













Preview text:
CHEMISTRY LABORATORY REPORT
EXPERIMENT 1: CHEMICAL REACTIONS Group: 4 Section: 1910 Group members: Lê Nguyễn Vân Anh Hoàng Nguyễn Minh Châu Lê Thanh Như Quỳnh Trần Băng Châu Tạ Thị Minh Thu Date: February 18th, 2019. Score:
International University, Vietnam National University - HCMC 2 GENERAL CHEMISTRY LABORATORY I.Introduction:
Chemical changes result in the formation of new substances. When a chemical reaction
occurs, substances called reactants are transformed into different substances called products that
often have different appearances and different properties. Observable signs of chemical reactions
can be a change in color, the formation o f a solid, t he re lease o f g as, a nd t he p roduction o f h eat a nd
light. We also learn how to classify chemical reactions. One classification system involves five general types of reactions: s ynthesis, d
ecomposition, single displacement, double displacement, and
combustion. Chemical reaction always happens in the living system, typically the formation and transformation of matter. II.Learning Objective:
-To perform different types of chemical reactions (acid-base, precipitate, gas forming,
complex compound forming, and oxidation-reduction reactions).
-To identify some of the products in these reactions and describe the chemical changes.
-To write and balance the chemical equations for the reactions observed. III.Materials and Equipment: Equipment Test tubes Alcohol lamp Test tube rack Looped wire Test tube holders Distilled water bottle Beakers Materials Semester II: 2018-2019
