



Preview text:
Experiment 5: Factors affecting reaction rate Mai Quý Khang BTFTIU22049 1. Purpose
General purpose: to examine the effects of concentration, temperature, and catalysts on reactions rates.
Applications: The rate of a reaction is a powerful diagnostic tool. By finding out how fast
products are made and what causes reactions to slow down we can develop methods to improve
production. This information is essential for the large-scale manufacture of many chemicals
including fertilisers, drugs and household cleaning items. 2. Procedure
2.1 Effect of concentration on reaction time 2.1.1 Preparation
Take ~90mL 0.1M (NH4)2S2O8 into beaker #1 by cylinder. Take ~60mL 0.005M Na2S2O 3 into
beaker #2 by cylinder. Take ~90mL 0.2M KI into beaker #3 by cylinder. 2.1.2 Procedure
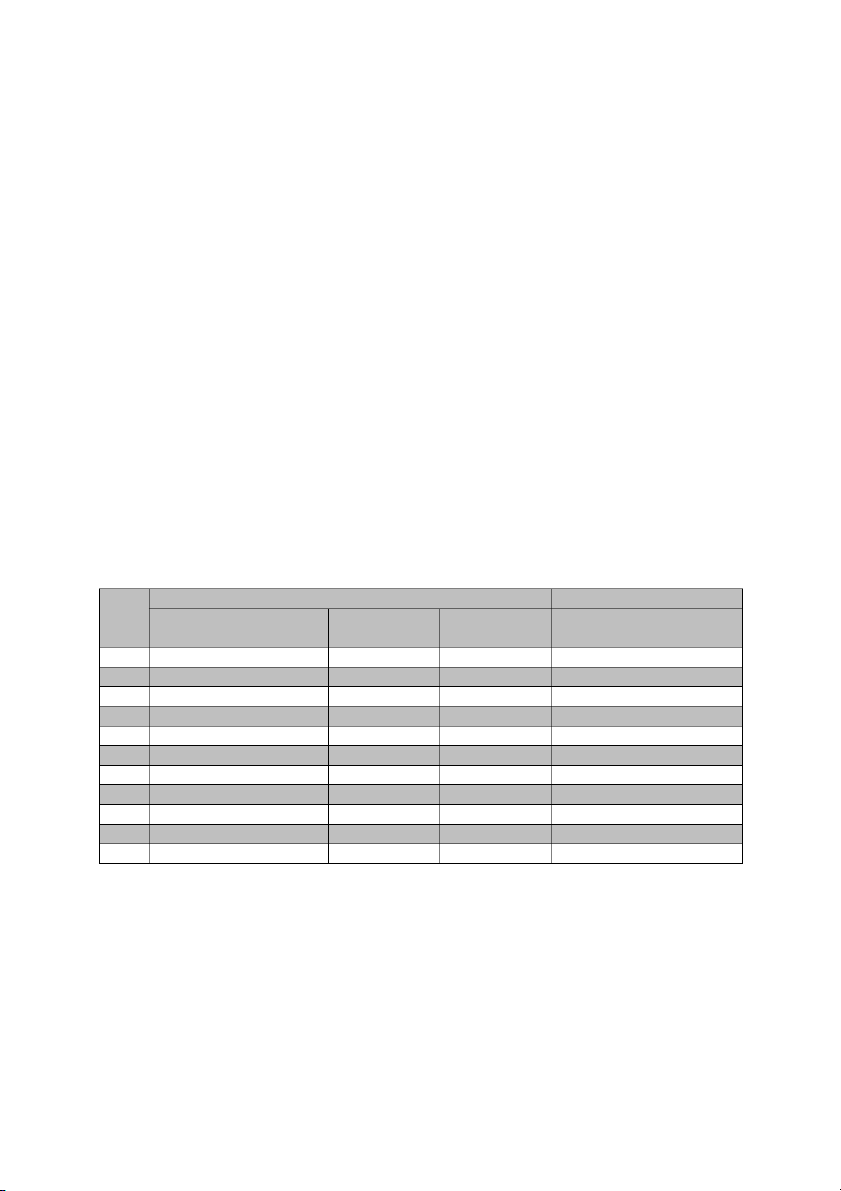
Table 1: Chemical preparation for the experiment on the effect of concentration on reaction time. Test tube #A1 - #A11 Test tube #B1- #B11 No (NH4)2S2O8 + Distilled Na water (mL) 2S2O3 (mL) Starch (mL) KI + Distilled water (mL) 1 10.0 + 0.0 water 5.0 ~4.0 10.0 + 0.0 water 2 10.0 + 0.0 water 5.0 ~4.0 8.5 + 1.5 water 3 10.0 + 0.0 water 5.0 ~4.0 7.0 + 3.0 water 4 10.0 + 0.0 water 5.0 ~4.0 5.5 +4.5 water 5 10.0 + 0.0 water 5.0 ~4.0 4.0 + 6.0 water 6 10.0 + 0.0 water 5.0 ~4.0 2.5 + 7.5 water 7 8.5 + 1.5 water 5.0 ~4.0 10.0 + 0.0 water 8 7.0 + 3.0 water 5.0 ~4.0 10.0 + 0.0 water 9 5.5 +4.5 water 5.0 ~4.0 10.0 + 0.0 water 10 4.0 + 6.0 water 5.0 ~4.0 10.0 + 0.0 water 11 2.5 + 7.5 water 5.0 ~4.0 10.0 + 0.0 water
Special remarks on reactivity of KI: moisture sensitive; light senstitive; air sensitive (Air
causes decomposition to iodine). Step 1: Prepare solution A Add 0.1M (NH4)2S2O8 and
distilled water to each tube based on figure of table 1. And
then add 5mL 0.005M Na2S2O and 4mL 3 starch per tube. 1. 10mL 0.1M (NH4)2S2O 8 + 5mL Na2S2O + 4mL 3 starch. 2. 10mL 0.1M (NH4)2S2O 8 + 5mL Na2S2O + 4mL 3 starch. 3. 10mL 0.1M (NH4)2S2O 8 + 5mL Na2S2O + 4mL 3 starch. 4. 10mL 0.1M (NH4)2S2O 8 + 5mL Na2S2O + 4mL 3 starch. 5. 10mL 0.1M (NH4)2S2O 8 + 5mL Na2S2O + 4mL 3 starch. 6. 10mL 0.1M (NH4)2S2O 8 + 5mL Na2S2O + 4mL 3 starch.
