








Preview text:
lOMoAR cPSD| 58562220 Le Ngoc Lieu Office: A1.706 Email: lnlieu@hcmiu.edu.vn lOMoAR cPSD| 58562220
a process in which the temperature of the food is lowered so that some of its water crystallizes as ice
Preservation: extend the storage life Freeze drying Freeze concentration
Firming up meat for slicing or grinding
a process of reducing heat and maintaining the temperature of a product below
the general temperature of its surroundings Preservation lOMoAR cPSD| 58562220 Terminology
: not just a store for cold produce (as the term is used domestically)
but a piece of equipment specifically designed to turn fresh products into
frozen ones by leading it through a clearly defined freezing process
: used to cool product down without taking it through freezing
: store!room or warehouse operated below 0oC for holding frozen
products, usually run at a temperature somewhere between −10oC and
−30oC depending on the product: typically at about −23oC for most foods
and down to −29oC for ice cream
: similar for chilled products, with 0oC usual for fresh meat, 2oC
for general chill and dairy produce and 10oC for bakery products
The formation of ice crystals can downgrade the quality of the food lOMoAR cPSD| 58562220 Mechanical damage to the food structure Cross!linking of proteins
Limited re!absorption of water on thawing Drip loss Other issues Frozen!thawed fresh products Added water in frozen fish
Effect of freezing on food quality Meat, poultry and fish lOMoAR cPSD| 58562220 Nutritional aspect
Total fat and individual fatty acids were not significantly reduced Even
though the irreversible aggregation of the actin and myosin protein
myofibrils may occur, this does not adversely affect the nutritional value of the protein
Loss of water!soluble proteins, vitamins and minerals may occur due to the “drip!loss”
Polyunsaturated fatty acids (in fish) might suffer oxidation
systematic effects of freezing and frozen storage on nutrient retention in meat,
poultry and fish products is not extensive
Effect of freezing on food quality lOMoAR cPSD| 58562220 Meat, poultry and fish Micro-organism aspect lOMoAR cPSD| 58562220
Effect of freezing on food quality Meat, poultry and fish Micro-organism aspect
Gram!negative bacteria survive less well than Gram!positive organisms
Some enzyme activity may continue. E.g. histidine decarboxylase Response to freezing
Spore formation: spores show little or no change in numbers (some bacteria or mould)
Production of cold!shock proteins to improve survivability (vegetative
bacteria such as E. coli, B. subtilis, Vibrio cholerae and some lactic acid bacteria
Effect of freezing on food quality Vegetable and fruits
Factors affecting the nutrition of frozen vegetable and fruits
: may differ between those selected for lOMoAR cPSD| 58562220
freezing and those to be consumed as fresh, canned or dried food
: washing, cutting, peeling and storage !
: They are heated, usually in water or steam for a variable period (e.g. 95!100 oC in 3!10 min) Inactivate metabolic enzymes Ensure microbiological safety
Optimum condition: ensure inactivation of the enzymes responsible
for oxidation while minimizing loss of sensory quality and nutrients Blanching or not? Frozen storage
Effect of freezing on food quality Vegetable and fruits Micro-organism aspect
Frozen vegetables are not regarded as high!risk foods and are rarely
associated with food!borne diseases " #
Contamination with Enterobacteriaceae is common lOMoAR cPSD| 58562220
Moulds are often found on vegetables and can be a good indicator of quality
Lactic acid bacteria are usually present in the highest numbers
The micro!organisms associated with fruit include moulds, yeasts, protozoa, viruses and bacteria lOMoAR cPSD| 58562220