International University, Vietnam National University - HCMC 3 GENERAL CHEMISTRY LABORATORY 0.5M CuSO Concentrated CH COOH 4 3 2M NaOH 0.5M Na SO 2 3 6M NaOH 0.1M KSCN 0.1M AgNO 2M KOH 3 0.5M KBr 0.5M K [Fe(CN) ] 4 6 0.5M KI 0.5M FeSO4 0.1M KI 0.5M FeCl3 2M NH OH 0.5M Al (SO ) 4 2 4 3 3% H O 2M HCl 2 2 2M H SO 0.5M LiCl 2 4 MnO 0.5M NaCl 2 Saturated FeSO 0.5M KCl 4 0.1M NaNO 0.5M CaCl 3 2 0.1M NaNO 0.5M BaCl 2 2 0.1M KMnO4 0.5M K Cr O 2 2 7 C H OH 96% H SO 2 5 2 4 2M H SO 2 4
IV. Experimental Procedure: Data and Observations: 1. Reactions of Cu2+
-First, put 10 drops of 0.5M CuSO into each of three test tubes. 4 -Then added 10 drops of 2M N aOH i nto t he fi rst test t ube; 2 M N H OH i nto t he s econd t est t ube; 4
0.5M K [Fe(CN) ] into the third one. 4 6 - A fe w s econds l ater, added more 1 0 d rops of 2 M NaOH into t he fi
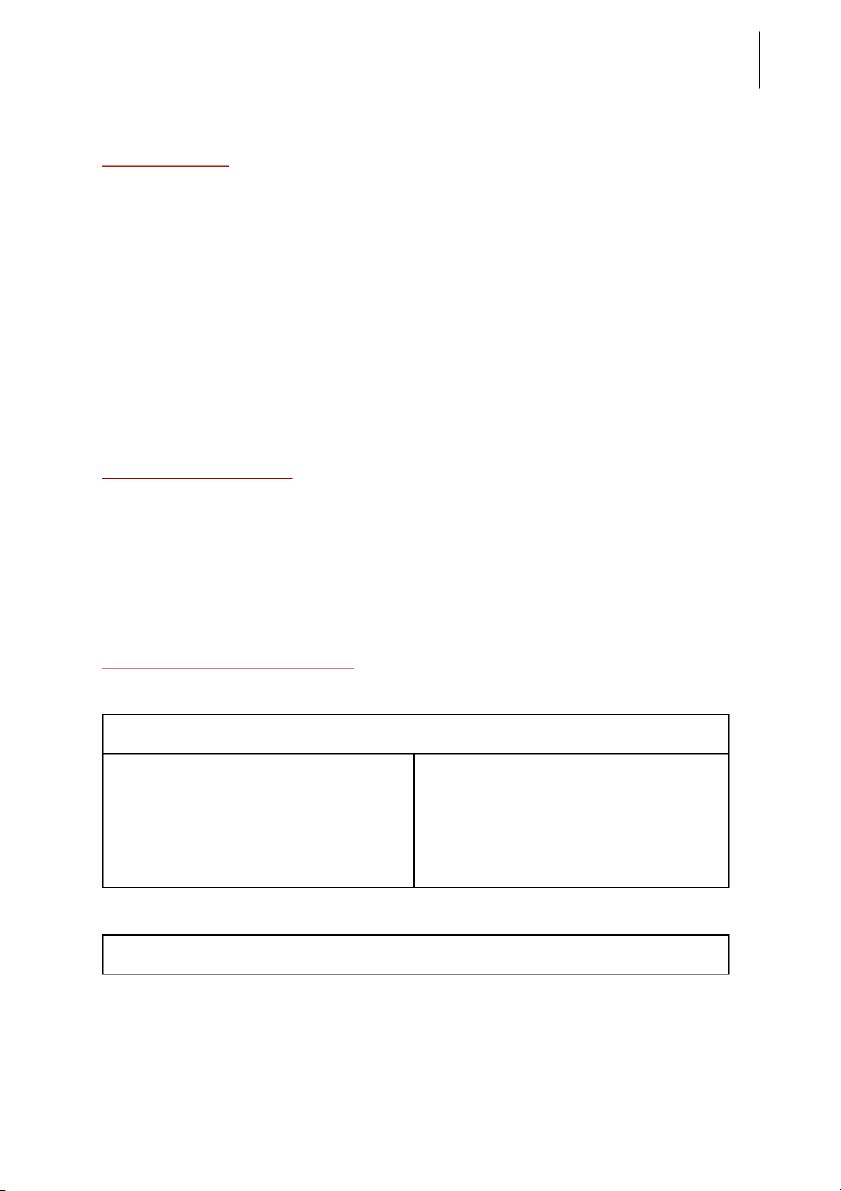
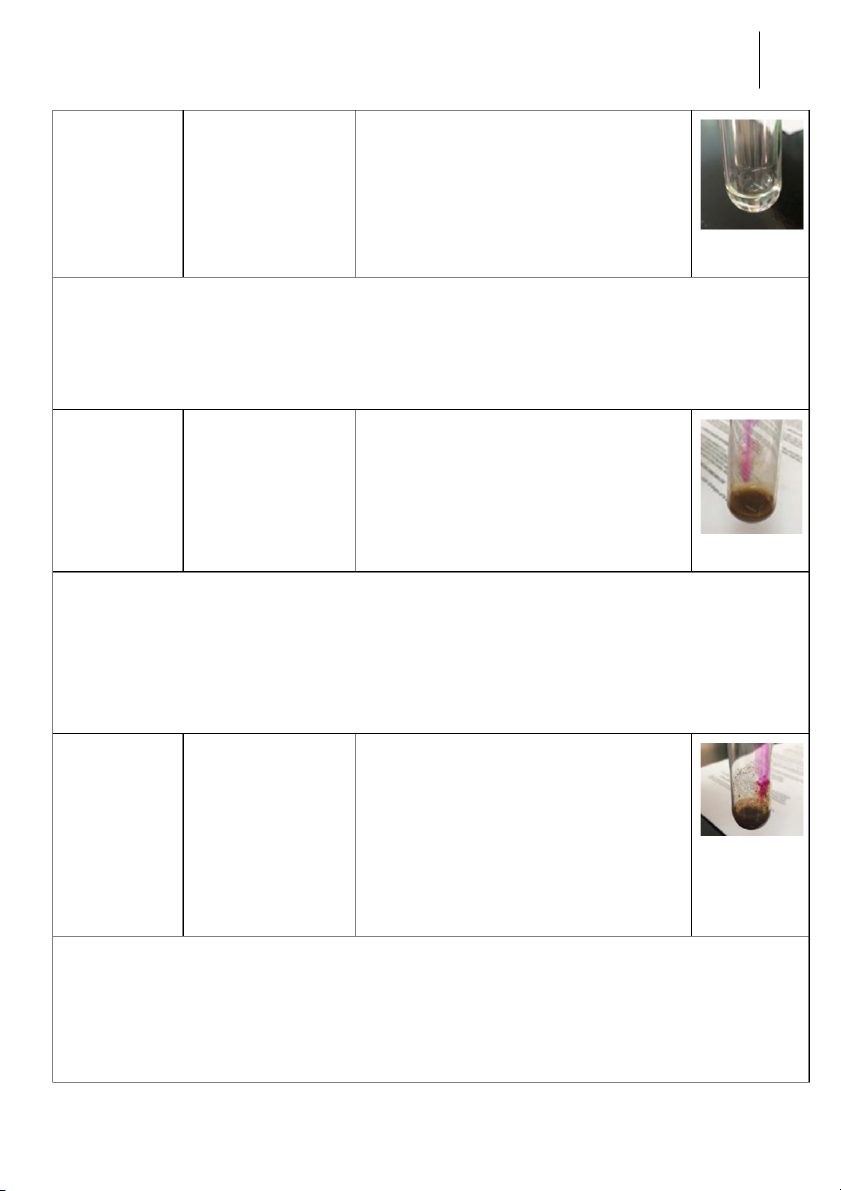
rst test t ube; 2M NH OH into 4 the second test tube. Reaction Observation Chemical Equation Image 0.5M CuSO
-The blue precipitate CuSO + 2 NaOH → Cu(OH) 4 4 2 + 2M NaOH and colloidal + Na SO 2 4 phenomena were formed Semester II: 2018-2019
International University, Vietnam National University - HCMC 4 GENERAL CHEMISTRY LABORATORY Explanation:
* CuSO is dissociated completely: 4
CuSO → Cu2++ SO 2- 4 4
* NaOH is also dissociated completely to form Hydroxide ion OH-:
NaOH → Na+ + OH-
*Ion Cu2+and OH-in the solution combine together to form Cu(OH) , a blue precipitate. 2 0.5M CuSO -First, the blue
CuSO + 2NH OH → Cu(OH) + 4 4 4 2 + 2M NH OH precipitate dissolved (NH ) SO 4 4 2 4 after being formed. Then, the solution Cu(OH) + 4NH 2 → 3 turned into dark blue. [Cu(NH ) ](OH) 3 4 2 Explanation:
* CuSO is dissociated completely: 4 CuSO 2+ 2-. 4 → Cu + SO 4
* NH OH is also dissociated completely: 4
NH OH → NH4++ OH- 4
*Ion Cu2+and OH-in the solution combine together to form blue precipitate Cu(OH) because it has 2 very low solubility.
