











Preview text:
lOMoAR cPSD| 58562220 Food Unit Operation 1 Lê Ngọc Liễu Office: A1-706 Email: lnlieu@hcmiu.edu.vn 1 lOMoAR cPSD| 58562220 Introduction
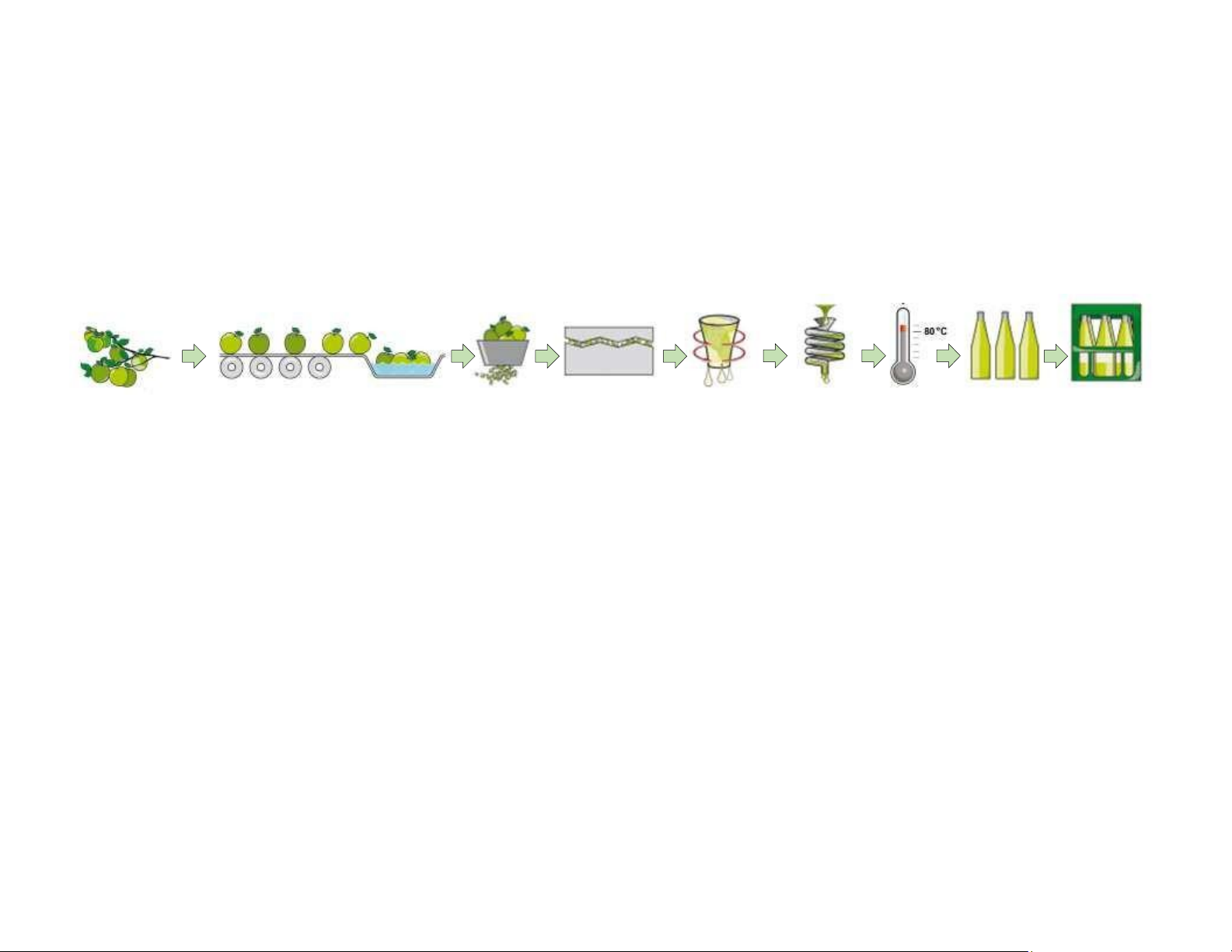
What is difference between food science and food technology? Apple juice processing Apple Receiving Washing/ Grinding Pressing Separation/
Filtration Pasteurization Fil ing Packaging harvest Sorting Centrifugation Unit operation
Definition: a basic step in a process, in common with many industrial
processes because it has common techniques and is based on the same scientific principles 2 lOMoAR cPSD| 58562220
Unit operation - Classification
Depend on the nature of transformation
Physical stages: grinding, sieving, mixture, fluidization, sedimentation, flotation,
filtration, rectification, absorption, extraction, adsorption, heat exchange, evaporation, drying, etc.