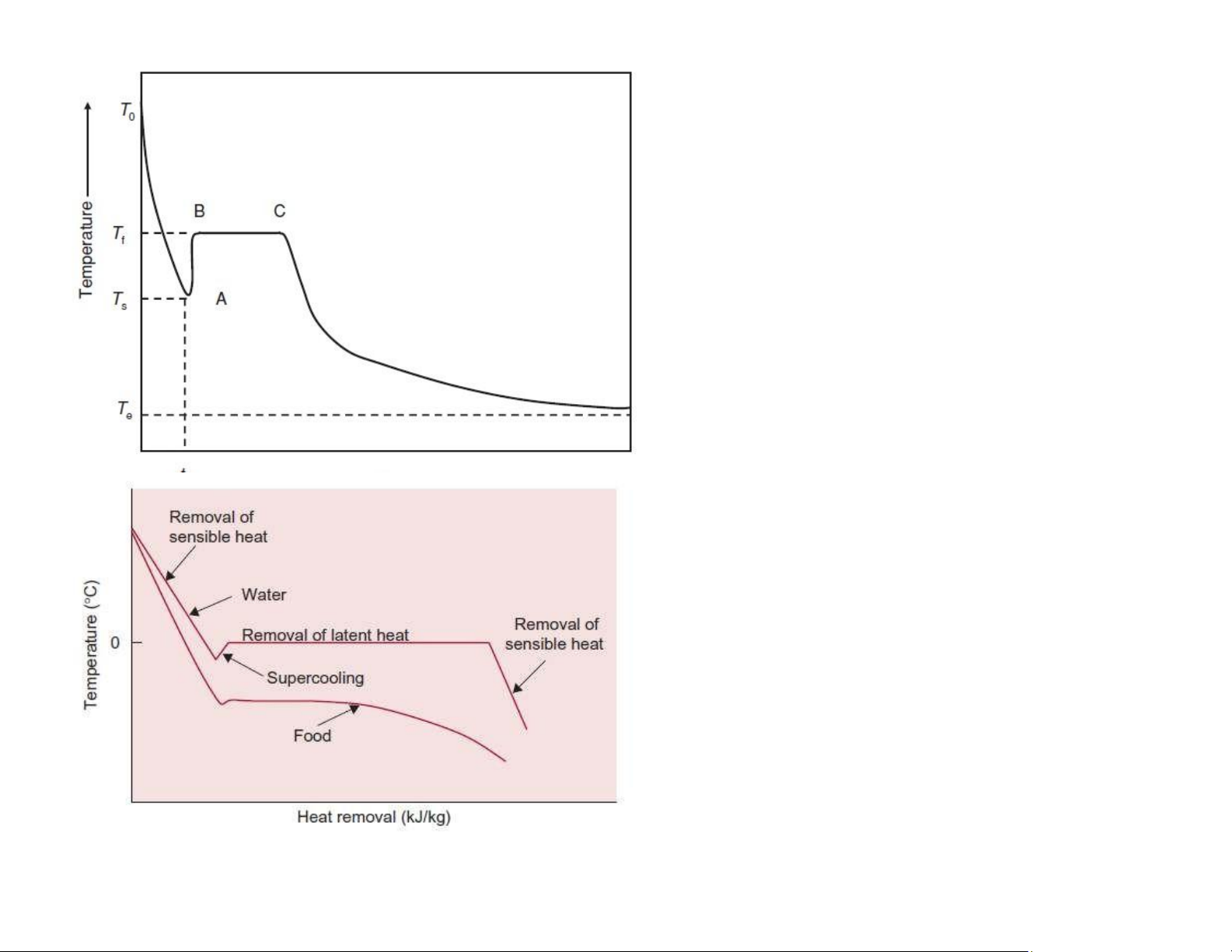
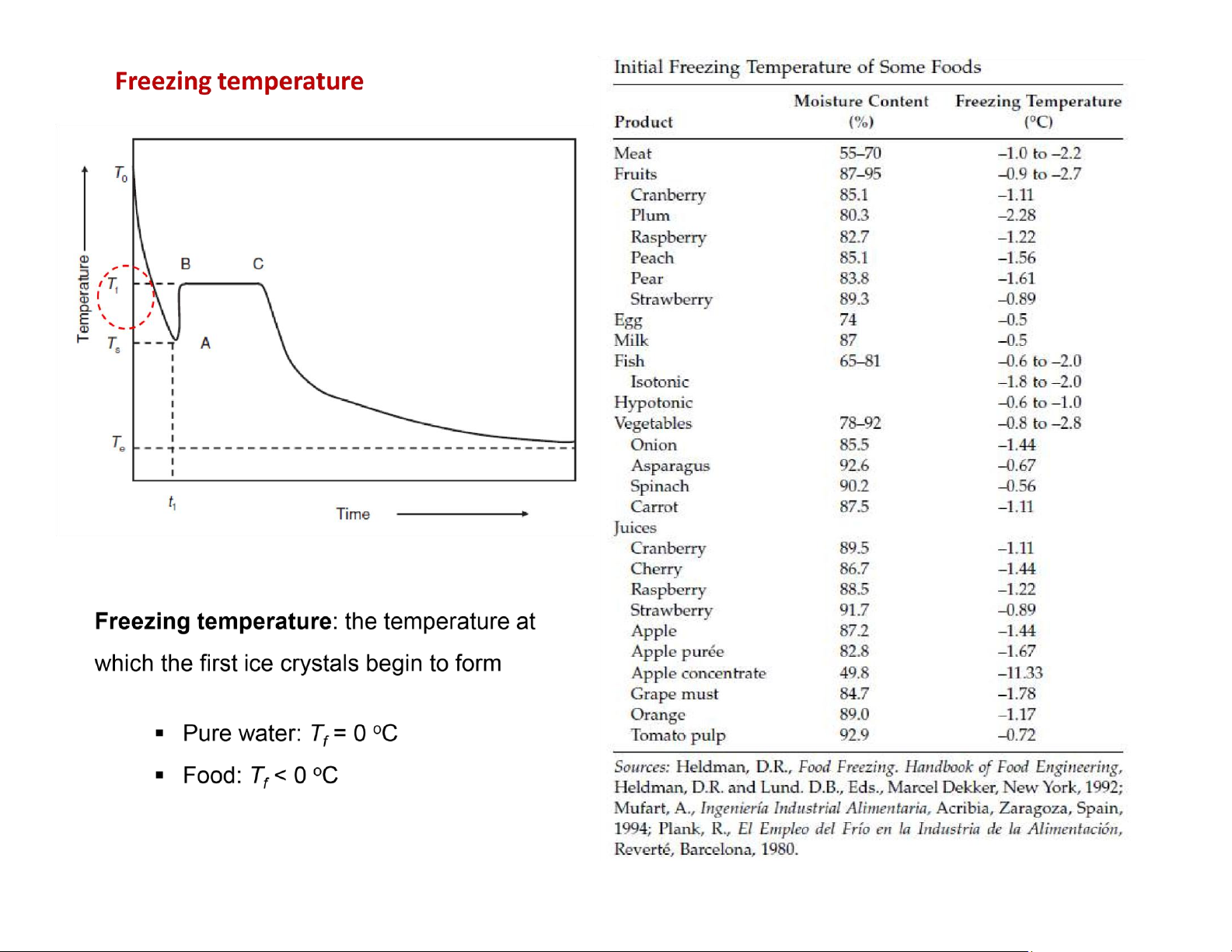
T0: the starting temperature
Tf: the initial freezing temperature Ts: the
temperature to which the food may supercool B!C: the freezing plateau
Te: the equilibrium temperature
1. Freezing involves removal of both sensible and latent heat.
2. Freezing of pure water exhibits sharp transitions
between the different freezing periods, whereas
with foods, the transitions are more gradual.
3. At the endpoint temperature for freezing foods,
