
GENNADIOS, A. and WELLER, C.L. 1990. Edible films and coatings
WELLER, C.L. 1994. Fatty acid concentration effect on tensile
from wheat and corn proteins. Food Technol. 10(44), 63–69.
strength, elongation, and water vapour permeability of
GONTARD, N., GUILBERT, S. and CUQ, J.L. 1993. Water and
laminated edible films. J. Food Sci. 59, 916–919.
glycerol as plasticizer affect mechanical and water-vapor
ROCKLAND, L.B. 1960. Saturated salt solution for static control
transactions of the ASAE. J. Food Sci. 17, 206–211.
of relative humidity between 5 and 40C. Anal. Chem. 32,
HABIG MCHUG, T., AVINA-BUSTILOS, R. and KROCHTA, 1375–1376.
J.M. 1993. Hydrophilic edible films: Modified procedure for
SAUCEDO-POMPA, S., ROJAS-MOLINA, R.,
water vapor permeability and explanation of thickness effects.
GUILERA-CARBO, A.F., SAENZ GALINDO, A., DE DELA J. Food Sci. 58(4), 899–903.
GARZA, H., JASSO-CANTUB, D. and AGUILAR, C.N. 2009.
HAN, J.H. 2005. Innovations in Food Packaging, Elsevier, San
Edible film based on candelilla wax to improve the shelf life Diego, CA.
and quality of avocado. Food Res. Int. 42, 511–515.
JENKINS, J.A. 1980a. A Fundamentals of Soil Mechanics, vol. I,
SOBRAL, P.J.A., MENEGALLI, F.C., HUBINGER, M.D. and John Wiley and Sons, New York.
ROQUES, M.A. 2001. Mechanical, water vapor barrier and
JENKINS, J.A. 1980b. A Fundamentals of Soil Mechanics, vol. II,
thermal properties of gelatin based edible films. Food John Wiley and Sons, New York. Hydrocolloids, 15, 423–432.
KAMPER, S.L. and FENNEMA, O. 1984. Water vapour
SOTHORNVIT, R. and KROCHTA, J.M. 2000. Plasticizer effect
permeability of edible bilayer films. J. Food Sci. 49, 1478–1485.
on oxygen permeability of beta-Lactoglobulin films. J. Agric.
LOTTI, C., ISAAC, C.S., BRANCIFORTI, M.C., ALVES, R.M.V.,
Food Chem. 48(12), 6298–6302.
LIBERMAN, S. and BREATS, R.E.S. 2008. Rheological,
SIRACUSA, V., ROCCULI, P., ROMANI, S. AND ROSA, M.D.
mechanical and transport properties of blown films of high
2008. Biodegradable polymer for food packaging: A review.
density polyethylene nanocomposites. Eur. Polymer J. 44(5),
Trends Food Sci. Technol. 19, 634–643. 1346–1357.
SRINIVASA, P.C., RAMESH, M.N. and THARANATHAN R.N.
MAHAMADOU, E.G., SHI-YING, X.U. and WANG, Z. 2007.
2002. A process for production of biodegradable films from
Whey protein isolate-based edible films as affected by protein
polysaccharides. Indian Patent, 85/DEL/2002.
concentration, glycerol ratio and pullulan addition in film
WYPYCH, G. 2004. Handbook of Plasticizers, ChemTec
formation. J. Food Eng. 83, 521–530. Publishing, Toronto. 10
Journal of Food Processing and Preservation 00 (2016) 00–00 V C 2016 Wiley Periodicals, Inc.