Chemical stages: refining, chemical peeling
Biochemical stages: fermentation, sterilization, pasteurization, enzymatic peeling
Depend on the nature of transferred property
Mass transfer: distillation, absorption, adsorption, extraction, ionic exchange.
Heat transfer: sterilization, pasteurization, evaporation, heat exchanger, oven.
Momentum transfer: pumps, compressors, blowers, fans, fluidization, sedimentation, filtration.
Simultaneous mass–heat transfer: Humidification and dehumidification, crystallization, dehydration
Complementary unit operations: grinding, milling, sieving, mixing of solids and pastes 3 lOMoAR cPSD| 58562220 Course content Chapter 1: Dehydration Chapter 2: Evaporation Chapter 3: Freezing
Chapter 4: Thermal preservation Chapter 5: Food Irradiation 4 lOMoAR cPSD| 58562220 Course evaluation Method Frequency Percentage Lab report 1 35% Mid-term exam 1 30% Final exam + seminar 1 35% lOMoAR cPSD| 58562220 References
1. Toledo, R.T. 1999. Fundamentals of Food Process Engineering, Aspen Publ. MD. USA
2. R. Paul Singh, Dennis R. Heldman. 2009. Introduction to food engineering. Academic Press. 4th Edition.
3. Ibarz, A., Barbosa-Cánovas, G.V. 2003. Unit operations in food engineering, Boca Raton, Fla., CRC Press, 889p.