the frozen food may still have some water present
as a liquid; in fact, up to 10% water may be in liquid
state for foods frozen to !18oC. lOMoAR cPSD| 58562220
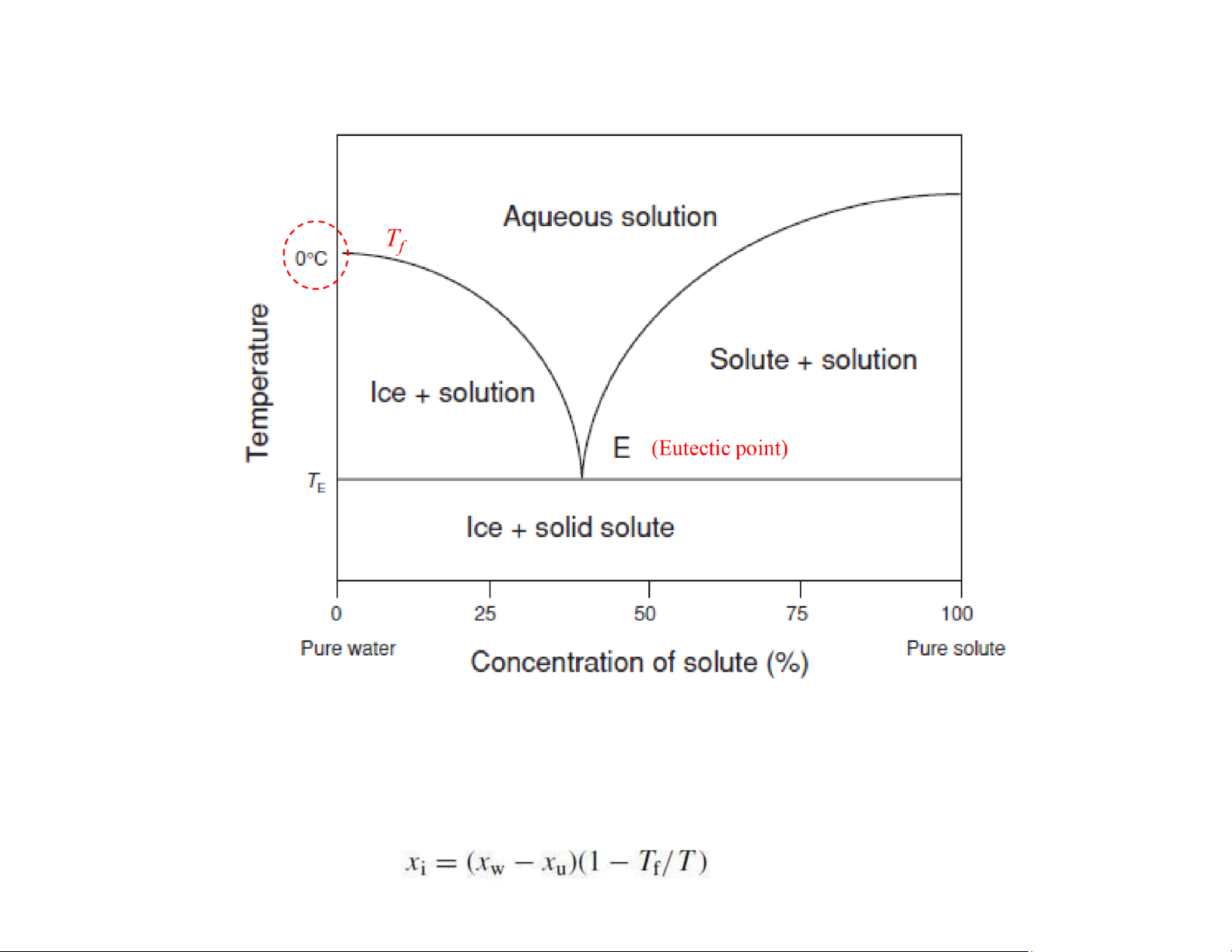
Diagram for aqueous binary solution
$ : the lowest possible melting temperature over all of the mixing ratios for the involved component species $
: the system that have the component ratio to be melted as a whole, at TE
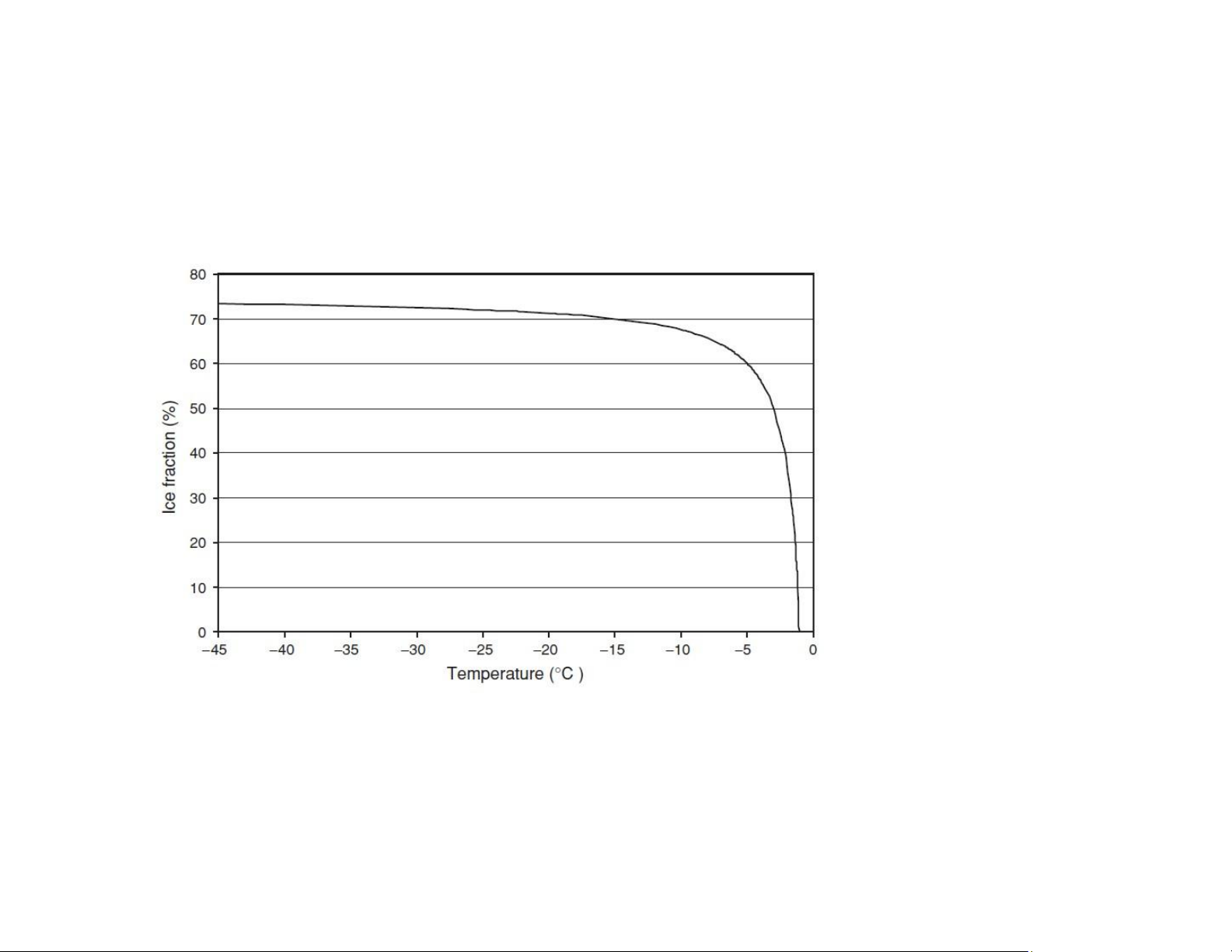
Ice fraction frozen out lOMoAR cPSD| 58562220
Tf: freezing temperature (oC) T:
operating temperature (oC) xw: the
total water content of the food xu: the unfreezable water content xw = 80% xu = 5% lOMoAR cPSD| 58562220
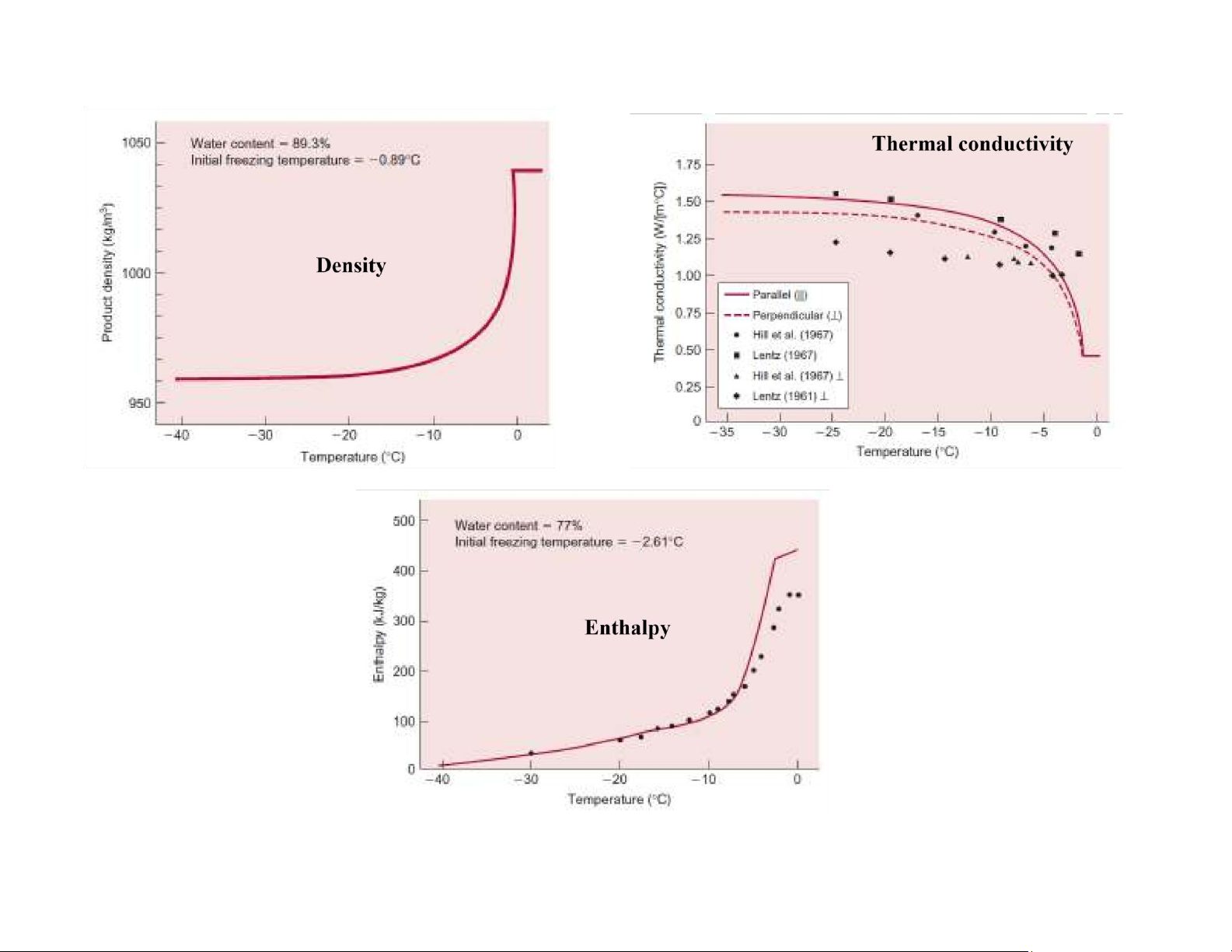
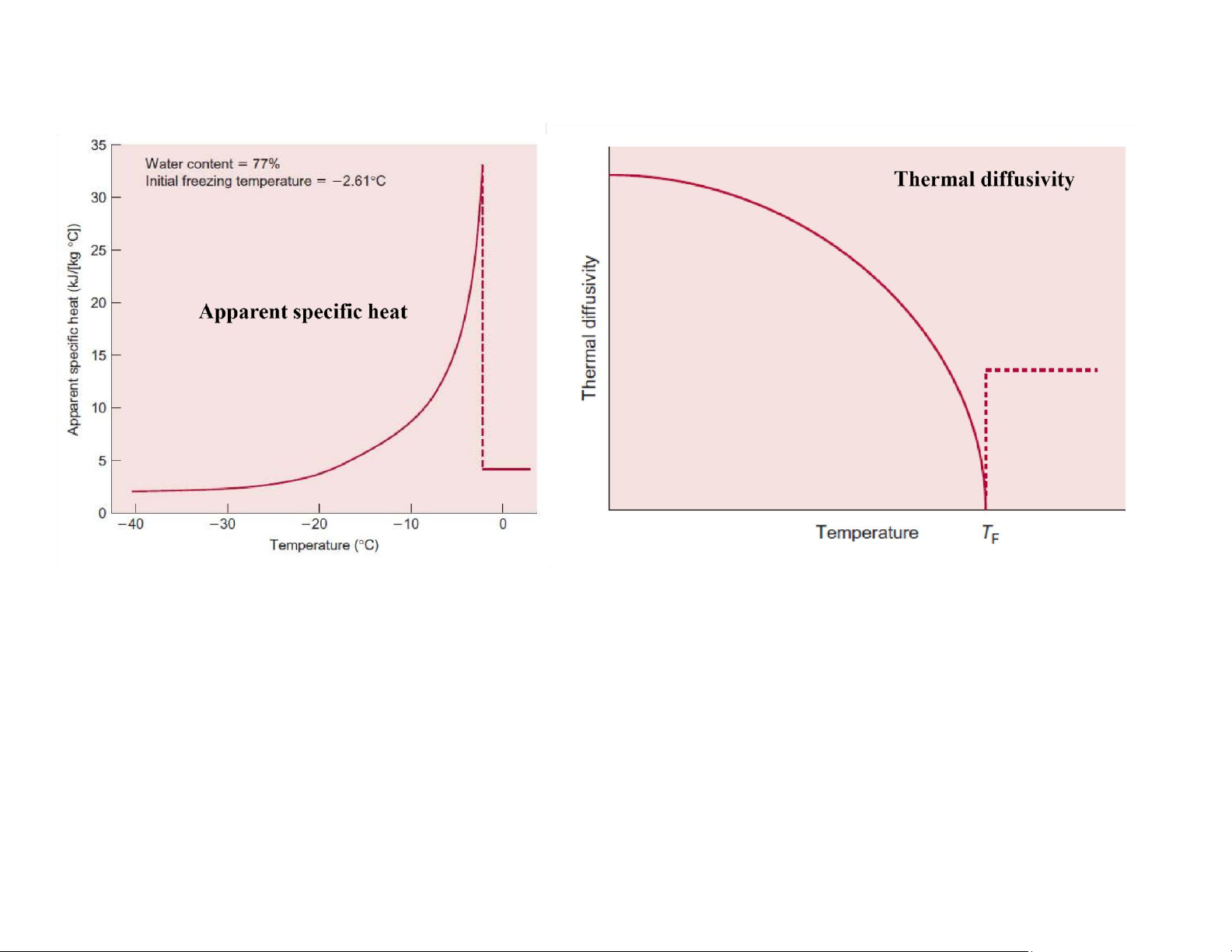
Thermophysical properties of frozen foods lOMoAR cPSD| 58562220
Thermophysical properties of frozen foods lOMoAR cPSD| 58562220 lOMoAR cPSD| 58562220
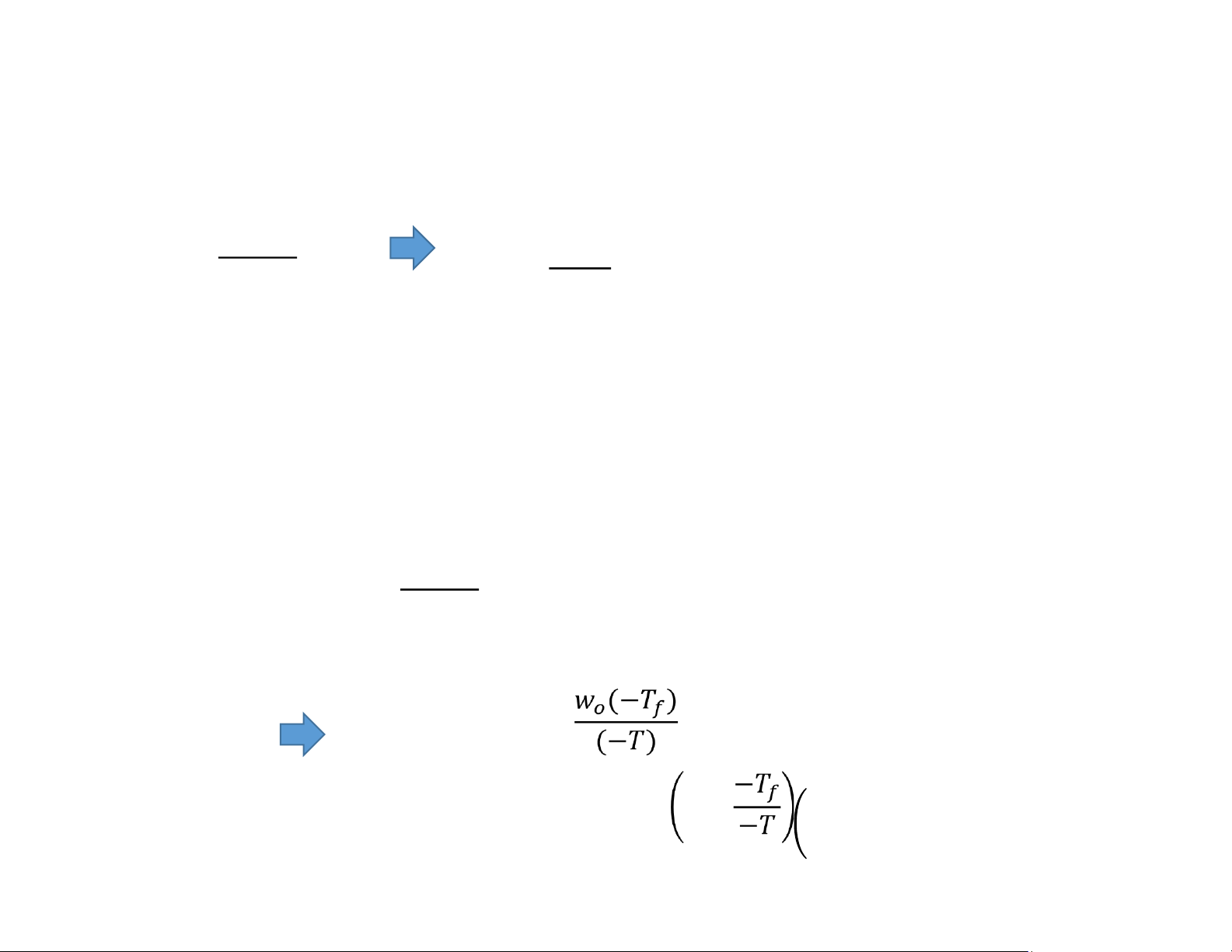
Amount of Liquid Water and Ice at Temperatures Below Freezing 1860 − = = − 1860
n: mole of solutes after ionization (mole)
