Preview text:
lOMoARcPSD|364 906 32
International University, VNU HCMC GENERAL CHEMISTRY LABORATORY 1
Instructor: MSc. Le Tran Hong Ngoc DATA SHEET
EXPERIMENT 2: pH AND BUFFERS
Group: __________ Section: 2_pH and Buffers
Date: ___________________ Group members:
___________________________ ID: _____________________
___________________________ ID: _____________________
___________________________ ID: _____________________
___________________________ ID: _____________________
Instructor’s/TA’s signature and comments: Experiment 1: Experiment 4: Experiment 2: Experiment 5: Experiment 3: Approval
Broken glassware (If any): Equipment Quantity
1. ________________________________________________ _________________
2. ________________________________________________ _________________
3. ________________________________________________ _________________
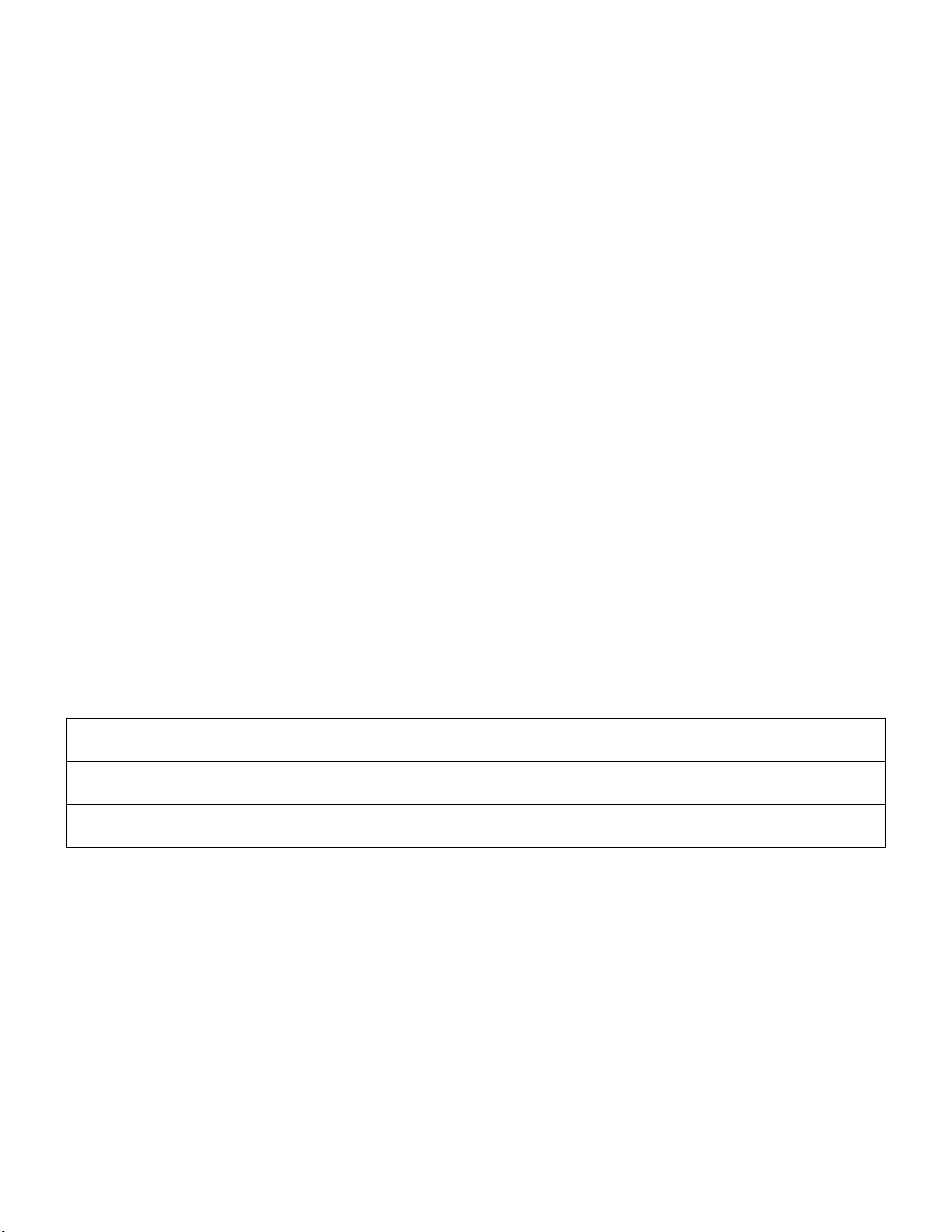
1. pH of Deionized Water lOMoARcPSD|364 906 32
International University, VNU HCMC GENERAL CHEMISTRY LABORATORY 2
Instructor: MSc. Le Tran Hong Ngoc Observed pH Time 1st
2nd (other group in the class) (second) (Your group) (Group ___ ) 0 20 40 60 80 100 120 140 160 … 2. pH of Strong Acid Measure pH Theoretical Solution 1st 2nd (Group pH (Your group) ___ ) 10 mL of 0.1M HCl + 90 mL of distilled water + 90 mL of 0.1M NaOH + 90 mL of 0.01M NaOH lOMoARcPSD|364 906 32
International University, VNU HCMC GENERAL CHEMISTRY LABORATORY 3