4. Evans J.A. 2008. Frozen Food Science and Technology. Wiley-blackwell Publishing lOMoAR cPSD| 58562220 Chapter 1: Dehydration Lê Ngọc Liễu Office: A1-706 Email: lnlieu@hcmiu.edu.vn lOMoAR cPSD| 58562220 Chapter 1 content Water in food
Heat transfer and psychrometric chart
Drying: Principle and Application Drying rate and drying time lOMoAR cPSD| 58562220 Chapter 1 content Water in food
Heat transfer and psychrometric chart
Drying: Principle and Application Drying rate and drying time lOMoAR cPSD| 58562220 Water in foods
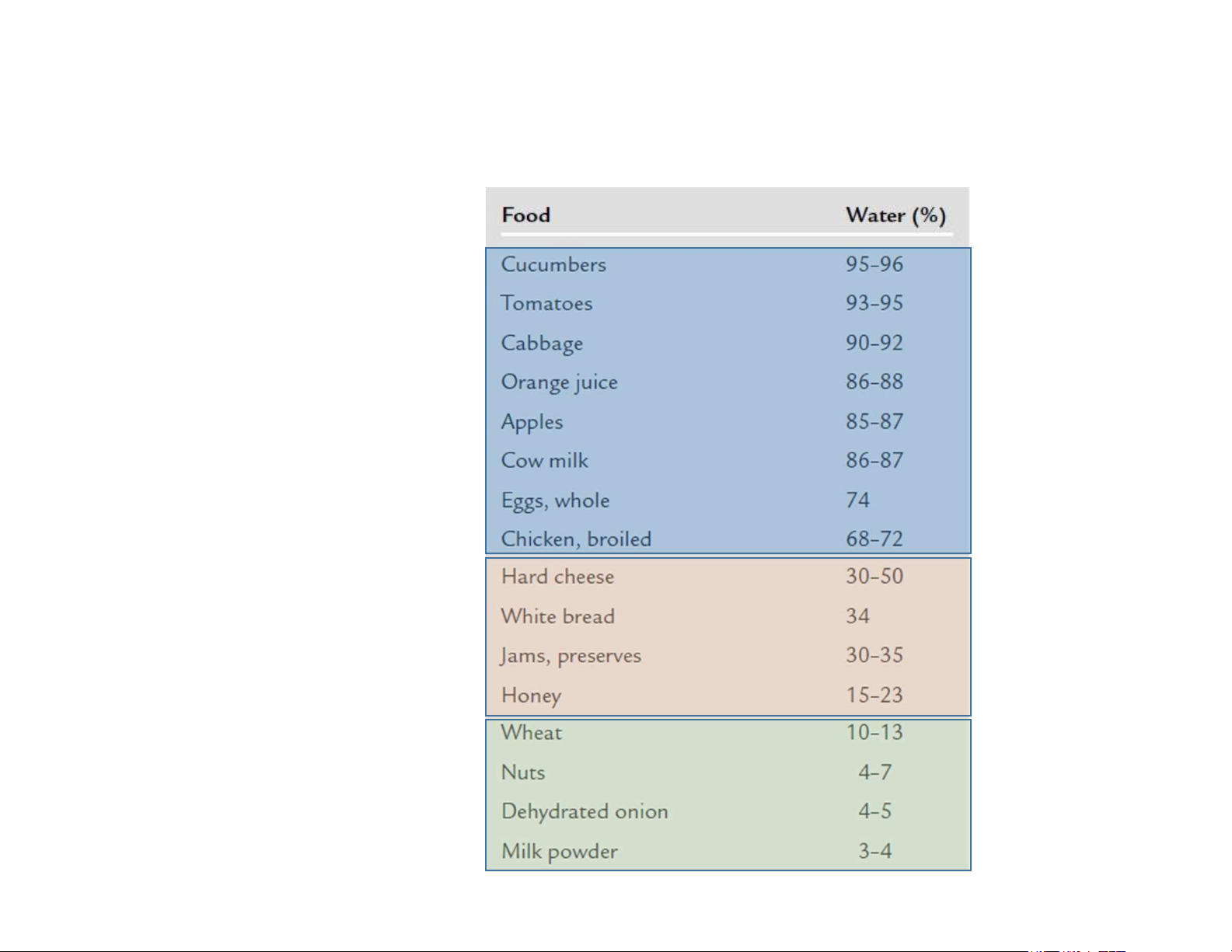
Classification of foods according to their water content High moisture foods Intermediate moisture foods Low moisture foods 10 lOMoAR cPSD| 58562220
Functional importance of water in foods
Good: essential for the good texture and appearance of fruits and vegetables
Prevent the loss of water during storage
Bad: essential for chemical reactions and microbial growth, responsible for the
microbial, enzymatic and chemical deterioration of food.
Dehydrate to preserve food during storage or shelf-life 11 lOMoAR cPSD| 58562220 Water activity
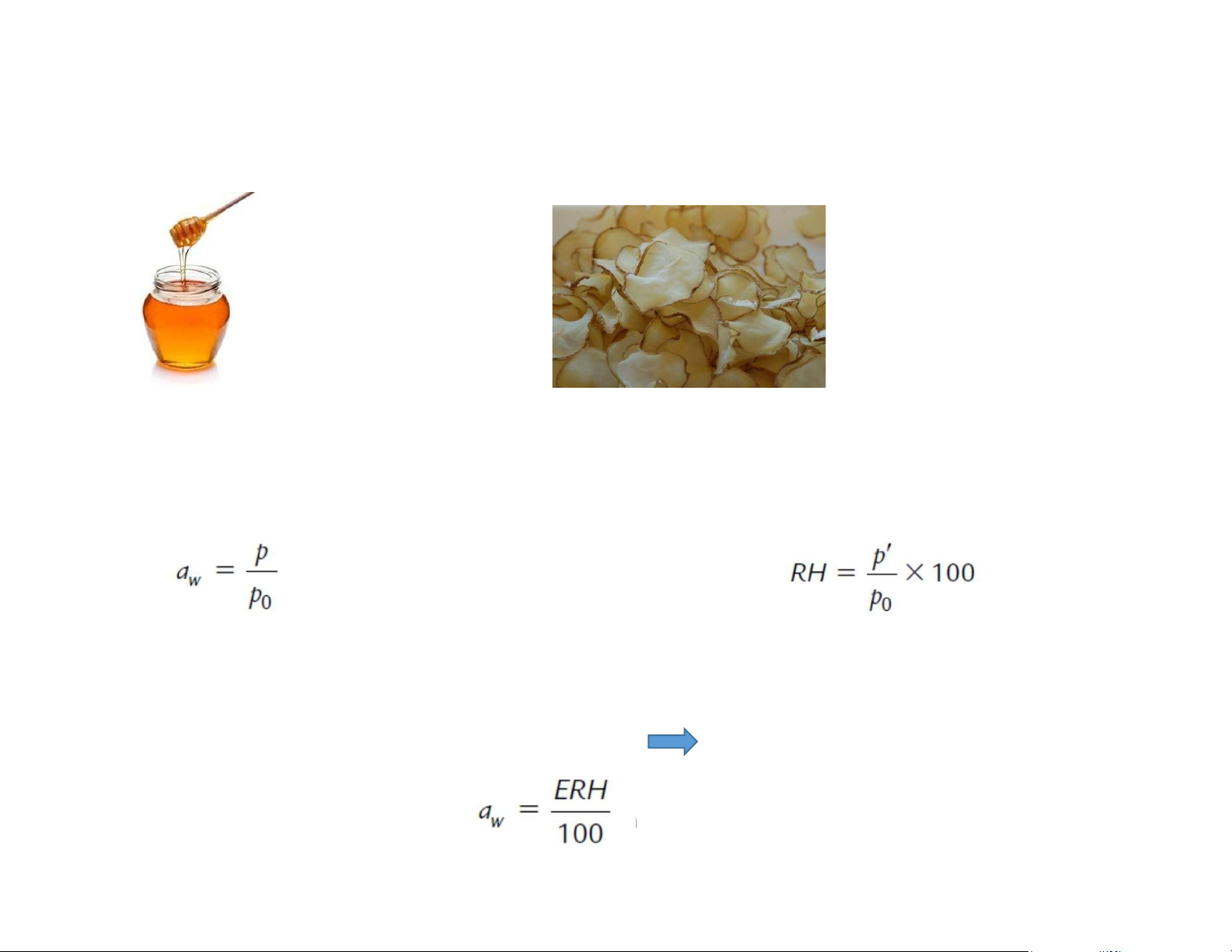
Does only the quantitative water content determine the stability of foods? Honey: 23 % water Dehydrated potato: 5-10%
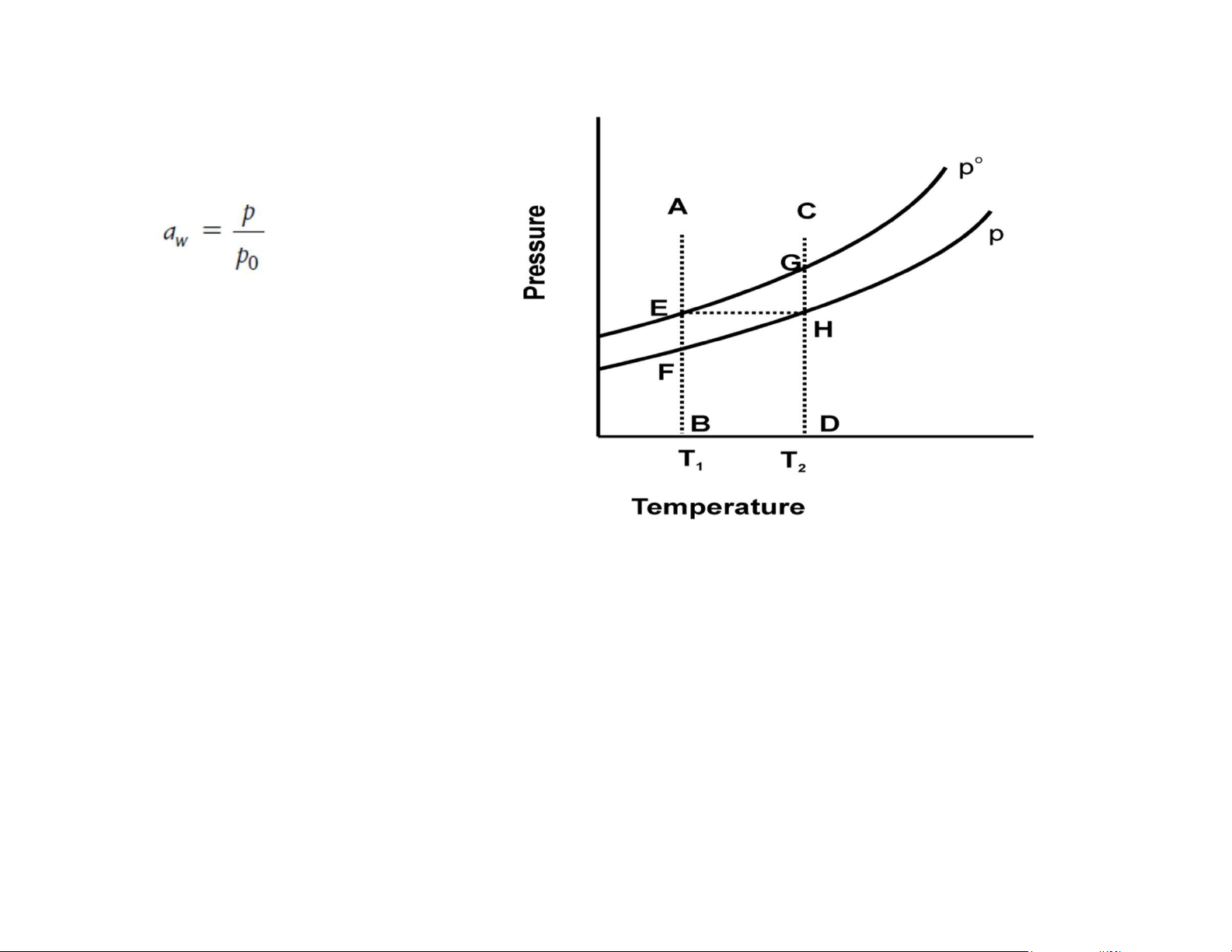