wo: original water mass in the mixture before freezing (g) % 1860
n: mole of solutes after ionization (mole) − =
w: water mass that still is in liquid phase (g) % = = − = 1 − lOMoAR cPSD| 58562220
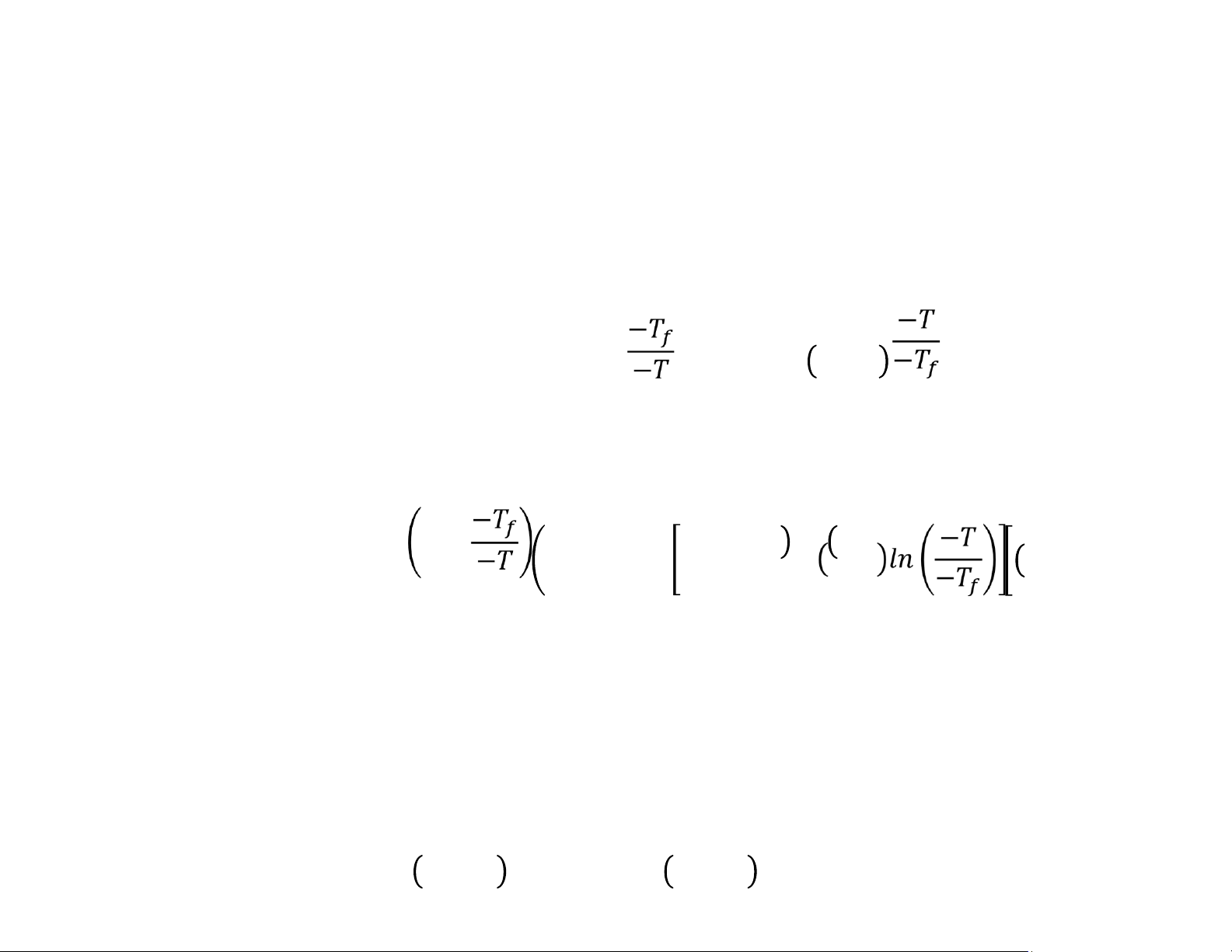
Sensible Heat of Water and Ice at Temperatures Below the Freezing Point
Change in sensible heat with temperature =
Change in sensible heat for liquid water from Tf to T = = = −
Change in sensible heat for liquid water from Tf to T = 1 − = − − − Total Enthalpy Change