*Then, the remaining NH OH reacts with the precipitate Cu(OH) to form a 4 2
complex-compound [Cu(NH ) ]SO4 to form the dark blue of the solution. 3 4 0.5M CuSO -The
brown 2CuSO +K Fe(CN) → 4 4 4 6 0.5M K [Fe(CN) ] precipitate
and Cu [Fe(CN) ] + 2K SO 4 6 2 6 2 4 colloidal phenomena were formed. Explanation:
* Copper (II) sulfate CuSO is dissociated completely: 4
CuSO → Cu2+ + SO42- 4 Comments:
● The feature of Cu2+ is it can react with OH- form blue precipitation.
● The solution Cu(OH) also react with NH OH to form a c omplex c ompound s o reaction 2 has 2 4 the dark blue at the end.
● The last precipitate solution has brown color because the solution is compound consist of Fe2+
● Double displacement reaction occurs when part of one reactant is replaced by part of another reactant. Semester II: 2018-2019
International University, Vietnam National University - HCMC 5 GENERAL CHEMISTRY LABORATORY ● Comparing: -
The color of second precipitate is less dark than in practice. -
The color of third precipitate is just only brown unlike red-brown in practical.
2. Reactions of silver halides
-Prepared 0.5 M solutions of KCl, KBr, and KI.
-Then added 10 drops of 0.1M silver nitrate to 10 drops of each salt solution: KCl, KBr, and KI. Observing the result
-Next, we divided each of the solutions equally into two test tubes labeled # 1-3 A and # 1-3 B
*With test tube #1-3 A: did not add anything, just observe.
*With test tube # 1-3 B: added 5 drops of 2M NH OH to each of test tubes. 4
-Waited till the end of the solution and recorded the observation. Reaction Observation Chemical Equation Image 0.5M KCl
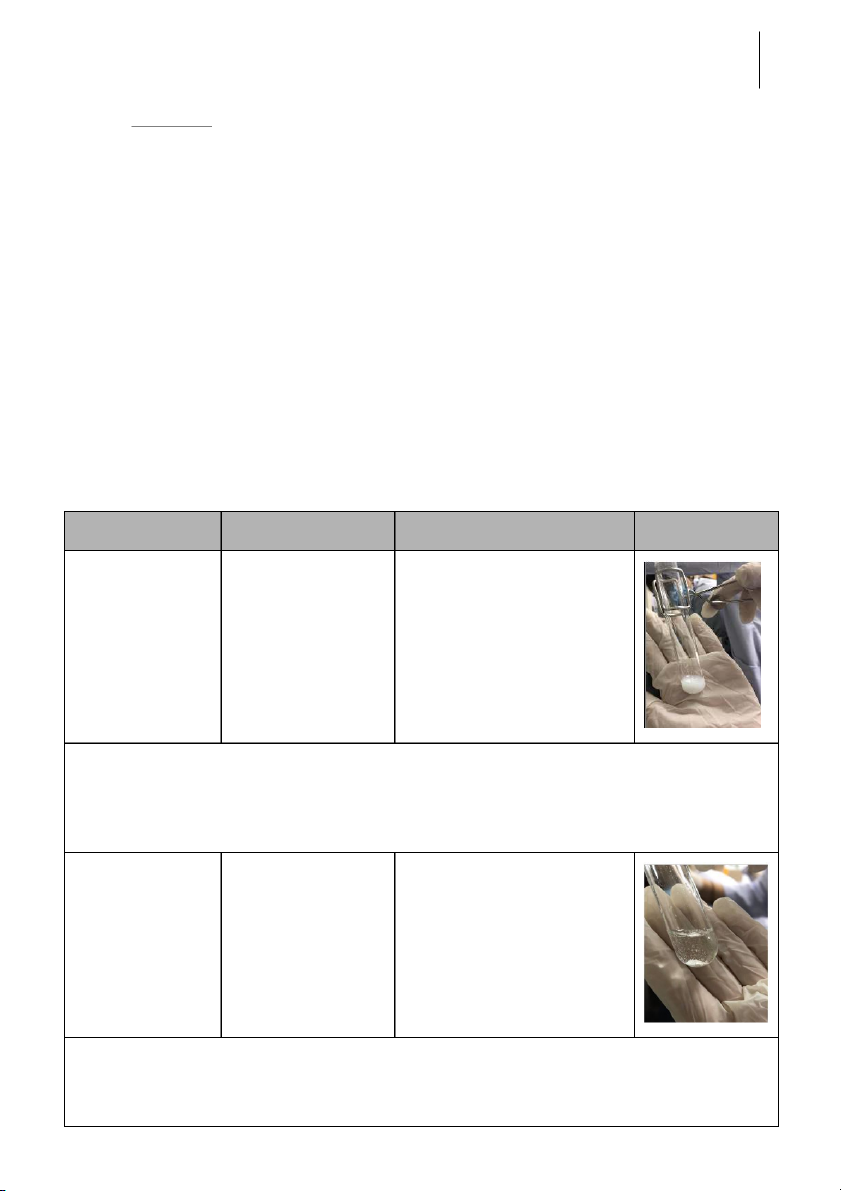
-The white precipitate AgNO + KCl → AgCl + KNO 3 3 + 0.1M AgNO was formed. 3 Explanation:
*KCl dissociate completely to form Cl-
*In the solution, ion Cl- combine with ion Ag+ to fo rm A
gCl which i s a white precipitate because they have low solubility. 0.5M KCl
-The white precipitate AgNO + KCl → AgCl + KNO 3 3 + 0.1M AgNO was formed. 3 + 2M NH OH -After adding NH OH, AgCl + 2NH OH → 4 4 4
the precipitate was [Ag(NH ) ]Cl + H O 3 2 2 dissolved slowly. Explanation:
*The precipitate AgCl reacts with Ammonium hydroxide NH OH to create the complex – compound 4
[Ag(NH ) ]Cl, which solute completely in the solution. 3 2 Semester II: 2018-2019
