






Preview text:
International University, Vietnam National University - HCMC CHEMISTRY LABORATORY Page 1 REPORT
EXPERIMENT 1: CHEMICAL REACTIONS Group: 1 Section: 1
Date: 07/09/2022 Sq. Full name Student ID % contribution Signature Score ( total = 100% ) 1 Nguyễn Khánh Hà ITCSIU21004 20 2 Nguyễn Tiến Anh BTFTIU21137 20 3 Tạ Thị Thuỳ Dương ITCSIU21053 20 4 Võ Ngọc Khương ITITIU19108 20 Duy 5 Bùi Nguyễn Phương ITITIU19113 20 Giao Total score: _______/15
International University, Vietnam National University - HCMC CHEMISTRY LABORATORY Page 2 I. Introduction
Today, chemicals play a significant role in everyday life. The atoms that make up the reactants of a
chemical reaction are rearranged to create products, which are substances with various properties. In this
experiment, several activities will be performed and observed to determine whether chemical reactions
have occurred. One of the observable signs of a chemical reaction is a change in attributes that can be seen
with the naked eye, such as a change in color, evaporation, precipitation, condition, physicality, temperature difference, and others.
And this report will discuss seven main experiments: performing different types of chemical
reactions and identifying products in these reactions by identifying their chemical changes and balancing
the chemical equations for the reactions observed.
Chemical reactions always happen in the living system, so understanding the nature of chemical
reactions allows people to apply their knowledge to create necessary substances that are helpful for humans
to live through the chemical reactions. II. Method: Equipments 10 test tubes 1 tes t tube holder 2 beakers 1 dispo sable pipet 1 tes t tube rack 1 alc ohol lamp 1 dist illed water bottle 1 flame test loop 1 tes t tube brush Materials 0.5 M CuSO 4 6 M NaOH 2 M NaOH Distilled Water 2 M NH 4 OH 2 M KOH 0.5 M KCl M FeCl 0.5 3 M 0.1 AgNO 3 0.5 M FeSO 4 0.5 M KBr 0.5 M Al 2 ( SO 4 ) 3 0.1 M KMnO 4 LiCl 2 M H 2 SO 4 NaCl 3 % H 2 O 2 KCl 0.1 M KI CaCl
International University, Vietnam National University - HCMC CHEMISTRY LABORATORY Page 3 MnO 2 BaCl 0.5 M Na 2 SO 3 1. Reactions of Cu2+
Two clean test tubes were prepared, then 10 drops of 0.5M CuSO4 were added to each tube. In the
next step, 10 drops of 2M NaOH were added to the first tube while the same amount of 2M NH4OH was
added into the second one. After that, both tubes were mixed gently and observed. After taking note of the
results of observation, the second step was performed again. Finally, two tubes were mixed gently and
observed. The results were recorded one more time.
2. Reactions of Silver halides
+ Section 1: Reactions of Potassium Chloride (KCl)
To begin with, two clean test tubes were prepared to be added 10 drops of 0.5M KCL in
each one. Then, 10 drops of 0.1M AgNO3 were added to each tube. After that, only the second tube
was added 10 drops 2M NH4OH into. Finally, both tubes were mixed gently and observed their status.
+ Section 2: Reactions of Potassium Bromide (KBr)
First of all, two clean test tubes were prepared. Then 10 drops 0.5M KBr and the same
amount of 0.1M AgNO3 were added into both test tubes sequentially. After that, 10 drops of 2M
NH4OH were added to the second tube only. Finally, both tubes were mixed gently and observed
their status after least 2 minutes. 3. Reactions of H2O2
To begin with, three clean test tubes were prepared. Then, 1 drop of 0.1M KMnO4, 5 drops of 0.1M
KI, 10 drops 3% H2O2 were added into each tube sequentially. After that, a pinch of MnO2 was
added into the third tube which contained H2O2 ; while 5 drops of 2M H2SO4 and 5 drops of 3% H2O2 were
added into both other tubes sequentially. Finally, all tubes were mixed gently and observed their status after least 2 minutes.
International University, Vietnam National University - HCMC CHEMISTRY LABORATORY Page 4 4. Reactions of KMnO4
To begin with, three clean test tubes were prepared to contain 10 drops of 0.5M Na2SO3 in each of
three test tubes. Then, 5 drops 2M H2SO4, 5 drops 6M NaOH and 5 drops distilled water were added into
the first, second and third test tube respectively. After that, 5 drops of 0.1M KMnO4 were added into all
tubes. Finally, the tubes were mixed gently and observed.
5. Reactions of Fe2+ and Fe3+
+ Section 1: Ferric ion (Fe3+)
First of all, two clean test tubes were prepared to be added 10 drops 0.5M FeCl3 into each
one. Then, 5 drops 2M KOH and 5 drops 2M NH4OH were added into the first and second test
tube respectively. Finally, the tubes were mixed gently and observed.
+ Section 2: Ferrous ion (Fe2+)
To begin with, two clean test tubes were prepared to be added 10 drops 0.5M FeSO4 into
per tube. Then, 5 drops 2M KOH and 5 drops 2M NH4OH were added into the first and second test
tube respectively. Finally, the tubes were mixed gently and observed. 6. Reactions of Al3+
First of all, two clean test tubes were prepared to be added 10 drops 0.5M Al2(SO4)3 into per tube.
Then, 5 drops 2M NaOH were added into each of two test tubes. After both tubes were mixed gently, they
were observed. In the next step, 20 drops 2M HCl and 20 drops 2M NaOH were added into the first and
second tube respectively. Finally, the tubes were mixed gently and observed. 7. Flame tests
To begin with, the Bunsen burner was lighted. Then, the loop was cleaned with distilled water and
dipped into the tested solution. After that, it was held in the flame. Finally, the color of the flame was observed and recorded .
