

Preview text:
lOMoARcPSD|364 906 32
International University, Vietnam National University - HCMC 1 CHEMISTRY LABORATORY REPORT
EXPERIMENT 1: CHEMICAL REACTIONS
Group: ______________ Class: ______________ Date: ____________ Group members: Full name Student ID Declaration of Contribution Signature 1 2 3 4 5 Total score: _______/100 Part 1. Introduction
(Introduce the general background and summarize the aims/objectives of the experiment) Part 2. Materials and Methods
(Summarize the experimental design/structure of your experiment)
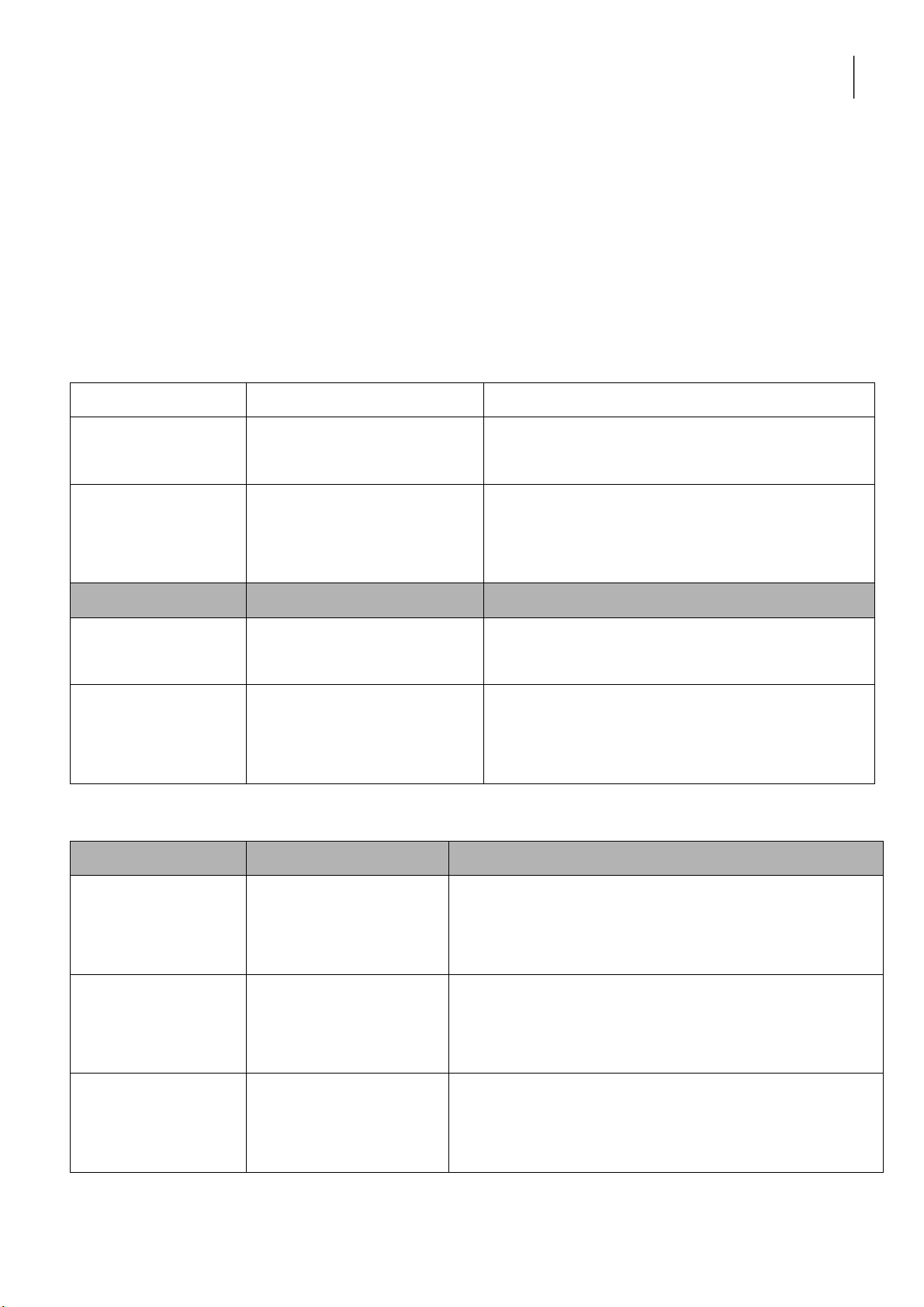
Part 3. Results and Discussion 1. REACTIONS OF Cu2+ Reaction Observation Chemical Equation 0.5M CuSO4 + 2M NaOH 0.5M CuSO4 + 2M NH4OH lOMoARcPSD|364 906 32
International University, Vietnam National University - HCMC 2 CHEMISTRY LABORATORY Discussion:
(Discuss: i) Explanation for the above observation; ii) Possible reasons for any misconducted results)
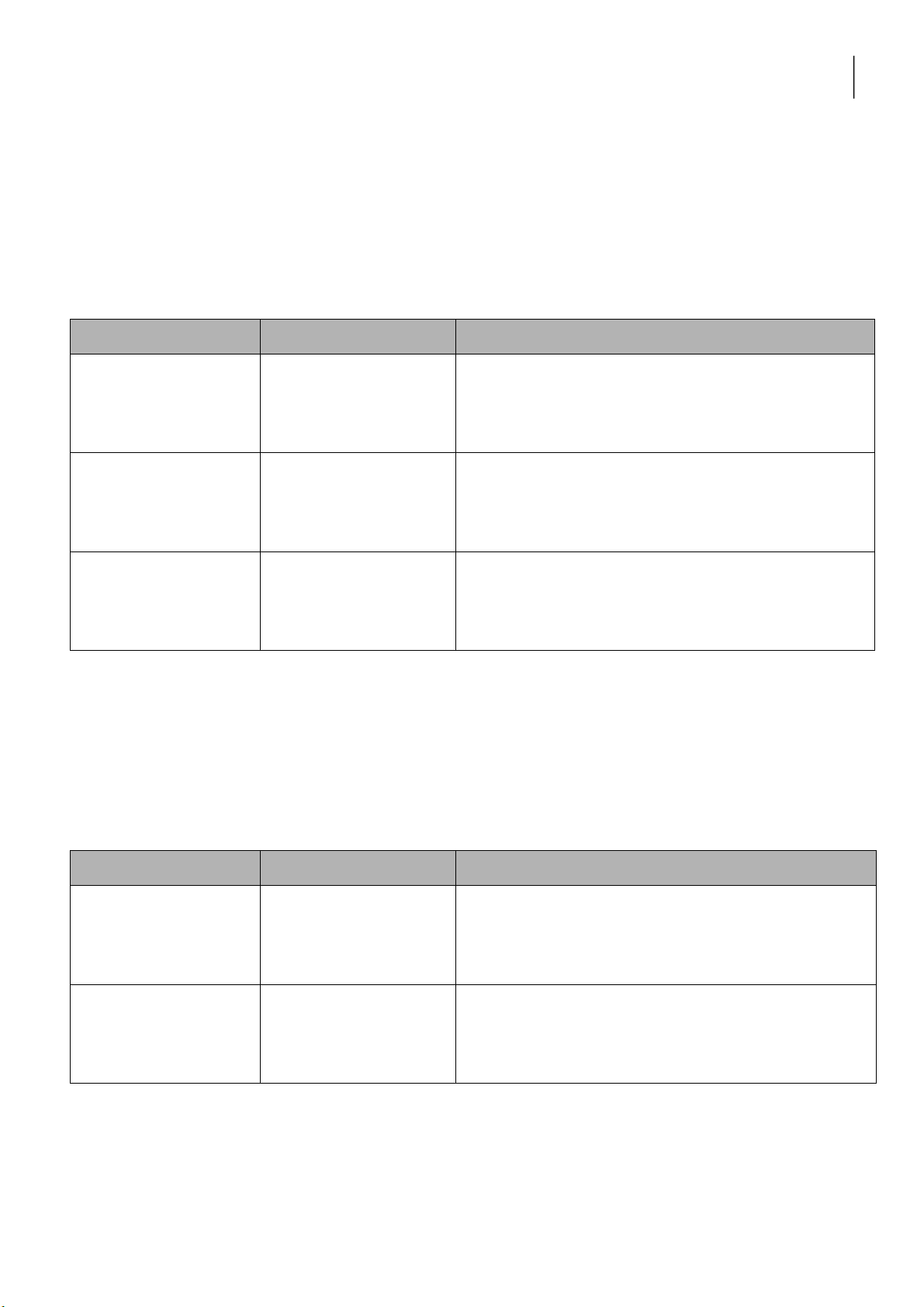
2. REACTIONS OF SILVER HALIDES Reaction Observation Chemical Equation 0.5M KCl + 0.1M AgNO3 0.5M KCl + 0.1M AgNO3 + 2M NH4OH 0.5M KBr + 0.1M AgNO3 0.5M KBr + 0.1M AgNO3 + 2M NH4OH Discussion: 3. REACTIONS OF H2O2 Reaction Observation Chemical Equation 0.1M KMnO4 + 2M H2SO4 + H2O2 0.1M KI + 2M H2SO4 + H2O2 H2O2 + MnO2 Discussion: lOMoARcPSD|364 906 32
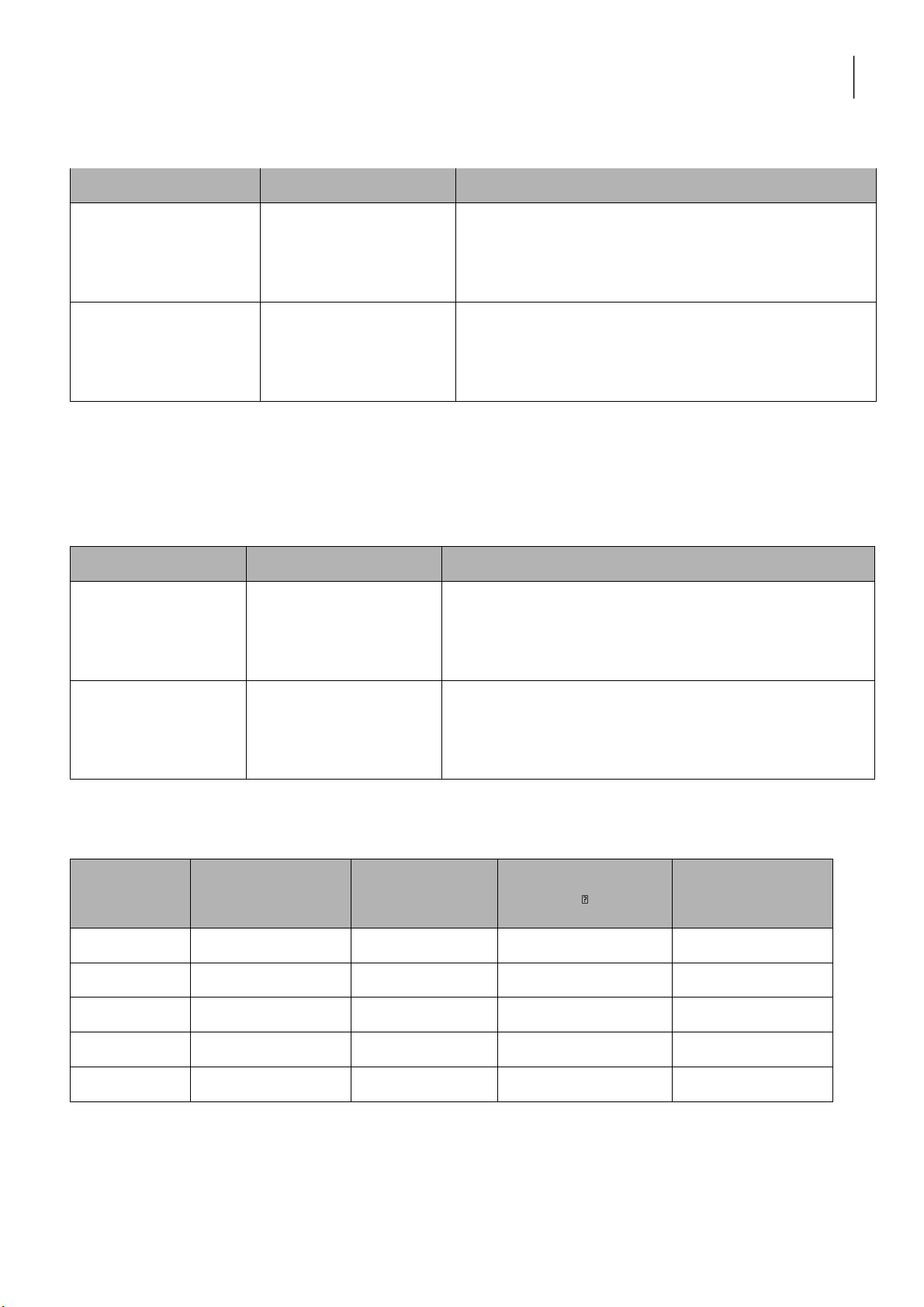
International University, Vietnam National University - HCMC 3 CHEMISTRY LABORATORY 4. REACTIONS OF KMnO4 Reaction Observation Chemical Equation 0.5M Na2SO3 + 2M H2SO4 + 0.1M KMnO4 0.5M Na2SO3 + 6N NaOH + 0.1M KMnO4 0.5M Na2SO3 + H2O + 0.1M KMnO4 Discussion: 5. A. REACTIONS OF Fe3+ Reaction Observation Chemical Equation 0.5M FeCl3 + 2M KOH 0.5M FeCl3 + 2M NH4OH Discussion: lOMoARcPSD|364 906 32
International University, Vietnam National University - HCMC 4 CHEMISTRY LABORATORY 5. B. REACTIONS OF Fe2+ Reaction Observation Chemical Equation 0.5M FeSO4 + 2M KOH 0.5M FeSO4 + 2M NH4OH Discussion: 6. REACTIONS OF Al3+ Reaction Observation Chemical Equation 0.5M Al2(SO4)3 + 2N NaOH + 2M HCl 0.5M Al2(SO4)3 + 2M NaOH + 2M NaOH Discussion: 7. FLAME TEST Solution Dominant flame Wavelength Frequency Photon energy (J) color (nm) (s 1) LiCl NaCl KCl CaCl2 BaCl2 Discussion: lOMoARcPSD|364 906 32
International University, Vietnam National University - HCMC 5 CHEMISTRY LABORATORY Part 4. Conclusions END.
Document Outline
- REPORT
- (Summarize the experimental design/structure of your experiment)
- (Discuss: i) Explanation for the above observation; ii) Possible reasons for any misconducted results)