International University, Vietnam National University - HCMC 6 GENERAL CHEMISTRY LABORATORY 0.5M KBr -The light yellow AgNO + KBr →AgBr + KNO 3 3 + 0.1M AgNO precipitate was 3 formed. Explanation:
*KBr dissociate completely to form BRr-
*In the solution, ion Br- combine with ion Ag+
to form AgBr which is a white-yellow precipitate because they have low solubility. 0.5M KBr -The light yellow AgNO +KBr → AgBr + KNO 3 3 + 0.1M AgNO precipitate was 3 + 2M NH OH formed. AgBr + 2NH OH → 4 4
-After adding NH OH, [Ag(NH ) ]Br + H O 4 3 2 2 the precipitate was dissolved a little. Explanation:
*The precipitate AgBr reacts with NH OH to create the complex-compound [Ag(NH ) ]Br, which 4 3 2
solute completely in the solution. 0.5M KI
-The milky- white AgNO + KI → AgI + KNO 3 3 + 0.1M AgNO yellow. 3 Explanation:
*KI dissociate completely to form I-
*In the solution, ion I- combine with ion Ag+
to form AgI which is a light yellow precipitate because they have low solubility. Semester II: 2018-2019
International University, Vietnam National University - HCMC 7 GENERAL CHEMISTRY LABORATORY 0.5M KI -The milky- white
AgI+2NH OH → [Ag(NH ) ]I 4 3 2 + 0.1M AgNO3 yellow precipitate + H O 2 + 2M NH OH appeared. 4 -After adding NH OH, 4 the precipitate dissolved hardly. Explanation:
*The precipitate AgI reacts with NH OH to create a milky yellow complex – compound [Ag(NH ) ]I, 4 3 2
which solute completely in the solution. Comments:
● All those experiments prove the properties of silver halides, which is: -
Forming precipitate when reacts with salt. -
All kind of precipitate dissolved a little in NH OH liquid. 4 -
The complex compound forming and precipitation reaction are determined by this experiment. ● Comparing: -
The precipitation color of AgI, AgBr is more lightly, muddy than in practical -
The precipitation didn’t dissolve completely and fastly like in practical 3. Reactions of H O 2 2
-First, we put 5 drops of 0.1 M KMnO solution into a test tube.To continue, we added 5 drops of 4
2M H SO and then added 5 drops of 3 % H O solution into the test tube above. Finally, we 2 4 2 2
observed the change in the color and the release of gas -With a new test tube which c ontain 5 d rops of 0 .1 M K I s olution, w e c ontinued t o add 5 drops o f 2
M H SO t o acidify the KI solution a bove. Later o n, w e a dded 5 drops o f 3 % H O solution a nd had 2 4 2 2 an observation.