III. Results and discussion Reaction Observation 1. Reactions of Cu2+
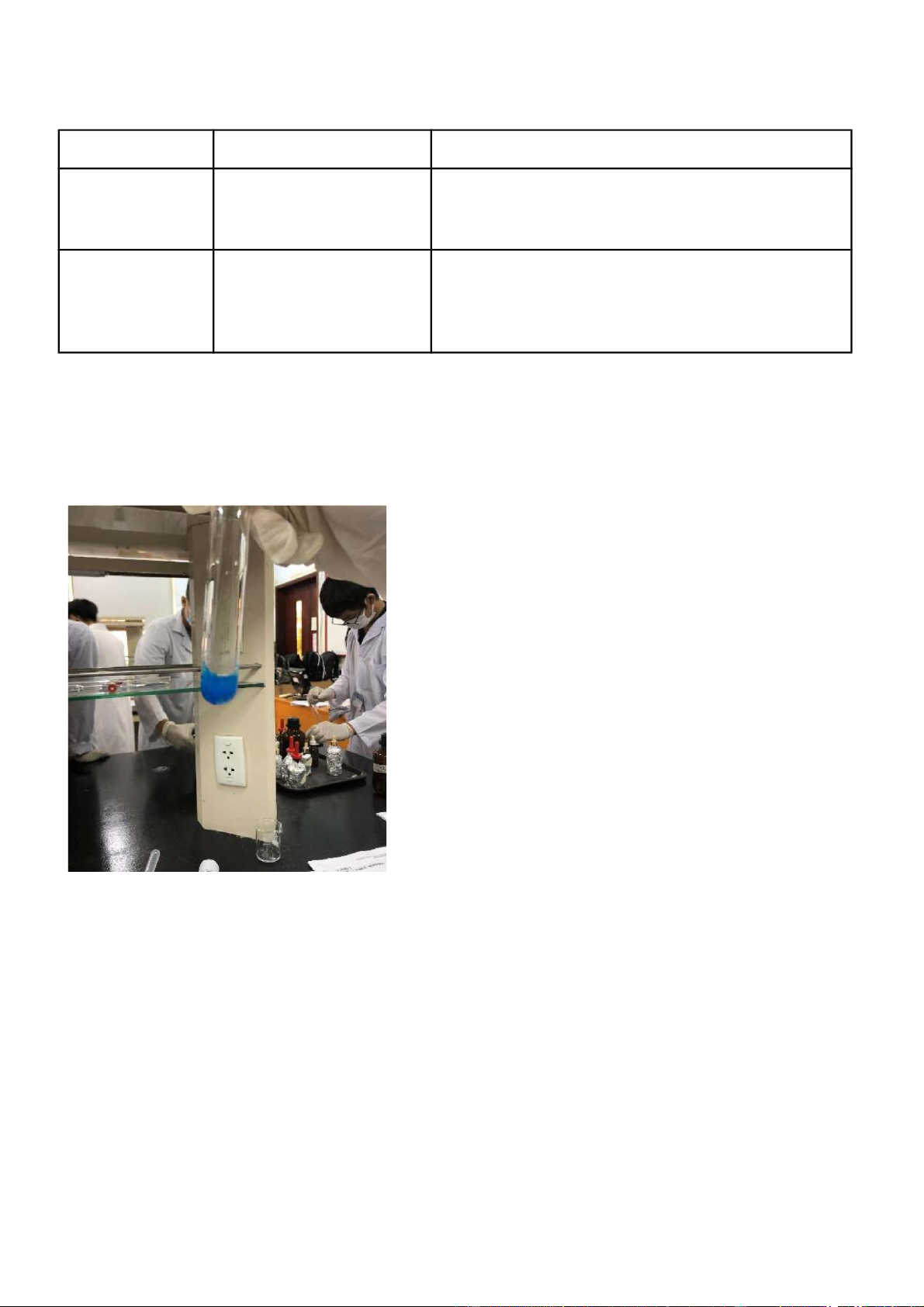
0.5M CuSO4 + 2M NaOH gradually dissolved NaOH in solution and blue
International University, Vietnam National University - HCMC CHEMISTRY LABORATORY Page 5 precipitate appeared. Chemical Equation 0.5M CuSO4 +
Blue precipitate dissolves CuSO4 + 2NaOH → Cu(OH)2↓ + Na2SO4 2M NaOH + 2M partly when NH4OH was NH4OH added, a dark blue solution appeared.
CuSO4 + 2NaOH → Cu(OH)2↓ + Na2SO4 Comment:
Cu(OH)2↓ + 4NH4OH → 4H2O + [Cu(NH3)4](OH)2 1.
As displayed in the substance condition, the response among CuSO4 and NaOH created Cu(OH)2
(1). Cu(OH)2 is a purplish blue encourage, as found in picture 1, and this is a two fold substitution/hasten response.
Figure 1.1 Reaction of CuSO4 + NaOH 2.