Water activity: reflect both quantity and the effectiveness Water activity aw Relative humidity of air RH
p: partial pressure of water vapor of p‘: partial pressure of water vapor in air the food at temperature
T po: equilibrium vapor pressure of pure water at temperature T
If the food is in equilibrium with air: p = p’
water activity is expressed as the
equilibrium relative humidity ERH lOMoAR cPSD| 58562220 Water activity pure water aqueous solution
p: partial pressure of water vapor of the food at temperature T po:
equilibrium vapor pressure of pure water at temperature T
Factors affecting the vapor pressure of water in foods:
water–solute interaction binding of water molecules to the polar sites of
polymer constituents (e.g. polysaccharides and proteins) adsorption of
water on the surface of the solid matrix and capillary forces lOMoAR cPSD| 58562220
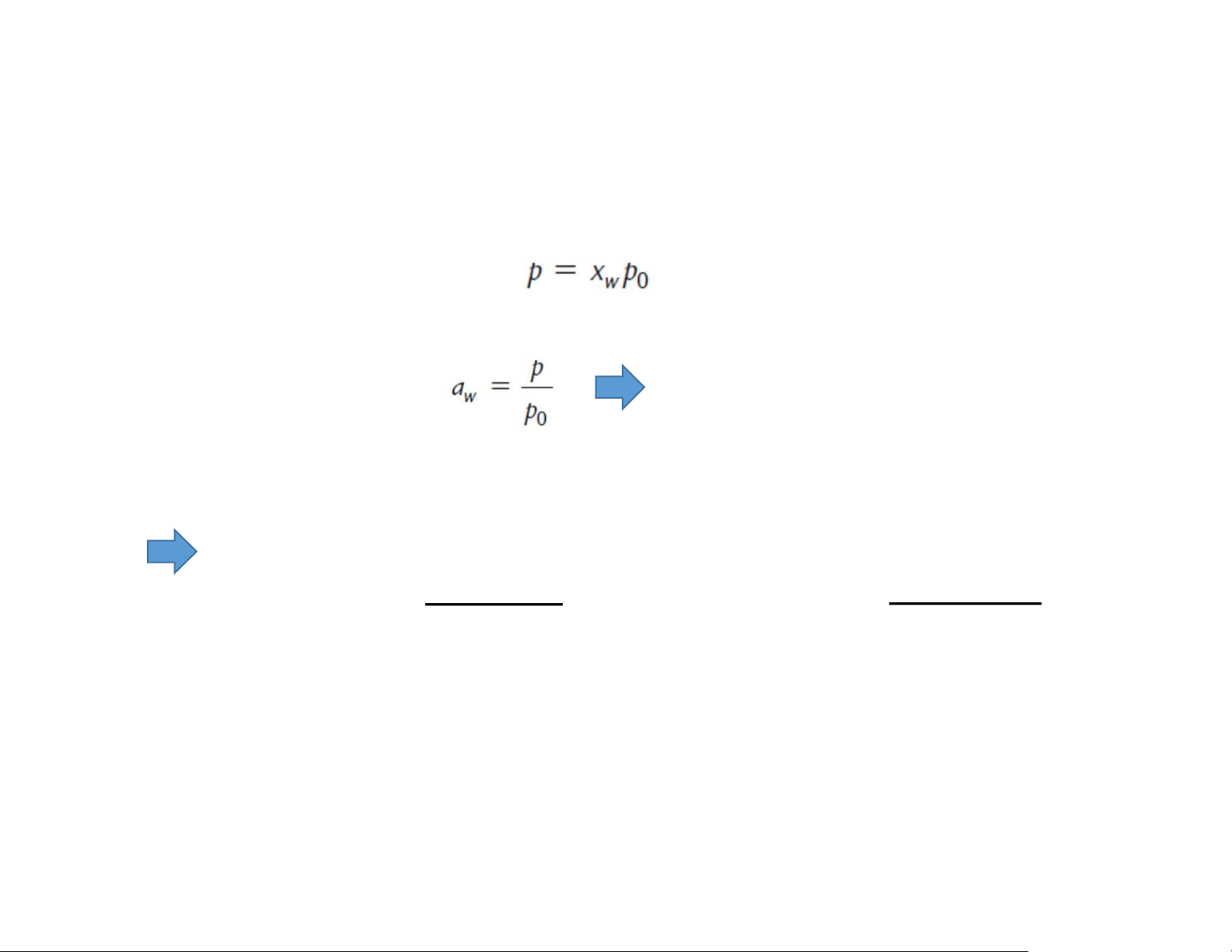
Water activity: determination Raoult’s law
Ideal solutions: The depression of vapor pressure is attributed entirely to water– solute interaction where xw is
the water content (in molar fraction) of the food a w = xw Non-ionized solutes: sugar Ionized solutes: salt or ψ where n: mole ψ: degree of ionization
Example 1.1 Estimate the water activity of honey. Consider honey as an 80% w/w aqueous