-In the third reaction, we added a “pinch” of solid Mn O i nto 1 mL o f 3 % H O s olution. T hen, w e 2 2 2
observed the released gas from the tube. Reaction Observation Chemical Equation Image Semester II: 2018-2019
International University, Vietnam National University - HCMC 8 GENERAL CHEMISTRY LABORATORY 0.1M KMnO
-The violet color of 2KMnO + 3H SO + 5H O → 4 4 2 4 2 2 + 2M H SO KMnO did n
ot change 8H O + 2MnSO + 5O + K SO 2 4 4 2 4 2 2 4 + H O when H SO was 2 2 2 4 added. -After adding H O , 2 2 the solution changed into transparent. The gas (as bubbles) and heat were released. Explanation:
*Due to the reducing properties of H O ; therefore, when reacting with a strong oxidizer like KMnO 2 2 4
in the acidic solution, H O is oxidized to Oxygen O which released gas as bubbles, and KMnO 2 2 2 4
loses its color. So, the solution is transparent (K SO and MnSO is non-color). 2 4 4 *In this reaction, oxygen in H O h as o xidation number -1 i s o xidized t o 0 i n O , and Mn (in K MnO ) 2 2 2 4 +7 is reduced to Mn +2. 0.1M KI
-After adding H O , 2KI + H SO + H O → K SO 2 2 2 4 2 2 2 4 + 2M H SO the transparent + 2H O + I 2 4 2 2 + H O solution quickly 2 2 changed into orange-yellow color, and a dark purple precipitate formed. Explanation:
*Because H O has oxidizing properties; therefore, when reacting with KI- a reducing agent in the 2 2
acidic solution, O-1 is reduced to O-2 in KOH, then KOH reacts with H SO to create K SO . KI is 2 4 2 4
oxidized to I , which makes the orange-yellow solution and precipitate is created. 2
*In this reaction, oxygen in H O with oxidation number -1 i s reduced t o -2 , and iodide I (in KI) -1 is 2 2 oxidized to 0 in I . 2 H O
-After adding MnO , 2H O → O + 2H O 2 2 2 2 2 2 2 + MnO the gas and heat were 2 released. The black solid did not dissolve. Semester II: 2018-2019
International University, Vietnam National University - HCMC 9 GENERAL CHEMISTRY LABORATORY Explanation: *MnO is a catalyst. 2
*H O is unstable and easy to be decomposed. When adding a “pinch” of MnO to the Hydrogen 2 2 2
peroxide (H O ) solution, H O is decomposed, with MnO as a catalyst, forming H O and releases 2 2 2 2 2 2
the gas – Oxygen (O ) (Self-redox reaction). Besides, it is also an e xothermic reaction d ue t o the heat 2 released. Comments:
● From the table above, we can see that H O has oxidizing properties also reducing properties, 2 2
and easy to be decomposed. When reacts with different substance involved, H O represents 2 2
different chemical properties of it.
● The last reaction is a self-redox reaction. This reaction also let us know that MnO is a catalyst. 2
● All reactions help us understand clearly about chemical properties of H O , the self-redox 2 2
reaction and learn more new chemical equations. 4. Reactions of Nitrate -At first, we added 1 mL o f saturated Fe SO into o ne t est t ube a nd 1 mL of 1 M NaNO s olution w as 4 3 added into another.
-Next, we poured concentrated sulfuric acid (96%) s lowly a nd c arefully d own the inside wall of t he
test tube. After a few seconds, we recorded the change of color at the interface b etween the nitrate
solution and the concentrated sulfuric acid.
-In the second stage, we replaced FeSO by NaNO . 4 3 Reaction Observation Chemical Equation Image 1M NaNO -The heat and reddish 3 2NaNO + 6FeSO + 4 + FeSO brown gas were 3 4 4 H SO → Na SO + + concentrated H SO released. 2 4 2 4 2 4 -The
transparent 2NO + 3Fe (SO ) + 2 4 3 solution changed to 4H O 2 brownish yellow. 2NO + O → 2NO 2 2
Explanation: *This is a redox reaction:
*Iron (II) sulfate (FeSO ), Sulfuric acid (H SO ) and Sodium nitrate (NaNO ) are dissociated 4 2 4 3 completely to ions:
FeSO → Fe2+ + SO 2- 4 4
H SO → 2H+ + SO 2- 2 4 4 Semester II: 2018-2019
International University, Vietnam National University - HCMC 10 GENERAL CHEMISTRY LABORATORY
NaNO → Na+ + NO - 3 3
*In the solution, with the presence o f H+, Fe 2+ is o
xidized t o Fe3+, and N w ith t he o xidation n umber + 5 in ion NO - +2
, is reduced to N in NO and NO is gas, so NO is released. 3
*The non-color gas released is NO, NO is oxidized by O , forming NO , which is reddish brown. 2 2
*After the reaction, Fe (SO ) is created and its color is b rownish y ellow w hile N a SO is t ransparent, 2 4 3 2 4
so the color of the solution after reacting depends on the color of Fe (SO ) . 2 4 3 1M NaNO -The liquid was * 2NaNO + 2FeSO + 2 2 4 + FeSO brown-black
when 3H SO → 2NaHSO 4 2 4 4 + concentrated H SO FeSO was added. + Fe (SO ) + 2NO + 2 4 4 2 4 3 -After adding 96% 2H O 2 H SO , the heat and 2 * 2NO + O → 2NO 4 2 2 reddish brown gas were released, and the color of liquid changed into brownish yellow.