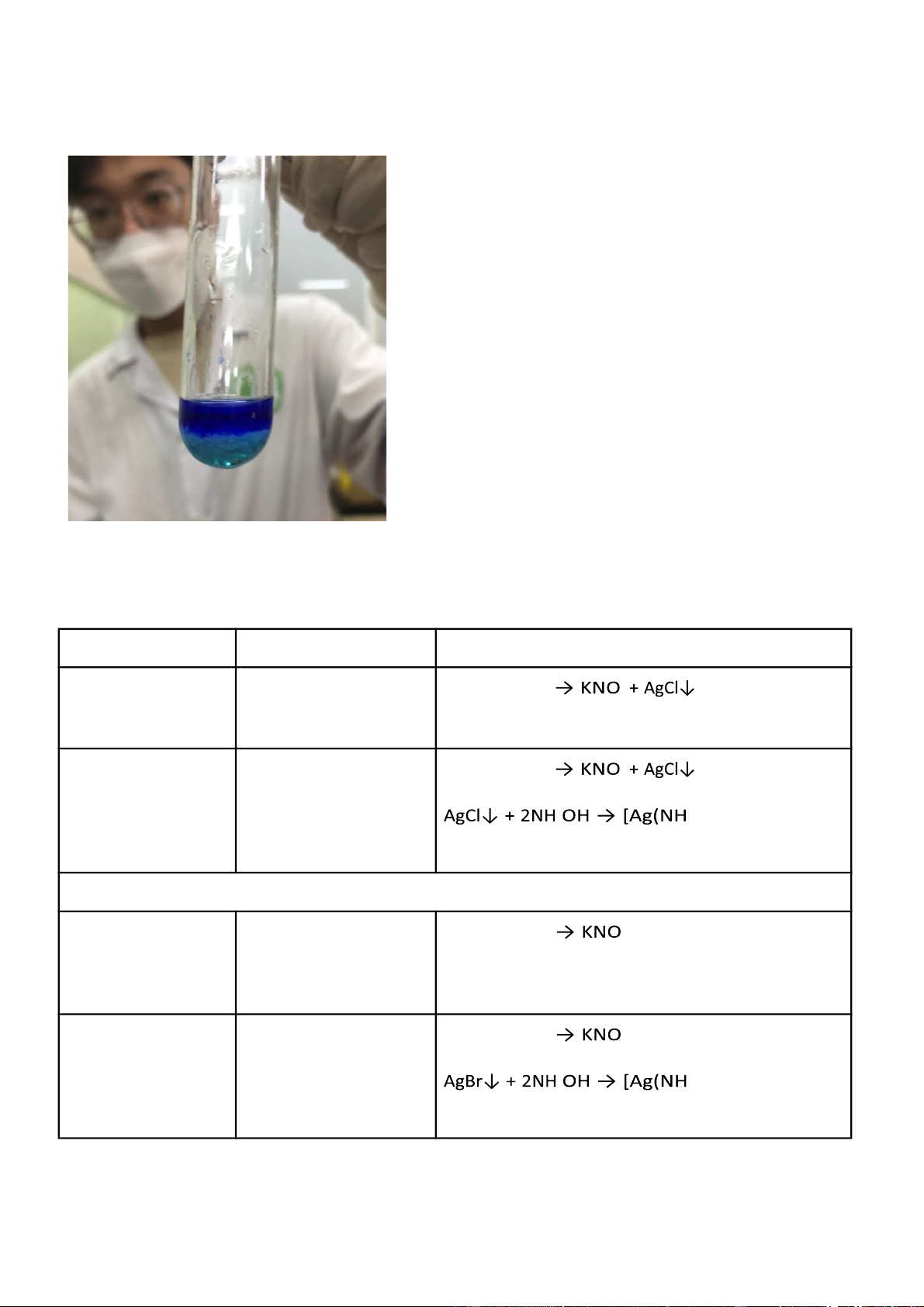
The mix of CuSO4 with NH4OH is a twofold uprooting response that ought to bring about a light
blue encouragement. At the point when an overabundance of NH4OH was added, the encourage
disintegrates, bringing about a dark blue arrangement. Because of a procedural slip-up, smooth blue
precipitation has gotten all things considered.
Figure 1.2 Reaction of CuSO4 + NH4OH
International University, Vietnam National University - HCMC CHEMISTRY LABORATORY Page 6
2 . Reactions of silver halides AgNO 3 Reaction Observation Chemical Equation 0.5 M KCl + 0.1M KCl reacted with KCl + AgNO 3 3 AgNO 3 AgNO 3 and a white precipitate appeared. M KC 0.5 l + 0.1M white precipitates KCl + AgNO 3 3 AgNO 3 + 2M dissolved when NH 4 OH NH 4 OH was added to the 4 3) 2 ]Cl + 2H 2 O mixture and the solution became colorless. 0.5 M KBr + 0.1M KBr reacted with KBr + AgNO 3 3 + AgBr↓ AgNO 3 AgNO3 and a white precipitate appeared at the bottom of the tube. M KB 0.5 r + 0.1M After NH 4 OH was KBr + AgNO 3 3 + AgBr↓ AgNO 3 + 2M added to the mixture, NH 4 OH there was no 4 3) 2 ] Br + 2H 2 O phenomenon change in the solution. Comments:
1) Figure 2.1 shows AgCl as a white encourage with a twofold substitution/hasten response.
International University, Vietnam National University - HCMC CHEMISTRY LABORATORY Page 7
2) There were two cycles in the response between KCl, AgNO3, and NH4OH: -
In the initial step, the response among KCl and AgNO3 created AgCl as displayed in the substance
condition. AgCl is a white encourage with a twofold substitution/hasten response type. -
In the subsequent step, AgCl responded with NH4OH answer for structure Ag(NH3)2Cl, which
brought about the white encourage AgCl dissolving as displayed in the substance condition. Figure 2.2
portrays Ag(NH3)2Cl, a mind-boggling material, and this sort of response is a perplexing compound development.
Even though the white encourage AgCl disintegrated, there was as yet a hasten in the photograph because AgCl was still there.
3) The substance condition shows that the response among KBr and AgNO3 made AgBr. AgBr is a smooth
white encourage with a twofold substitution/hasten response type, as displayed in Figure 2.3.
4) There were two cycles in the response of KBr, AgNO3, and NH4OH: -
In the initial step, the response among KBr and AgNO3 created AgBr, as displayed in the compound
condition. Figure 2.3 portrays AgBr as a smooth white (yellowish) encourage and this response is a twofold substitution/hasten response. -
In the subsequent step, AgBr collaborated with NH4OH to create Ag(NH3)2Br, which broke down
the smooth white (yellowish) hasten AgBr, as displayed in the substance condition. Ag(NH3)2Br is a
perplexing material, as displayed in Figure 2.4, and it is a mind-boggling compound creating a response
type. Albeit the smooth white encourage AgBr has broken down, there is as yet a hint of it noticeable.
Figure 2.1 Reaction of KCl + AgNO3
International University, Vietnam National University - HCMC CHEMISTRY LABORATORY Page 8
Figure 2.2 Reaction of KCl + AgNO3 + NH4OH
Figure 2.3 Reaction of KBr + AgNO3 Figure 2.4 Reaction of KBr + AgNO3 + NH4OH
International University, Vietnam National University - HCMC CHEMISTRY LABORATORY Page 9
3 . Reactions of H 2 O 2 Reaction Observation Chemical Equation 0.1 M KMnO 4 +
When H 2 O 2 was added to 2 KMnO 4 + 3H 2 SO 4 + 5H 2 O 2 2 M H 2
2 SO 4 + H 2 O 2 the mixture of KMnO4 4 + K 2 SO 4 and H 2 SO 4 , KMnO 4 ’s purple color disappeared. 0.1 M KI + 2M
When H 2 O 2 was added to H 2 O 2 + 2 KI+ H 2 SO 4 2 + K 2 SO 4 + 2 H 2 O H 2 SO 4 + H 2 O 2 the mixture, KMnO 4 ’s purple color disappeared. The solution turned into a reddish brown color and no precipitate appeared. H 2 O 2 + MnO 2 When H 2 O 2 was mixed H 2 O 2 + MnO 2 2 O + O 2 2 with MnO 2 , the reaction appeared bubbling and evaporating, then the solution appeared black precipitate Comments: 1.