solution of sugars (90% hexoses, 10% disaccharides). Answer: 0.725 lOMoAR cPSD| 58562220 Example 1.2
NaCl, sucrose or the NaCl-sucrose solutions are commonly used for osmotic dehydration of potatoes.
(a) Estimate the water activity of 20% sucrose solution.
(b) Estimate the water activity of 20% NaCl solution
(c) Estimate the water activity of solution containing 10% NaCl and 10% sucrose.
(d) Which solution do you think will be more efficient for osmotic dehydration of
potatoes having water activity of 0.942? Data
Molecular weight of water: 18 g/mole
Molecular weight of NaCl: 58.44 g/mole
Molecular weight of sucrose: 342 g/mole
NaCl ionizes and its maximum degree of ionization, ψ: 2 Answer a) 0.987 b) 0.867 c) 0.923
d) When 20% sucrose solution is used, potatoes will adsorb moisture. Using a 20% NaCl
solution is preferred to the solution containing 10% NaCl and 10% sucrose. lOMoAR cPSD| 58562220 Example 1.3
A dry food product has been exposed to a 30% relative-humidity environment at
15oC for 5 h without a weight change. The moisture content has been measured and
is at 7.5% (wet basis). The product is moved to a 50% relative-humidity
environment, and a weight increase of 0.1 kg/kg product occurs before equilibrium is achieved.
a. Determine the water activity of the product in the first and second environments.
b. Compute the moisture contents of the product on a dry basis in both environments. lOMoAR cPSD| 58562220 Colligative properties
Colligative properties depend on the number of solute molecules or ions added to the solvent
(but not the nature of the solute).
Colligative properties include: Vapor pressure lowering Boiling point elevation Freezing point depression Osmotic pressure
These properties are used to determine the molecular weights and to measure water activity.
Freezing point depression
When the solution is ideal or when the solute concentration is low, the freezing point depression is ∆
∆Tf is the freezing point depression relative to the freezing point of water, 0oC. Let Tf = freezing point, then: lOMoAR cPSD| 58562220 ∆ 0 − −
Kf is cryoscopic constant, Kf = 1.86 for water
M is molality = mole solute/(g water/1000) 1000
n: mole of solutes after ionization (mole) 1860 − w: mass of water (g)
Remark: For highly ionized solutes such as salts of sodium and potassium, multiply the actual moles of
solute by 2 to obtain n Example 1.4
Boneless broiler breast meat contains 70.6% water, 24.0% protein, 1.2% ash, and 4.2%
fat. The freezing point is −1.2oC. If this meat is marinated by adding salt solution to obtain
a weight gain over the unmarinated meat of 15% and a net salt (NaCl) content of 1.0%.