Explanation: *This is also a redox reaction
*Iron (II) sulfate (FeSO ), Sulfuric acid (H SO ) and Sodium nitrite (NaNO ) are dissociated 4 2 4 2 completely to ions:
FeSO → Fe2+ + SO 2- 4 4
H SO → 2H+ + SO 2- 2 4 4
NaNO → Na+ + NO - 2 2
*In the solution, Fe2+ is oxidized to Fe3+, and N in NaNO has the oxidation number + 3, it is reduced 2
to +2 in NO (gas), then the gas is released.
*The non-color gas released is NO, NO is oxidized by O , forming NO , which is reddish brown. 2 2
*After the reaction, Fe (SO ) i s c reated a nd its color i s b rownish y ellow w hile N aHSO is t ransparent, 2 4 3 4
so the color of the solution after reacting depends on the color of Fe (SO ) . 2 4 3 1M NaNO
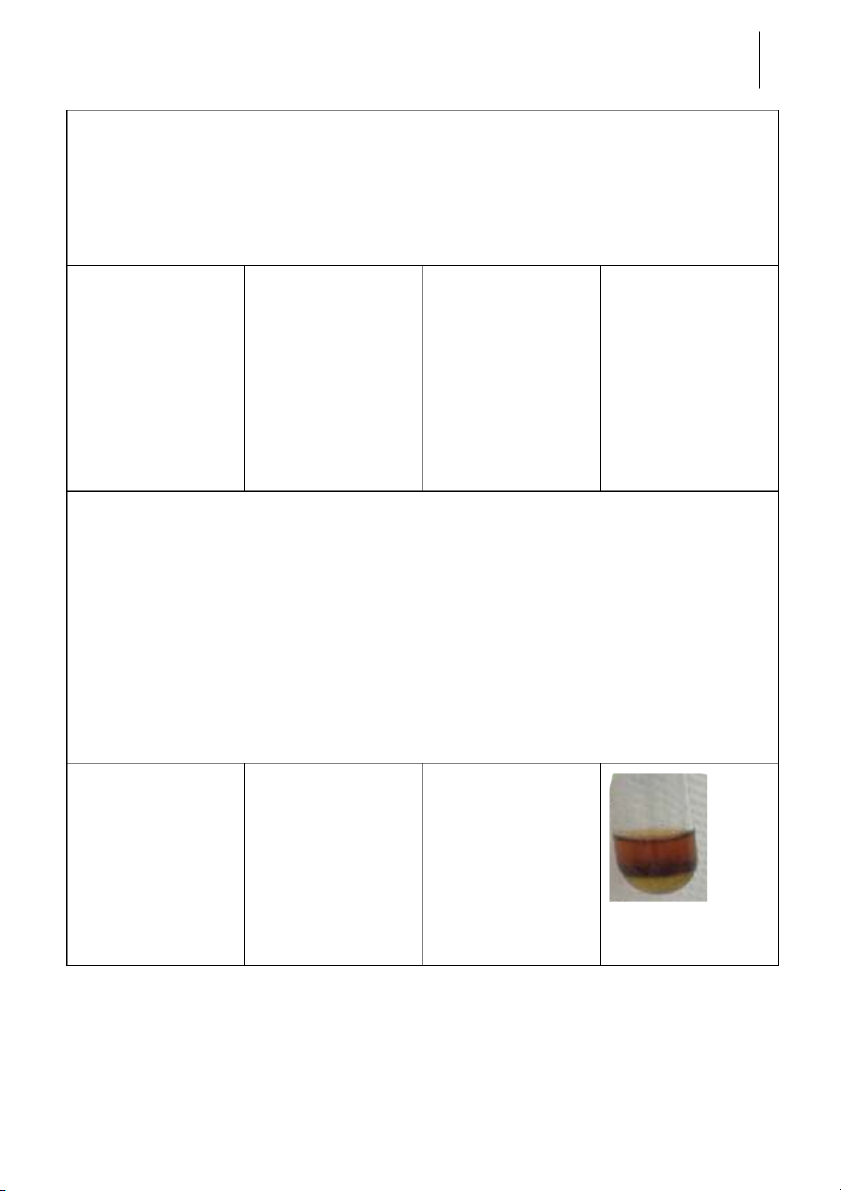
-The solution was split * NaNO + FeSO + 3 3 4 + FeSO
into two layers. The CH COOH → NaNO 4 3 2 +concentrated
upper was blood red, + 2(CH COO) Fe + 3 3 CH COOH
and the lower was 2SO + 3H O 3 3 2 transparent (maybe a * (CH COO) Fe + 3 3
little yellow). In the 3H O → Fe(OH) + 2 3 middle, some 3CH COOH 3 precipitate was created. Semester II: 2018-2019
International University, Vietnam National University - HCMC 11 GENERAL CHEMISTRY LABORATORY
Explanation: *This is another redox reaction.