As displayed in the substance condition, the response of KMnO4, H2SO4, and H2O2 is an oxidation
cycle in which KMnO4 is the oxidant and H2O2 is the reductant. The purple color of potassium
permanganate blurred into a clear fluid and created gas in this trial.
International University, Vietnam National University - HCMC CHEMISTRY LABORATORY Page 10
Figure 3.1 Reaction of KMnO4 + H2SO4 + H2O2 2.
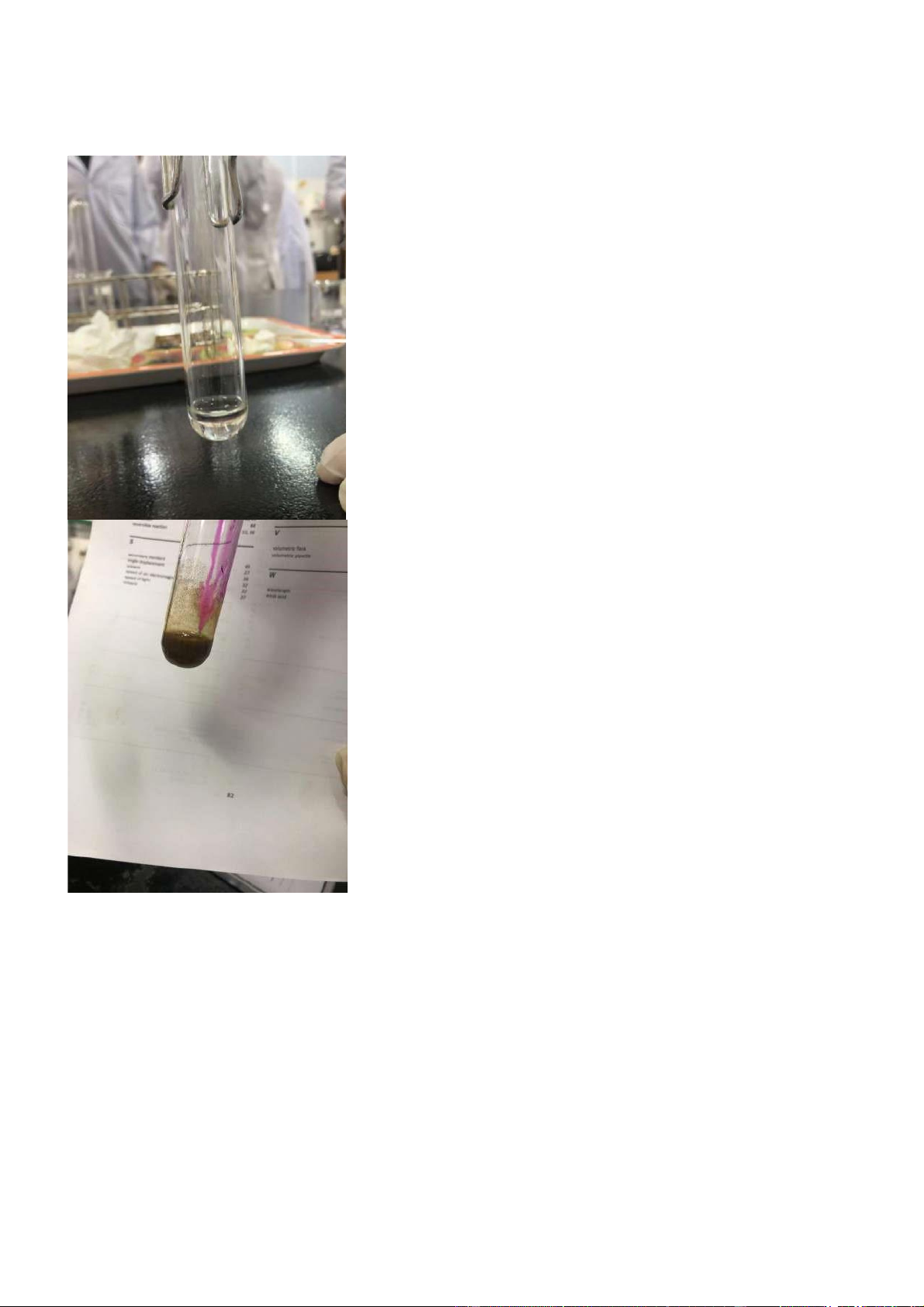
Displayed in the substance condition, the response of KI, H2SO4, and H2O2 is an oxidation cycle in
which KI is the oxidant and H2O2 is the reductant. The clear liquid in this examination became
yellowishbrown and created a dark encourage.