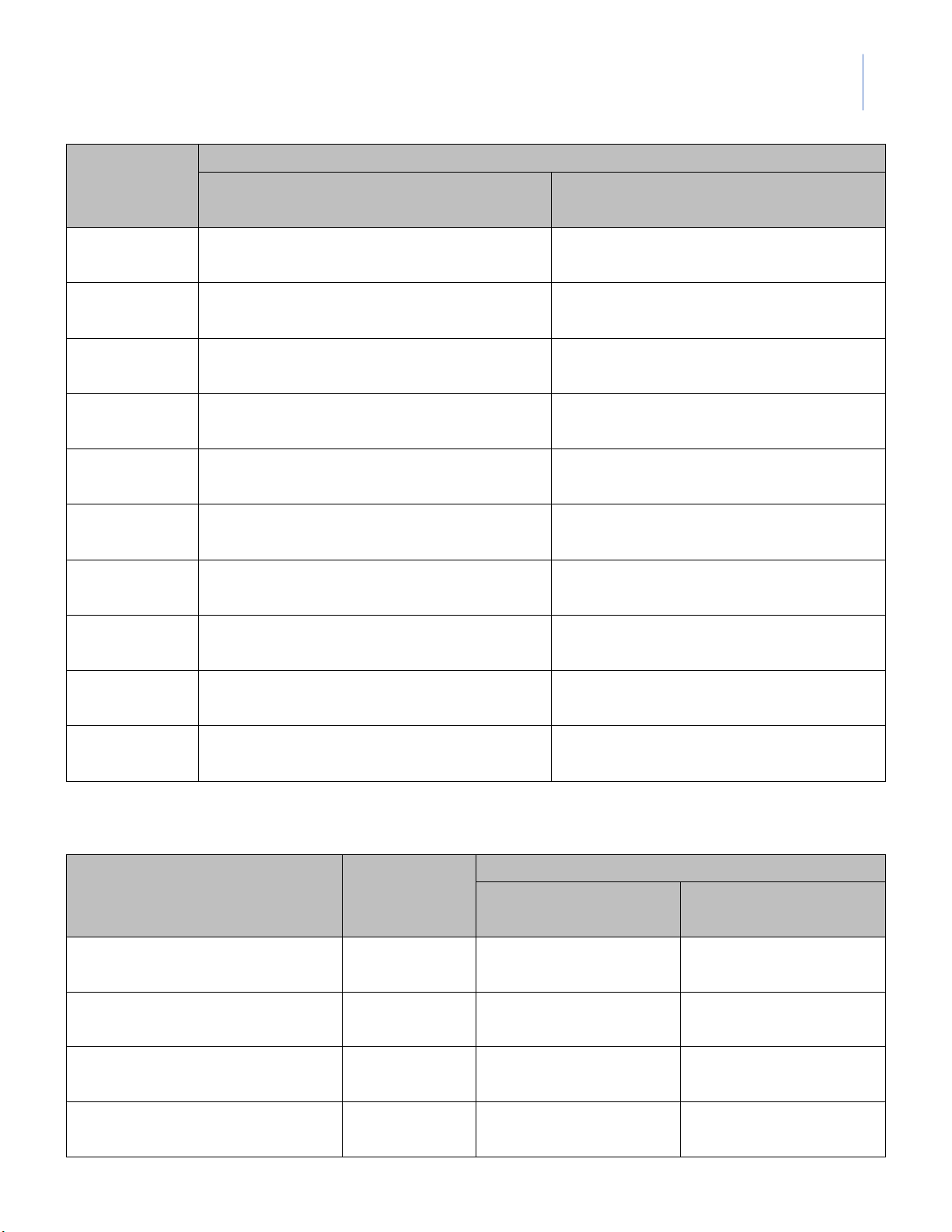
Instructor: MSc. Le Tran Hong Ngoc 3. pH of Weak Acid Measure pH Solution 1st 2nd (Group Average Ka (Your group) ___ ) 0.1M acetic acid 0.01M acetic acid 0.001M acetic acid 4. pH of Salts Measure pH Predicted Solution 1st 2nd (Group pH (Your group) ___ ) 0.1M NaCl 0.1M CH3COONa 0.1M NH4Cl 5. pH of Buffers Volume Volume Measured pH (mL) (mL) Calculated Buffer [Acid] [Base] 1st 2nd 0.1M 0,1M pH
(Your group) (Group___) CH3COOH CH3COONa A 10 40 B 40 10 lOMoARcPSD|364 906 32
International University, VNU HCMC GENERAL CHEMISTRY LABORATORY 4
Instructor: MSc. Le Tran Hong Ngoc
¥ Part I: Addition of 10 drops of 0.1M HCl Total volume of HCl pH from the start
pH after adding 10 drops Buffer
(drops) to change by one (pH0) of 0.1M HCl unit (pH0-1) A1 B1
¥ Part II: Addition of 10 drops of 0.1M NaOH Total volume of HCl pH from the start
pH after adding 10 drops Buffer
(drops) to change by one (pH0) of 0.1M NaOH unit (pH0+1) A2 B2 --- DATA SHEET
EXPERIMENT 3: REDOX TITRATION
Group: __________ Section: 3_Redox Titration
Date: ___________________ Group members:
___________________________ ID: _____________________
___________________________ ID: _____________________
___________________________ ID: _____________________
___________________________ ID: _____________________
Instructor’s/TA’s signature and comments: lOMoARcPSD|364 906 32