*Iron (II) sulfate FeSO , Acetic acid CH COOH 4 3
and Sodium nitrite NaNO are dissociated completely to ions: 2
FeSO → Fe2+ + SO 2- 4 4
CH COOH → CH COO- + H+ 3 3
NaNO → Na+ + NO - 3 3
*In the solution, Fe2+ is oxidized to Fe3+, and N in NaNO has the oxidation number + 5, it is reduced 3 to +3 in NaNO . 2
*The color of the upper layer is from (CH COO) Fe. The lower one may be from transparent 3 3
solutions: CH COOH, NaNO … influenced the color of the upper layer, so it has a light color. 3 2
*The precipitate is formed because (CH COO) Fe is not stable, it decomposes in solution to Fe(OH) 3 3 3
(the reddish brown precipitate ). Comments:
● From the table above, we can see when NaNO /NaNO reacts with FeSO and concentrated 3 2 4
H SO , the observation is similar. The reason is that concentrated H SO is a strong oxidizing 2 4 2 4
agent, so it can oxidize ions to the highest oxidation number. Furthermore, the substances in
these reactions do not change concentration and necessary volume to use, thus some products are the same.
● The last reaction is redox. It shows us that (CH COO) Fe is not stable. 3 3
● All reactions help us understand clearly about redox reaction and learn more new chemical equations. 5. Reactions of KMnO4
-Prepared 3 clean test tubes and labeled # 1-3.
-In the test tube # 1: we had a combination of 10 drops of 0.5 M Na2SO3and 5 drops of 2 M H2SO4.
-In the test tube # 2: we had a combination of 10 drops of 0.5 M Na 2SO3and 5 drops of 6N NaOH.
-In the test tube # 3: we had a combination of 10 drops of 0.5 M Na2SO3and 5 drops distilled water.
-In the final step, we added 5 drops of 0.1M KMnO4 to each of the test tubes. Reaction Observation Chemical Equation Image Semester II: 2018-2019
International University, Vietnam National University - HCMC 12 GENERAL CHEMISTRY LABORATORY 0.5M Na SO -The color of KMnO
2KMnO + 3H SO + 5Na SO → 2MnSO + 2 3 4 4 2 4 2 3 4 + 2M H SO disappeared.
K SO + 3H O + 5Na SO 2 4 2 4 2 2 4 + 0.1M KMnO 4 Explanation:
* In Na SO , S has the oxidation number +4, is oxidized by the strong oxidizer Potassium 2 3
permanganate KMnO in which Mn has the oxidation number +7, to form S with the oxidation number +6 4
and Mn with the oxidation number +2 in MnSO in the acidic environment (H SO ). So the color of turns 4 2 4 into non_color 0.5M Na SO
-The solution turns 2KMnO + 2NaOH + Na SO → K MnO + 2 3 4 2 3 2 4 + 6N NaOH
into a green-brown Na MnO + Na SO + H O 2 4 2 4 2 (l) + 0.1M KMnO solution and after a 4 few seconds, it turns into a brown solution. Explanation:
* In Na SO , S has the oxidation number +4, is oxidized by the strong oxidizer Potassium 2 3
permanganate KMnO in which Mn has the oxidation number +7, to form S with the oxidation number + 6 4
and Mn with the oxidation number +6 in the basic environment. Mn +6 is in the form of Potassium
manganate K MnO and Sodium manganate Na MnO , which make the solution has the green-brown color. 2 4 2 4
After a few seconds, adding more K MnO create MnO – a brown solution. 2 4 4 0.5M Na SO -The dark
brown 2KMnO + 3Na SO + H O → 2MnO + 2 3 4 2 3 2 2 + H O precipitate appears, 3Na SO + 2KOH 2 2 4 + 0.1M KMnO and the non-color 4 solution occurs when Potassium permanganate (KMnO ) loses its 4 color. Explanation:
*In Na SO , S has the oxidation number +4, is oxidized by the strong oxidizer Potassium 2 3
permanganate (KMnO ) in which Mn has the oxidation number +7, t o fo rm S with t he o xidation number + 6 4
and Mn with the oxidation number +4 in the neutral environment.
*Mn +6 is in the form of Manganese dioxide MnO which is a dark brown precipitate. 2 Comments: Semester II: 2018-2019
International University, Vietnam National University - HCMC 13 GENERAL CHEMISTRY LABORATORY
● All three equations are redox reaction, which changes the oxidation number of 1 or more elements.
● In the second experiment, in fact when adding more K MnO that create MnO – brown 2 4 4
precipitate, but we did not add enough K MnO so the solution became the brown solution 2 4
instead of the brown precipitate.
6. Reactions of Potassium Dichromate (K Cr O ) 2 2 7
-First, we added 10 drops of 0.5M K Cr O into a test tube. 2 2 7
-Second, we added 10 drops of 6M H SO and 5 drops of C H OH was added at the end. 2 4 2 5 Reaction Observation Chemical Equation Image 2M K Cr O
-When we put H SO 2K Cr O + 3C H OH + 8H SO 2 2 7 2 4 2 2 7 2 5 2 4 + 6M H SO
into K Cr O , the → 3CH COOH + 2Cr (SO ) + 2 4 2 2 7 3 2 4 3 + C H OH
color of the solution 2K SO + 11H O 2 5 2 4 2 is orange. -After we added C H OH to the test 2 5 tube, the color of the solution is dark green.
Comments: In C H OH, C has the oxidation number -1, is oxidized by the strong oxidizer 2 5
Potassium dichromate K Cr O – in which Cr has the oxidation number +6, to form C with the 2 2 7
oxidation number +1 and Cr with the oxidation number +3. The dark green color of the solution
comes from the color of the salt Cr3+ Cr (SO ) . 2 4 3 7. A. Reactions of Fe3+
-In the first step, we added 1 mL of 0.5M FeCl solution in each of the four test tubes. 3 Semester II: 2018-2019
International University, Vietnam National University - HCMC 14 GENERAL CHEMISTRY LABORATORY
-Later 5 drops of the following four reagents: 0.1M KSCN; 2M KOH;
0.5M K [Fe(CN) ]; 2M NH OH was added to four test tubes which contained FeCl solution in turn. 4 6 4 3
-At the end of the reaction, we had observations Reaction Observation Chemical Equation Image 0.5M FeCl -The solution c
hanged FeCl + 3KSCN→ K [Fe(SCN) ] + 3 3 3 6 + 0.1M KSCN the color into blood 3KCl color (red-brown color). Explanation:
FeCl reacts with potassium thiocyanate (KSCN) to form the complex compound K [Fe(SCN) ], 3 3 6
which is a red-brown solution. 0.5M FeCl -A brownish
red FeCl + 3KOH → Fe(OH) + 3KCl 3 3 3 + 2M KOH precipitate was formed. Explanation:
*FeCl and KOH dissociate in the solution to create ions Fe3+and OH-, then these two ions combine 3
together to form brownish-red precipitate Fe(OH) . 3
FeCl → Fe3+ + 3Cl- 3 KOH → K+ + OH- ⇒ F
e3+ + 3OH-→ Fe(OH) ↓ 3 0.5M FeCl -Forming a dark blue 2FeCl + 3K [Fe(CN) ] → 3 3 4 6
+ 0.5M K [Fe(CN) ] precipitate. Fe [Fe(CN) ] + 12KCl 4 6 4 6 3 Explanation:
FeCl reacts with potassium ferrocyanide (K [Fe(CN) ]) to form a complex compound Iron(III) 3 4 6
ferrocyanide (Fe [Fe(CN) ] ), which is dark blue precipitate. 4 6 3 Semester II: 2018-2019
International University, Vietnam National University - HCMC 15 GENERAL CHEMISTRY LABORATORY 0.5M FeCl -The orange
FeCl + 3NH OH → Fe(OH) + 3 3 4 3 + 2M NH OH precipitate appeared. 3NH Cl 4 4 Explanation:
*FeCl and NH OH dissociate i n the solution t o create ions Fe3+ and OH-, then these t wo i ons combine 3 4
together to form a light brownish red precipitate.
FeCl → Fe3+ + 3Cl- 3
NH OH → NH + + OH - 4 4 ⇒ F
e3+ + 3OH- → Fe(OH) ↓ 3 Comments:
● In fact, Fe(OH) has a brownish red color. But experiment 5 has different color precipitate. In 3
experiment 5, it creates light brownish red color because of NH OH 4 is a weak base.
● Furthermore, we can learn mo
re about the new chemical equations. 7. B. Reactions of Fe2+
-In the second stage, we did the same as what we had done before but FeSO was used instead of 4 FeCl . 3 Reaction Observation Chemical Equation Image 0.5M FeSO
-The solution changed FeSO + 2KSCN → K [Fe(SCN) ] 4 4 4 6 + 0.1M KSCN color into red-brown. + K SO 2 4 Explanation:
FeSO reacts with potassium thiocyanate K SCN t o fo
rm the complex compound K [Fe(SCN) ], w hich 4 4 6 is a red brown solution. 0.5M FeSO -A dark green
FeSO + 2KOH → Fe(OH) + 4 4 2 + 2M KOH precipitate appeared. K SO 2 4 Semester II: 2018-2019