Figure 3.2 Reaction of KI + H2SO4 + H2O2
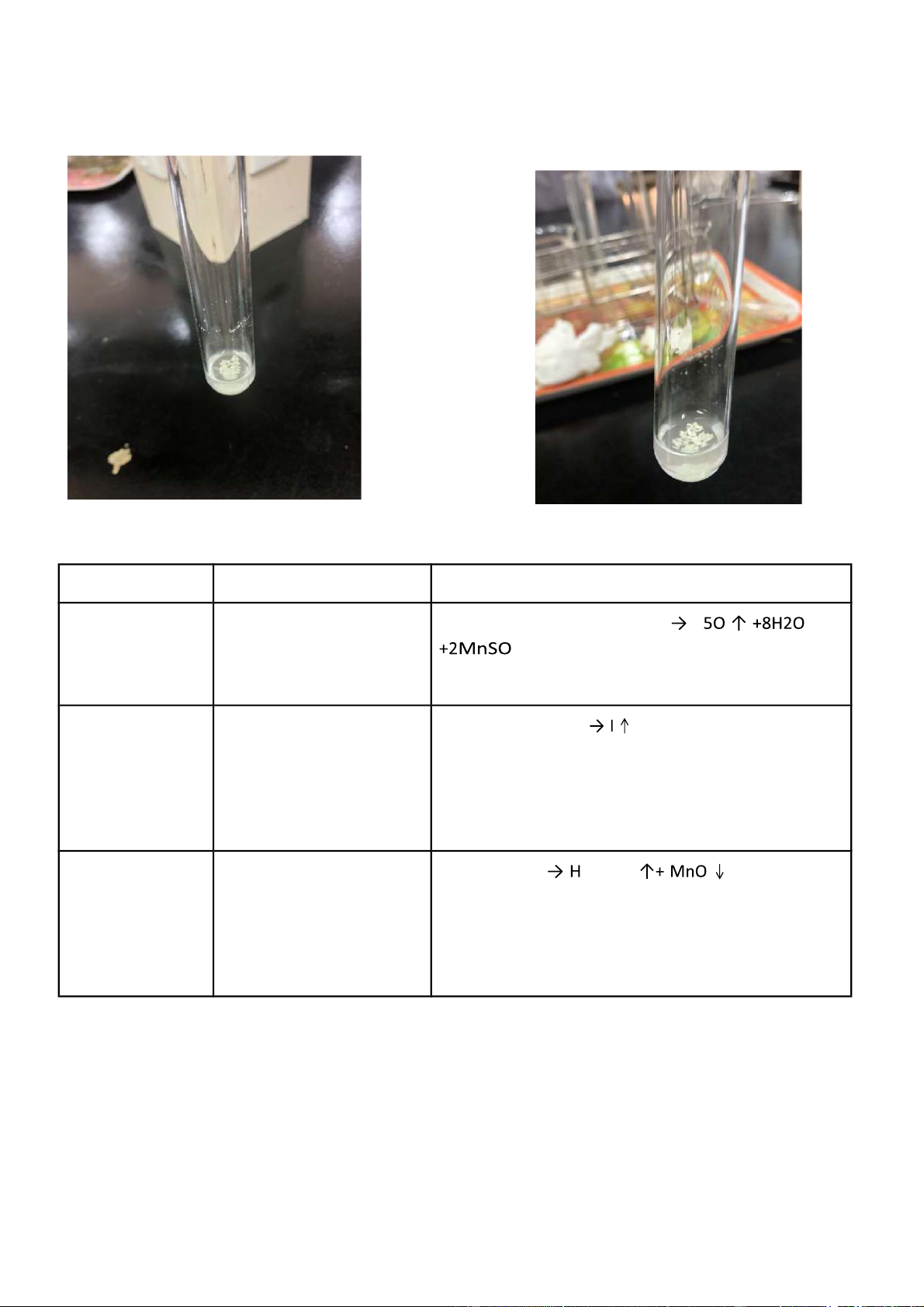
3. In the substance condition, the impetus for the H2O2 decay
process is MnO2. We saw the leftover MnO2 and the outflow of gas in this analysis.
Figure 3.3 Reaction of H2O2 + MnO2
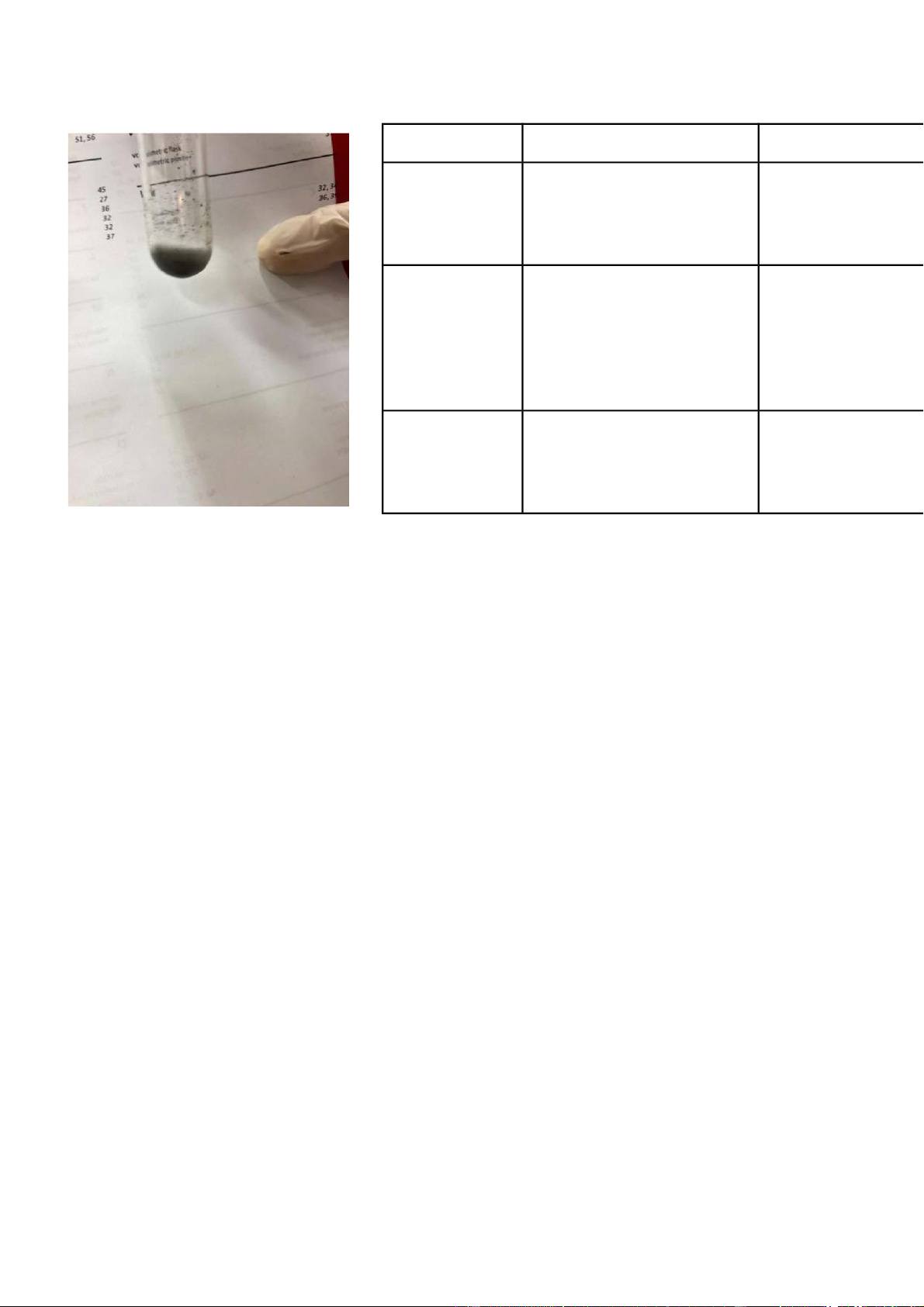
International University, Vietnam National University - HCMC CHEMISTRY LABORATORY Page 11 6M NaOH + solution containing Na2SO3 0.1M KMnO4
and NaOH, the solution appeared a dark green color and no precipitate was created.