International University, VNU HCMC GENERAL CHEMISTRY LABORATORY 5
Instructor: MSc. Le Tran Hong Ngoc Experiment 1: Experiment 3: Experiment 2: Experiment 4: Approval:
Broken glassware (If any): Equipment Quantity
4. ________________________________________________ _________________
5. ________________________________________________ _________________
6. ________________________________________________ _________________
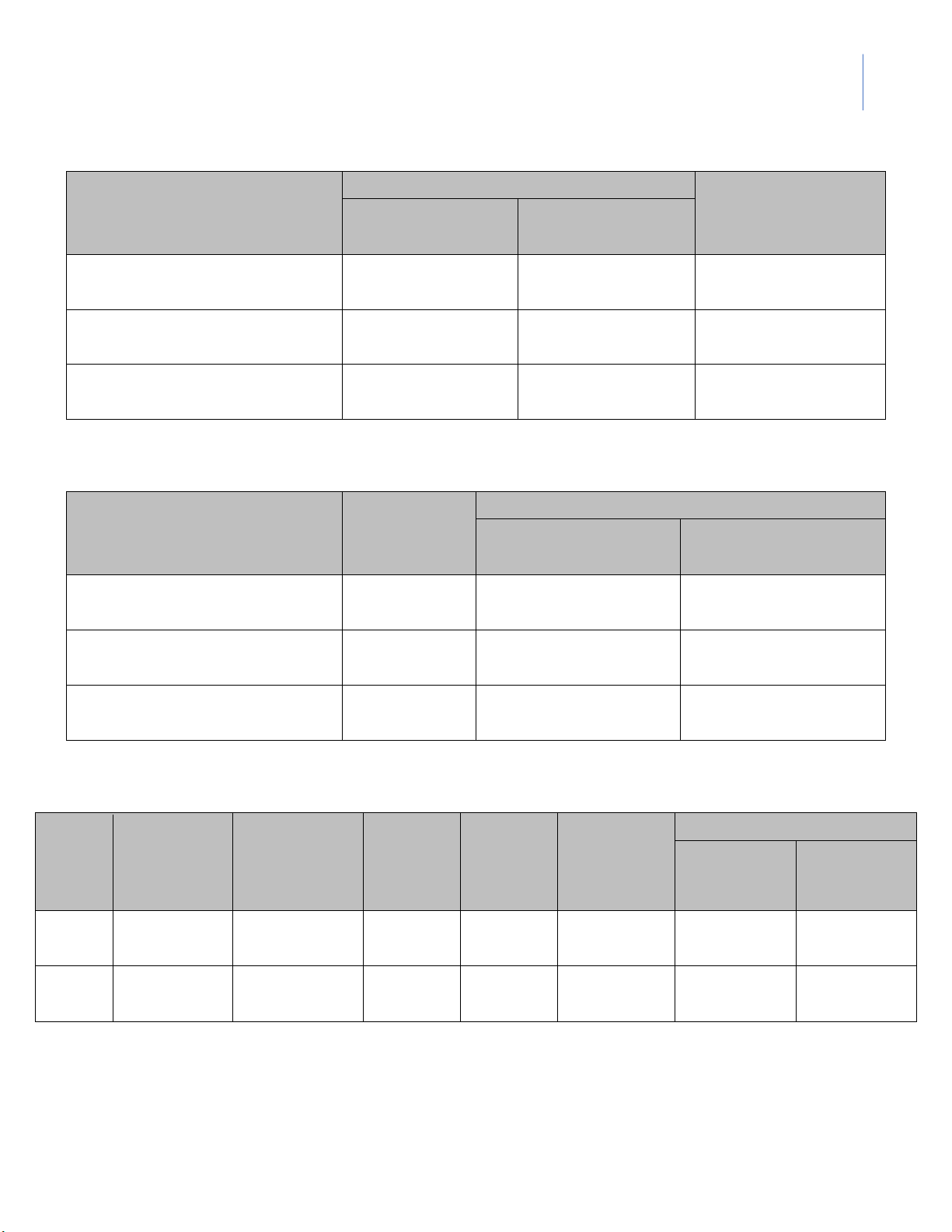
1. Titration of KMnO4 solution with standard H2C2O4 solution Burette reading Volume of KMnO4 Normality of KMnO4 Trial (mL) (mL) (N) #1 _______ - _______ #2 _______ - _______
Average Normality of KMnO4 = _________________________ (N)
2. Titration of unknown concentration H2C2O4 solution with standard KMnO4 solution Burette reading Volume of KMnO Normality of H Trial 4 2C2O4 (mL) (mL) (N) #1 _______ - _______ #2 _______ - _______
Average Normality of H2C2O4 = _________________________ (N) lOMoARcPSD|364 906 32
International University, VNU HCMC GENERAL CHEMISTRY LABORATORY 6
Instructor: MSc. Le Tran Hong Ngoc
3. Titration of unknown concentration of FeSO4 solution with standard KMnO4 solution Burette reading Volume of KMnO4 Normality of FeSO4 Trial (mL) (mL) (N) #1 _______ - _______ #2 _______ - _______
Average Normality of FeSO4 = _________________________ (N) --- DATA SHEET
EXPERIMENT 4: CHEMICAL EQUILIBRIUM