Calculate the new freezing point lOMoAR cPSD| 58562220
Boiling point evelation
Boiling-point elevation of a solution (liquid food) is defined as the increase in boiling point over that of pure water, at a given pressure. ∆ lOMoAR cPSD| 58562220
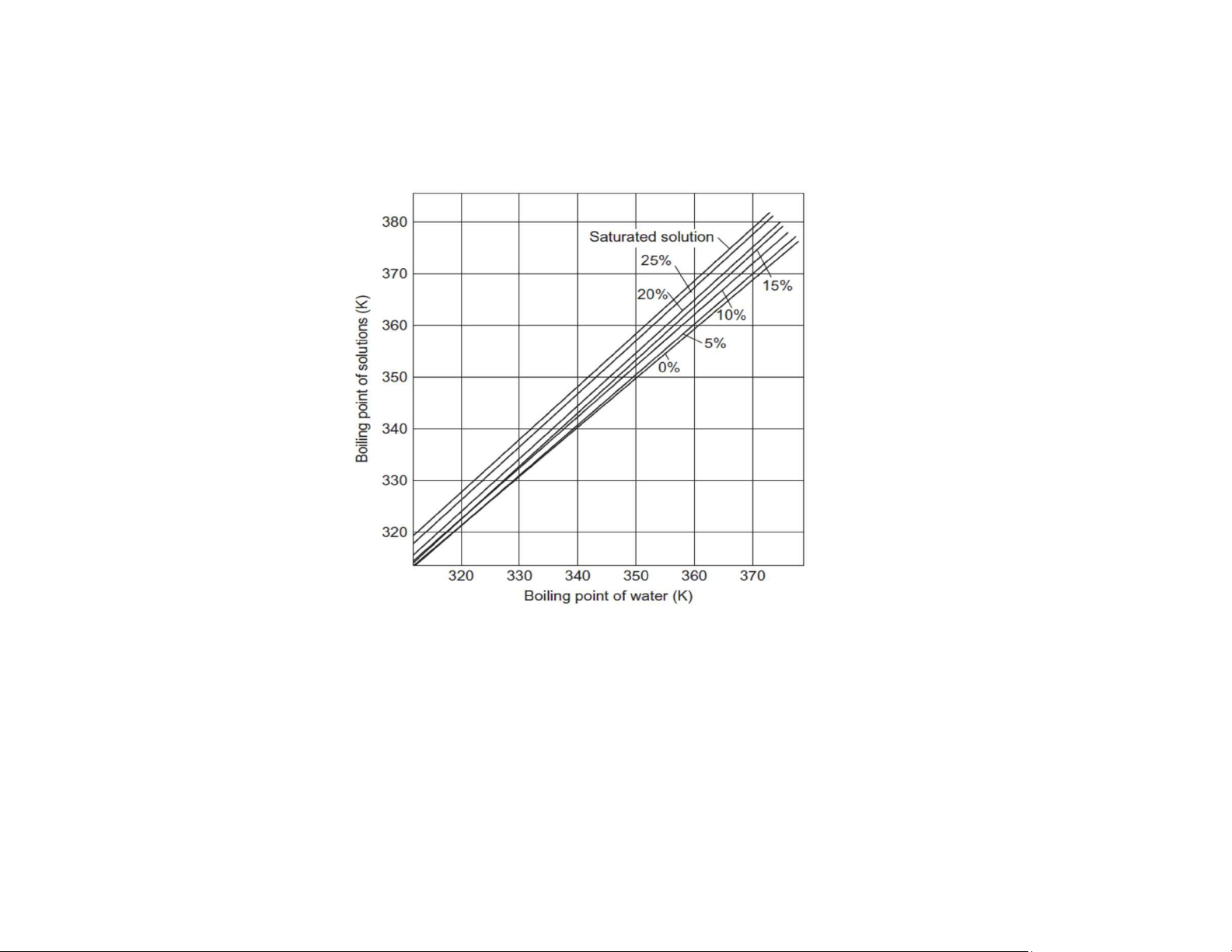
For real solutions, the boiling point rise can be calculated by the empirical rule of Dühring, which states that
the boiling point of the solution is a linear function of the boiling point of the pure solvent at the same pressure. For NaCl solution Pressure: 20 kPa Osmotic pressure What is osmosis?