0.5M Na2SO3 + KMnO4 was added to the H2O + 0.1M mixture of Na2SO3 and KMnO4 distilled water, and a dark brown precipitate appeared. Comments: Equation
5Na2SO3 + 3H2SO4 + 2KMnO4 → K2SO4 +5Na2SO4 + 2MnSO4 + 3H2O 4. Reactions of KMnO4
Na2SO3 + 2NaOH + 2KMnO4 → K2MnO4 +Na2SO4 + Reaction Observation Chemical Na2MnO4 + H2O 0.5M Na2SO3 + When KMnO4 was added to 2M H2SO4 + mixture Na2SO3 and H2SO4, 0.1M KMnO4 KMnO 4’s purple color disappeared.
3Na2SO3 + H2O + 2KMnO4 → 2MnO2↓ +3Na2SO4 +
0.5M Na2SO3 + Slowly adding KMnO4 to a 2KOH
- Observe that KMnO4 is a strong oxidizing agent because all 3 reactions are redox reaction
Figure 4.1 Na2SO3 + H2SO4 + KMnO4
International University, Vietnam National University - HCMC CHEMISTRY LABORATORY Page 12
Figure 4.2 Na2SO3 + NaOH +2KMnO4
Figure 4.3 Na2SO3 + H2O + KMnO4
International University, Vietnam National University - HCMC CHEMISTRY LABORATORY Page 13 .
5 A. Reactions of Fe 3+ Reaction Observation Chemical Equation
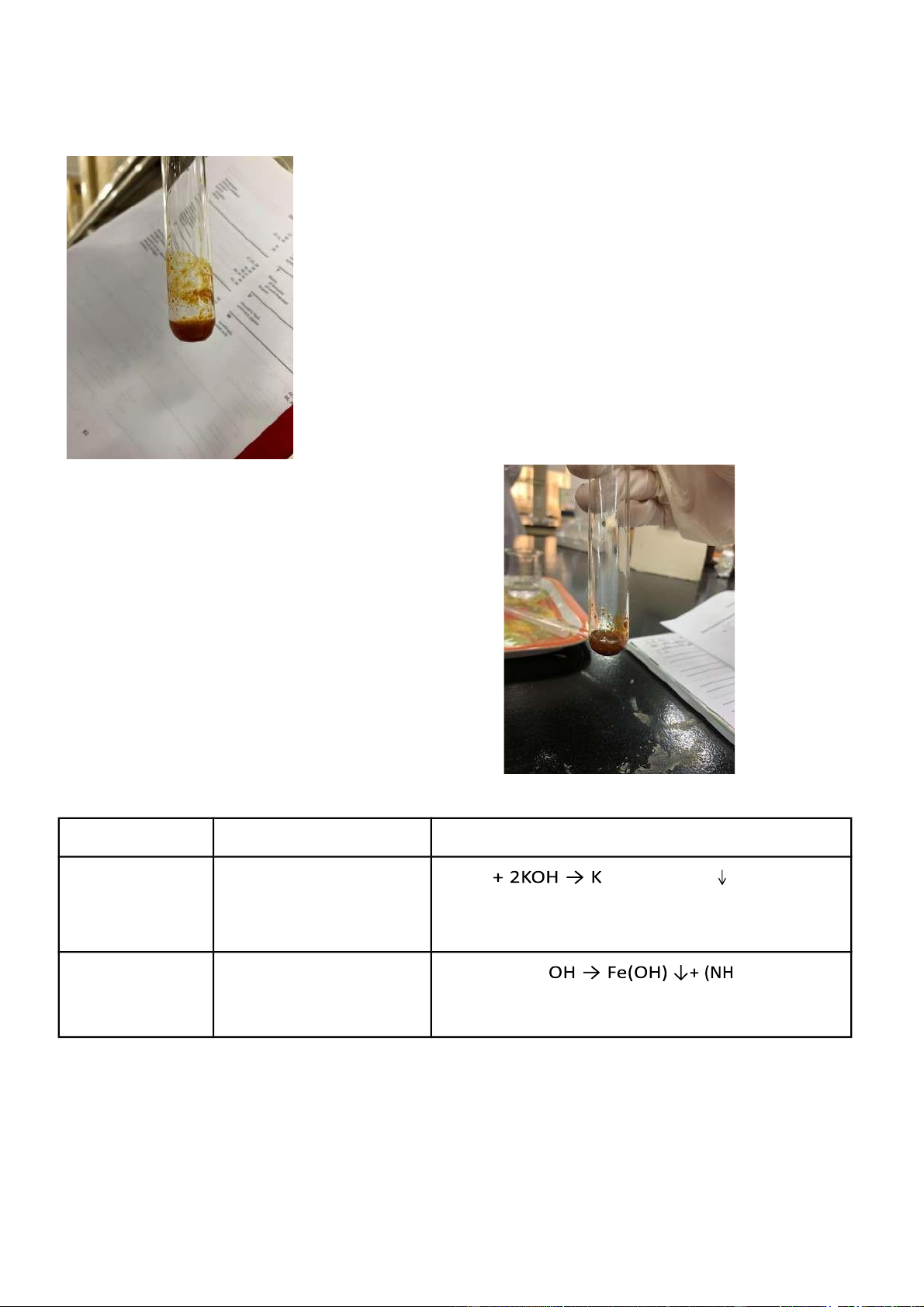
0.5 M FeCl 3 + 2M After FeCl 3 was mixed FeCl 3 3 3 KOH with KOH, a reddish brown precipitate appeared in the solution
0.5 M FeCl 3 + 2M Likewise, in this reaction, FeCl 3 + 3NH 4 3 4 Cl NH 4 OH a reddish brown precipitate appeared in the solution Comments:
- It is observed that Fe3+ has outstanding properties of oxidizing.