Group: __________ Section: 4_Chemical Equilibrium Date: ___________________ Group members:
___________________________ ID: _____________________
___________________________ ID: _____________________
___________________________ ID: _____________________
___________________________ ID: _____________________
Instructor’s/TA’s signature and comments: Experiment 1: Experiment 4: Experiment 2: Experiment 5: lOMoARcPSD|364 906 32
International University, VNU HCMC GENERAL CHEMISTRY LABORATORY 7
Instructor: MSc. Le Tran Hong Ngoc Experiment 3: Approval
Broken glassware (If any): Equipment Quantity
1. ________________________________________________ _________________
2. ________________________________________________ _________________
3. ________________________________________________ _________________
1. Acid/Base Equilibria
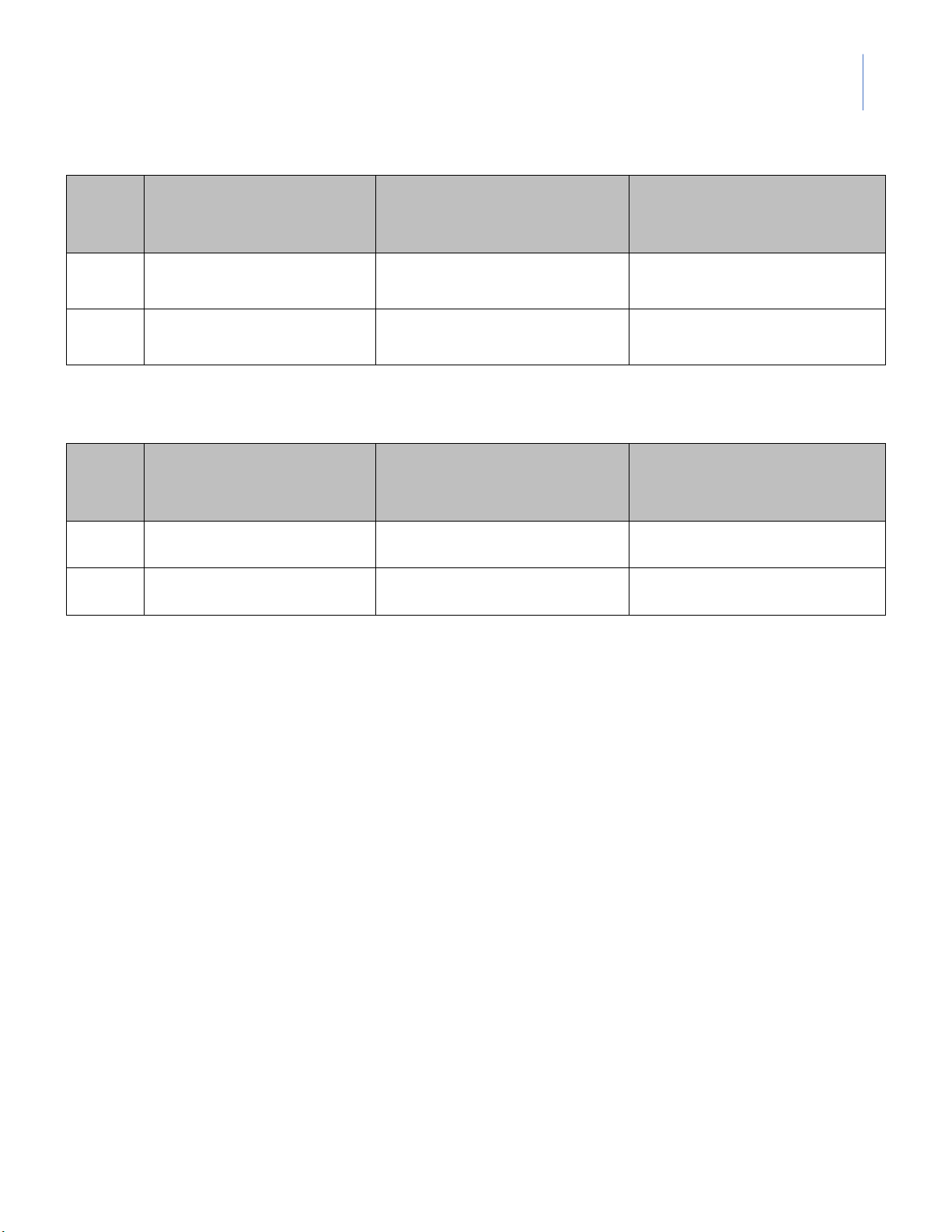
Equilibrium System: 2CrO 2- 4 (aq) + 2H+(aq) ⇌ Cr2O72-(aq) + H2O(l) Description of Predicted outcome Observation conditions Initial solution + Conc. HCl + 6N NaOH
2. Equilibria of Acid/Base Indicators
Equilibrium System: H(MV)(aq) + H2O(l) ⇌ H3O+(aq) + MV-(aq) Description of Predicted outcome Observation conditions None (Control) + 6M HCl lOMoARcPSD|364 906 32
International University, VNU HCMC GENERAL CHEMISTRY LABORATORY 8
Instructor: MSc. Le Tran Hong Ngoc + 6M NaOH + 6M HCl ---
3. Equilibria of Precipitation Reactions
Equilibrium System: Ca2+ 2- (aq) + C2O4 (aq) ⇌ CaC2O4(s) Description of Predicted outcome Observation conditions Test tube #1 + 0.1M Na2C2O4 Test tube #2 + 0.1M H2C2O4 + 6M HCl + 6M NH4OH
4. Temperature Effects on Equilibria
Equilibrium System: [Co(H2O)6]2+(aq) + 4Cl-(aq) ⇌ (CoCl4)2-(aq) + 6H2O(l) Description of Predicted outcome Observation conditions Test tube #1 lOMoARcPSD|364 906 32
International University, VNU HCMC GENERAL CHEMISTRY LABORATORY 9
Instructor: MSc. Le Tran Hong Ngoc Room temperature Test tube #2 Hot water bath ¯ Ice water bath Test tube #3 Ice water bath ¯ Hot water bath --- DATA SHEET
EXPERIMENT 5: FACTORS AFFECTING REACTION RATE
Group: __________ Section: 5_Reaction Rate
Date: ___________________ Group members:
___________________________ ID: _____________________
___________________________ ID: _____________________
___________________________ ID: _____________________
___________________________ ID: _____________________
Instructor’s/TA’s signature and comments: lOMoARcPSD|364 906 32
International University, VNU HCMC GENERAL CHEMISTRY LABORATORY 10
Instructor: MSc. Le Tran Hong Ngoc Experiment 1: Experiment 3: Experiment 2: Approval
Broken glassware (If any): Equipment Quantity
1. ________________________________________________ _________________
2. ________________________________________________ _________________
3. ________________________________________________ _________________ 1.
Effect of Concentration on The Reaction Time
Reaction #1: _______________________________________________________________
Reaction #2: _______________________________________________________________
Calculate the initial concentration of I- and S 2- 2O8 ions: Mixture #5: [I-] = [S2O82-]= Iodide ion Peroxydisulfate Mixture Time in seconds (mol/L) (mol/L) 1 2 3 4 5 lOMoARcPSD|364 906 32
International University, VNU HCMC GENERAL CHEMISTRY LABORATORY 11
Instructor: MSc. Le Tran Hong Ngoc 6 7 8 9 10 11 2.
Effect of Temperature on The Reaction Rate Equilibrium System: 5H ®
2C2O4(aq) + 2KMnO4(aq) + 3H2SO4 (aq)
2MnSO4(aq) + K2SO4(aq) + 10CO2(g) + 8H2O(l) Description of Reaction time Predicted outcome Observation conditions (second) Test tube #1A - B Room temperature Test tube #2A - B 50oC Test tube #3A - B 90oC 3.
Effect of a Catalyst on The Reaction Rate
Equilibrium System: 2H2O2(aq) ® 2H2O(l) + O2(g) Description of Observation Trial Predicted outcome conditions (Reaction rate) lOMoARcPSD|364 906 32
International University, VNU HCMC GENERAL CHEMISTRY LABORATORY 12
Instructor: MSc. Le Tran Hong Ngoc 1 + MnCl2 2 + MnO2 3 + NaCl 4 + CaCl2 5 + Zn 6 + KNO3 7 + Fe(NO3)3 ---
Document Outline
- DATA SHEET
- EXPERIMENT 2: pH AND BUFFERS
- DATA SHEET (1)
- EXPERIMENT 3: REDOX TITRATION
- DATA SHEET (2)
- EXPERIMENT 4: CHEMICAL EQUILIBRIUM
- DATA SHEET (3)
- EXPERIMENT 5: FACTORS AFFECTING REACTION RATE