The total enthalpy change will consist of: sensible heat of fat; sensible heat of non!fat solids;
sensible heat of ice; sensible heat of liquid water; and the latent heat of fusion of ice. = − + − + + + × ! lOMoAR cPSD| 58562220
Average specific heat of water above the freezing point, fat and solid!non!fat cpw = 4186.8 J/kg.K cpf = 837.4 J/kg.K cpsnf = 1674.7 J/kg.K
Latent heat of fusion of ice: Hf = 334.9 kJ/kg $& ’()
Boneless broiler breast meat contains 70.6% water, 24.0% protein, 1.2% ash, and
4.2% fat. The freezing point is −1.2◦C. If this meat is marinated by adding salt
solution to obtain a weight gain over the unmarinated meat of 15% and a net salt
(NaCl) content of 1.0%, calculate (a) the new freezing point and (b) the enthalpy
change as the marinated meat is frozen to −18◦C from the new freezing point per kg of marinated meat. lOMoAR cPSD| 58562220
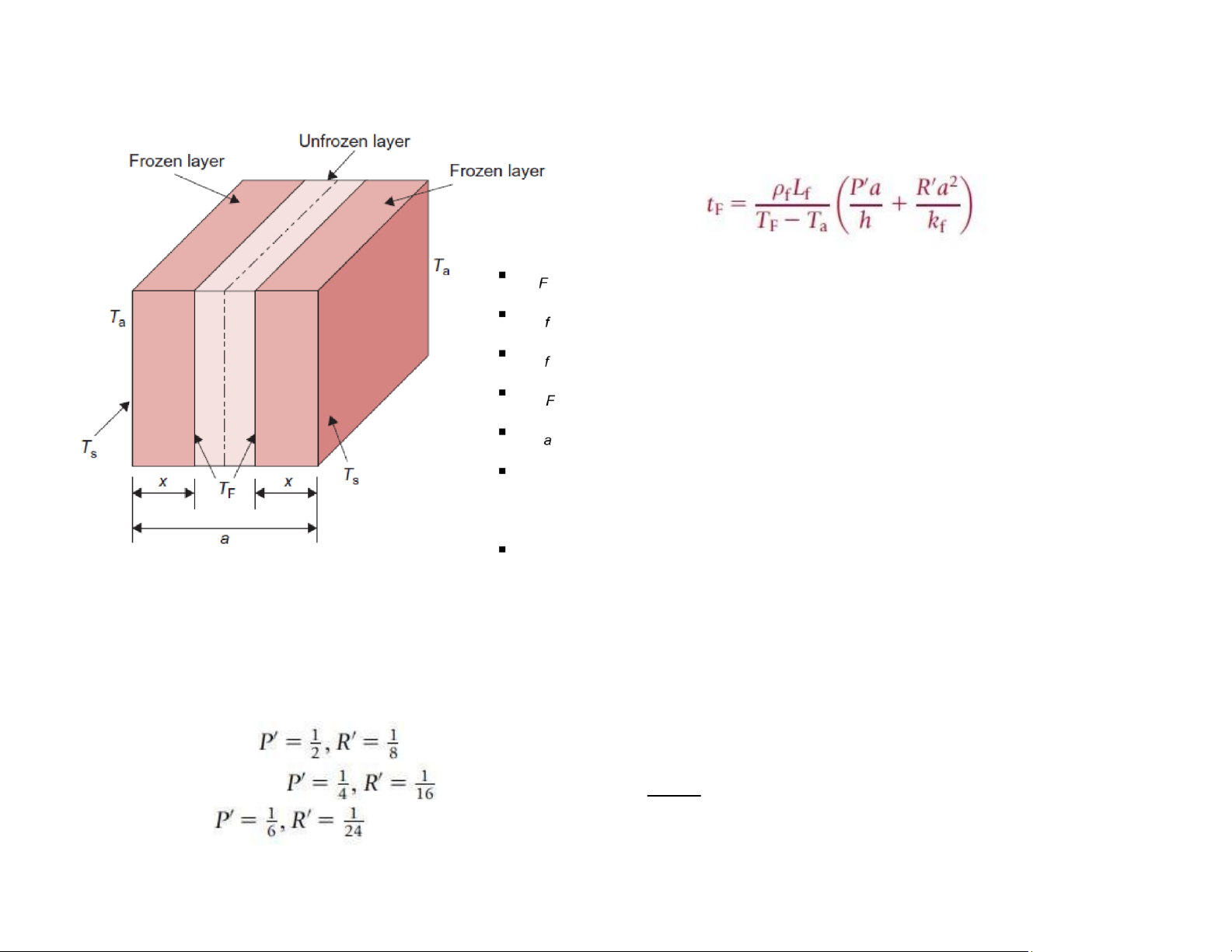
Freezing time - Plank’s Equation
Prediction of freezing time of infinite-shaped objects t : freezing time
ρ: the density of the frozen material
L: the change in the latent heat of the food (kJ/kg)
T : the freezing temperature (oC) T : the freezing air
temperature (oC) h: the convective heat transfer
coefficient at the surface of the material (W/[m2 oC]) a:
the thickness/diameter of the object (m)
k: the thermal conductivity of the frozen material (W/[m oC])
P' and R': constants, used to account for the influence of product shape Freezing power:
m: total quantity of food to be frozen Infinite slab: Infinite cylinder:
P w = mLt f tfreezing equipment, : the time that the food
stays in the t = tF lOMoAR cPSD| 58562220 Sphere:



