

Preview text:
Experiment 4: Chemical Equilibrium Mai Quy Khang BTFTIU22049 1. Purposes
General purpose: To observe the effect of applying stresses on chemical systems at
equilibrium and apply Le Chatelier’s Principle to explain the changes in the system.
Applications: The main application of Chemical Equilibria in industrial process is to
maximise the desired product concentration by minimising the leftover reactants. 2. Methods 2.1 Acid/Base equilibria Equilibrium system: 2CrO 2- + 2-
4 (aq) + 2H (aq) ⇌ Cr2O7 (aq) + H2O(l)
Test tube #1, #2, #3 contain 10 drops 0.5M K2CrO4. Observe the color of test tube #1.
Test tube #2: add 5 drops concentrated HCl observe the color and compare with test tube #1.
Test tube #3: add 5 drops concentrated HCl
observe the color and compare with test tube #1 add 10 drops 6M NaOH
observe the color and compare with test tube #2.
2.2 Equilibria of acid/base indicators Equilibrium system: H(MV) -
(aq) + H2O(l) ⇌ H3O+(aq) + MV (aq)
Test tube contain 2 drops methyl violet and 20mL distilled water. Divide equally into 2 test
tubes. Test tube #1: observe the color. Test tube #2: add wisely drop 6M HCl until no significant change
observe the color change add wisely drop 6M NaOH until no significant change
observe the color change add wisely drop 6M HCl until no significant change observe the color change.
2.3 Equilibria of precipitation reactions Equilibrium system: Ca2+ 2-
(aq) + Cr2O4 (aq) ⇌ CaC2O4(s)
Test tube #1 and #2 contain 5mL 0.1M CaCl .
2 Test tube #1: add 1mL 0.1M Na2C2O4
observe the color. Test tube #2: add 1mL 0.1M H2C2O4
observe the color compare with #1 add 10 drops 6M HCl
observe the color change add 10 drops 6M NH OH 4 observe the color change.
2.4 Temperature effects on equilibria Equilibrium system: [Co(H -
2O)6]2+(aq) + 4Cl (aq) ⇌ (CoCl4)2-(aq) + 6H2O(l)
Test tube contain 3mL 0.1 CoCl2, add conc. HCl drop-by-drop until solution turns to
purple-violet. (Do again if deep blue appears). #1: At room temperature, observe the color. #2: At warm temperature (hot water)
observe the color compare with #1 at cool temperature (ice bath) o bserve the color c
ompare with #1. #3: At cool temperature (ice bath) observe the color c ompare with #1 a
t warm temperature (hot water) o bserve the color c ompare with #1. 3. Safety precautions
Natri hydroxide (NaOH) is a dangerous chemical, it will corrode and cause skin blisters. It might
causes allergies, burns or maybe blindness. Causes allergies, burns or scars when exposed to skin.
Acid hydrochloric (HCl) is also highly corrosive when in contact with the body. Breathing in
fumes causes coughing, choking, and inflammation of the nose, throat, and upper respiratory system.
Calcium chloride (CaCl2) poses some serious health and safety hazards. If ingested, calcium
chloride can lead to burns in the mouth and throat, excess thirst, vomiting, stomach pain, low
blood pressure, and other possible severe health effects. It can also irritate skin by causing
excessive dryness or desiccating moist skin.
Therefore, when using natri hydroxide (NaOH), acid hydrochloric (HCl) or Calcium chloride
(CaCl2), we need to strictly follow the regulations on dosage and process. Prepare protective
equipment and tools for contact. 4. Suggested questions
4.1 What are the ojectives of today’s lab work?
To observe the effect of applying stresses on chemical systems at equilibrium and apply
Le Chatelier’s Principle to explain the changes in the system.
4.2 What is chemical equilibrium in a reversible chemical reaction? And when the equilibrium
state of a chemical reaction can be obtained?
For a reversible chemical reaction, an equilibrium state is attained when the rate at which
a chemical reaction is proceeding in forward direction equals the rate at which the reverse
reaction is proceeding. When the rates of the forward and reverse reactions have become equal to
one another, the reaction has achieved a state of balance.
4.3 Please define dynamic equilibrium and static equilibrium
Dynamic equilibrium exists once a reversible reaction occurs. Substances transition
between the reactants and products at equal rates, meaning there is no net change. Reactants and
products are formed at such a rate that the concentration of neither changes. It is a particular
example of a system in a steady state.
Static equilibrium refers to a condition where the reaction occurring in a system is
completely halted, and there exists no movement between the reactants and the products
corresponding to the chemical reaction.
4.4 Please describe factors that can disturb a reversible reaction at its equilibrium state?
Equilibriums are affected by changes in concentration, total pressure or volume, and temperature.
4.5 What is Le Chatelier's Principle about?
Le Chatelier's principle states that if a dynamic equilibrium is disturbed by changing the
conditions, the position of equilibrium shifts to counteract the change to establish an equilibrium.
4.6 Please write the Equilibrium equation
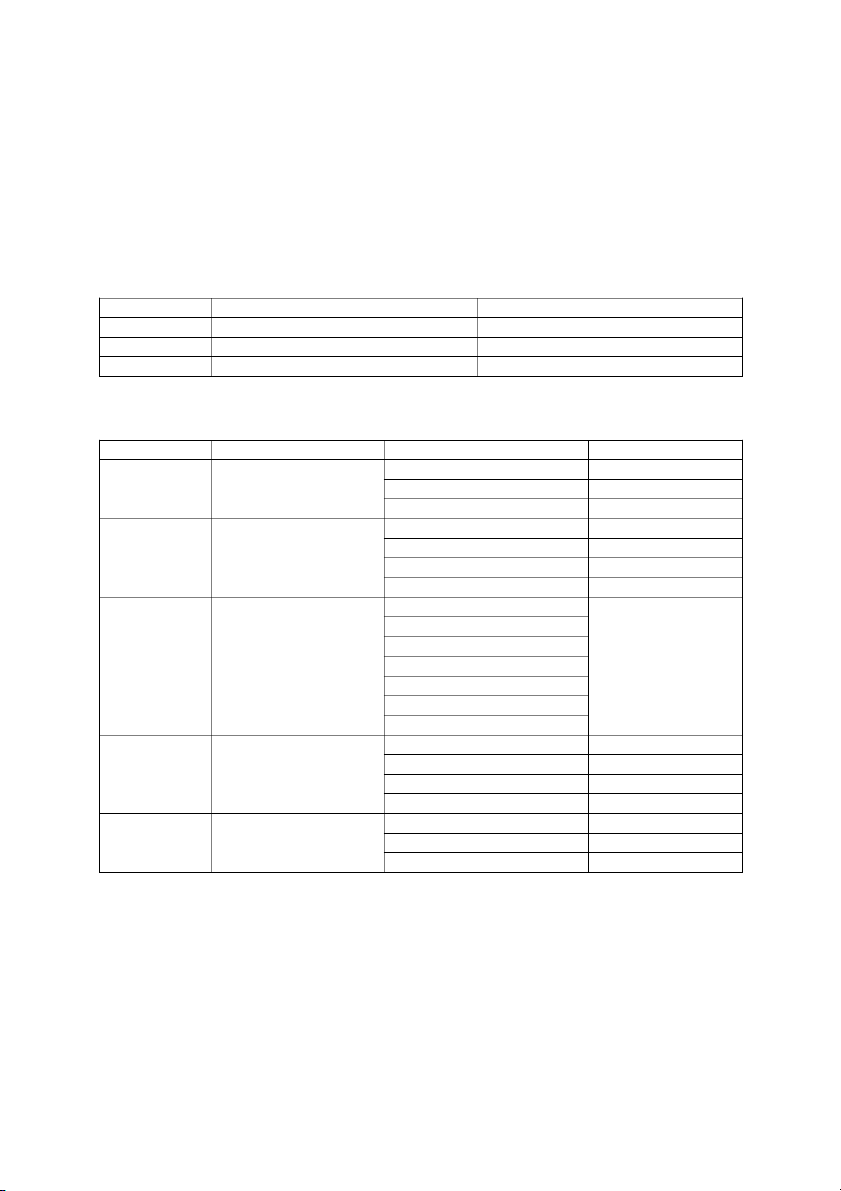
4.7 Please fill out the following table K Value
Reaction favor (reactants/products)
Reaction lies to (left/center/right) K << 1 Reactants Right K ~ 1 None Center K >> 1 Products Left
4.8 Please predict the outcome of today lab work and fill out the following table System No. System name Description of conditions Predicted outcome Initial solution Yellow 1 Acid/Base equilibria + Conc. HCl Orange + 6N NaOH Light yellow None (control) Purple Equilibria of acid/base 6M HCl Yellow-green 2 indicators 6M NaOH Blue 6M HCl Violet None (control) 0.01M FeCl3 0.01M KSCN 3 Complex ion formation 6M NaOH Cold Hot 0.1M AgNO3 Test tube 1: 0.1M Na2C2O4 White Equilibria of Test tube 2: + 0.1M H 4 2C2O4 White recipitation reactions Test tube 2: + 6M HCl Milky white Test tube 2: + 6M NH4OH Foggy white Nothing change (control) Blue Temperature effects on 5 Hot water bath Blue equilibria Ice water bath Pink