- Fe(OH)3 is a reddish brown precipitate and an insoluble base Figure 5.A.1: FeCl3 + KOH
International University, Vietnam National University - HCMC CHEMISTRY LABORATORY Page 14 Figure 5.A.2: FeCl3 + NH4OH
5 . B. Reactions of Fe 2+ Reaction Observation Chemical Equation
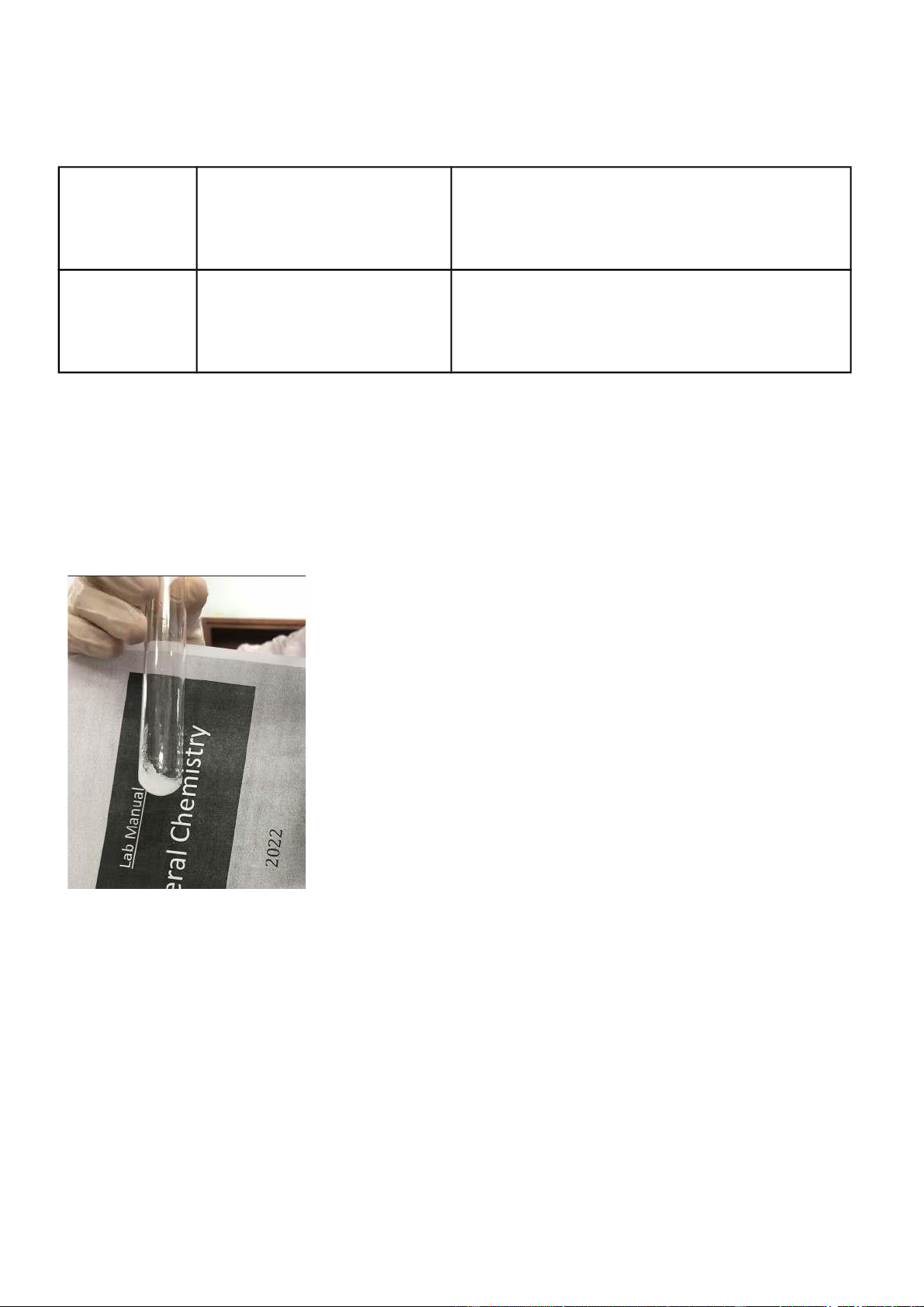
0.5 M FeSO 4 + 2M Added KOH to the FeSO 4 , FeSO 4 2SO 4 + Fe(OH) 2 KOH dark green precipitate appeared. M FeSO 0.5
4 + 2M Added NH 4 OH to FeSO 4 , FeSO 4 + 2NH 4 2 4 ) 2 SO 4 NH 4 OH and dark green precipitate appeared. Comments:
- It is observed that Fe2+ characteristic is the reduction.
- Fe(OH)2 is a dark green precipitate and an insoluble base Figure 5.B.1: FeSO4+ KOH
International University, Vietnam National University - HCMC CHEMISTRY LABORATORY Page 15 Figure 5.B.2: FeSO4+ NH4OH 6. Reactions of Al3+ Reaction Observation Chemical Equation 0.5M Al2(SO4)3
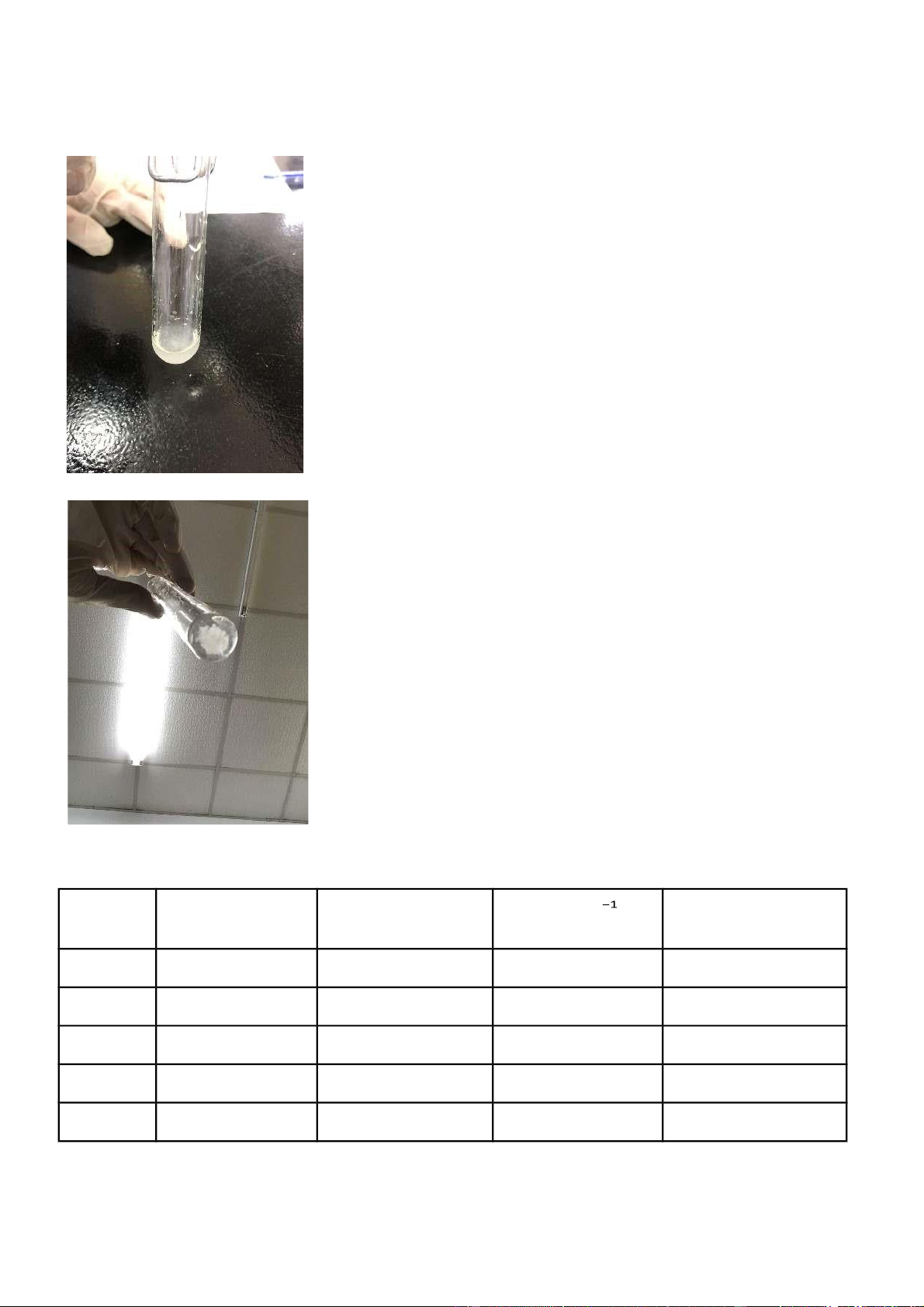
White colloidal precipitate of
Al2(SO4)3 + 6NaOH → 2Al(OH)3↓ + 3Na2SO4 + 2M NaOH Al(OH)3 appeared in the solution.
International University, Vietnam National University - HCMC CHEMISTRY LABORATORY Page 16 0.5M Al2(SO4)3
White colloidal precipitate of Al2(SO4)3 + 6NaOH → 2Al(OH)3↓ + 3Na2SO4 + 2M NaOH + Al(OH)3 dissolved slowly in 2M HCl solution after HCl was added. Al(OH)3 +HCl → AlCl3 + H2O
The solution had cloudy white color 0.5M Al2(SO4)3 After NaOH was added to the
Al2(SO4)3 + 6NaOH → 2Al(OH)3↓ + 3Na2SO4 + 2M NaOH + tube, the solution gradually 2M NaOH became colorless but a white
Al(OH)3 +NaOH → NaAlO2 + 2H2O solid did not dissolve Comments:
- Observed the chemical properties of Al(OH)3 and especially the amphoteric nature of Al(OH)3,
which can react with both bases and acid
Figure 6.1 Al2(SO4)3 + 6NaOH
Figure 6.2 Al(OH)3 +HCl
International University, Vietnam National University - HCMC CHEMISTRY LABORATORY Page 17
Figure 6.3: Al2(SO4)3 + NaOH + NaOH . Flame tes 7 t Solution Dominant flame Wavelength(nm) Frequency (s ) Photon energy (J) color LiCl Red 701 4.28x10 14 2.84 x 10 -19 NaCl Orange - Yellow 597 5.0x10 14
3.33 x 10 -19 KCl Violet 423 7.1x10 14 4.7 x 10 -19 CaCl 14 -19 2 Orange 609 4.93x10 3.3 x10 BaCl 14 -19 2 Green - Yellow 577 5.2x10 3.45 x 10 Comments:
International University, Vietnam National University - HCMC CHEMISTRY LABORATORY Page 18
- In this case, we can see the main flame color of LiCl, NaCl, KCl, CaCl2, and BaCl2 when they
have been heated. So, this is not a chemical reaction but a change in properties in physics
because electrons in different substances move and their colors when burned are also different.
Moreover, the above figures and the two formulas below are used to calculate the above table: 1. C = λ x v
- C: is the speed of light (3 x 108 m/s) - λ: is the wavelength (nm) - v: is a frequency 2. Ephoton = h x v
- Ephoton: is the energy per photon (J)
- h: is Planck’s constant (6.626 x 10-34 J.s) - v: is a frequency IV. Conclusion
After this experience, every chemistry reaction is now better understood by us. By analyzing the
changes that occur after the reaction and using theoretical chemistry equations, it is possible to identify the
special traits that each reaction possesses. It is also very clear how academic understanding and realworld
application differ. We gained some understanding of chemical reactions, learned safe experimentation
techniques, and enhanced lab-related skills. We also improve the possibility to improve our teamwork
abilities and learn how to handle members, and exactly how to work in a team in order to deliver the
greatest job results on first-day work together.