7. 8.5mL 0.1M (NH4)2S2O + 1.5mL 8
water + 5mL Na2S2O3 + 4mL starch.
8. 7.0mL 0.1M (NH4)2S2O + 3.0mL 8
water + 5mL Na2S2O3 + 4mL starch.
9. 5.5mL 0.1M (NH4)2S2O + 4.5mL 8
water + 5mL Na2S2O3 + 4mL starch.
10. 4.0mL 0.1M (NH4)2S2O + 6.0mL 8 water + 5mL Na2S2O + 4mL sta 3 rch.
11. 2.5mL 0.1M (NH4)2S2O + 7.5mL 8 water + 5mL Na2S2O + 4mL 3 starch. Step 2: Prepare solution B
Add 0.2M KI and distilled water to each tube based on figure of table 1. 1. 10mL KI 2. 8.5mL KI + 1.5mL water 3. 7.0mL KI + 3.0mL water 4. 5.5mL KI + 4.5mL water 5. 4.0mL KI + 6.0mL water 6. 2.5mL KI + 7.5mL water 7. 10mL KI 8. 10mL KI 9. 10mL KI 10. 10mL KI 11. 10mL KI
Step 3: Mix solution A and solution B Mixing A1 test tube and B1 test tube together, then stirring and observing time. When Mixing A1 test tube and B1 test tube together, then stirring and observing time. When
Mixing A1 and B1 together, then stirring and observing time. When appearing a first sign color (deep blue)
stop and record result. Continuing the remaining test tube with the same procedure
Step 4: Calculate the initial concentrations of iodide and peroxydisulfate ion for each of the mixtures. Step 5: Plot the data
Plotting the concentration of iodide ion versus time for mixtures #1 – 6. Time should be
on the x – axis and the contrations should be on the y – axis.
Plotting the concentration of peroxydisulfate ion versus time for mixtures #1, 7 – 11.
Time should be on the x – axis and the contrations should be on the y – axis.
2.2 Effect of temperature on the reaction rate 2.2.1 Preparation Add ~5mL 0.33M H2C2O
4 into per test tube #1A, #2A and #3A. Add ~1mL 0.01M KMnO4 and
5mL 3M H2SO4 into per test tube #1B, #2B and #3B. 2.2.2: Procedure
Place test tubes #1A and #1B at room temperature and then pour #1B into #1A. Record
the time for the purple color to disappear.
Place test tubes #2A and #2B in 50 C o
water bath (for ~3mins) and then pour #2B into
#2A. Record the time for the purple color to disappear.
Place test tubes #3A and #3B in 90 C o
water bath (for ~3mins) and then pour #3B into
#3A. Record the time for the purple color to disappear.
2.3 Effect of a catalyst on the raction rate 2.3.1 Preparation
Pour ~40mL 3% H2O2 into the beaker by the cylinder. 2.3.2 Procedure
Add 5mL of 3% H2O into per tube. 2 1. 5mL H2O + MnCl 2 2 2. 5mL H2O + MnO 2 2 3. 5mL H2O + NaCl 2 4. 5mL H2O + CaCl 2 2 5. 5mL H2O + Zn 2 6. 5mL H2O + KNO 2 3 7. 5mL H2O + Fe(NO 2 ) 3
Agitate the mixture. compare the reaction rate (the time for air bubbles to appear) and
record your observation. Rank them in the decreasing order: fastest (01) --> lowest (07). 3. Safety precautions
Na2S2O3: solutions or mist may cause skin irritation from repeated or prolonged contact.
Dust, solutions or mist may irritate or burn the eyes and cause temporary conjunctivitis.
KI: harmful if swallowed or if inhaled. Very toxic to aquatic life. Harmful in contact with
skin. If inhaled: Remove victim to fresh air and keep at rest in a position comfortable for breathing.
H2C2O4: it may cause burns, nausea, severe gastroenteritis and vomiting, shock and
convulsions. It is especially toxic when ingested. As little as 5 to 15 grams (71 mg/kg) may be fatal to humans.
H2SO4: can severely burn the skin and eyes. It may cause third degree burns and
blindness on contact. Exposure to sulfuric acid mist can irritate the eyes, nose, throat and lungs,
and at higher levels can cause a buildup of fluid in the lungs.
MnCl2: may cause skin irritation. Harmful if swallowed. May cause gastrointestinal
irritation with nausea, vomiting and diarrhea. May cause central nervous system effects and/or neurological effects.
MnO2: harmful if inhaled or swallowed. May cause eye, skin, and respiratory tract
irritation. May cause central nervous system effects.
CaCl2: poses some serious health and safety hazards. If ingested, calcium chloride can
lead to burns in the mouth and throat, excess thirst, vomiting, stomach pain, low blood pressure,
and other possible severe health effects. It can also irritate skin by causing excessive dryness or desiccating moist skin.
KNO3: can affect you when breathed in. Contact can cause eye and skin irritation.
Breathing Potassium Nitrate can irritate the nose and throat causing sneezing and coughing.
Fe(NO3): causes severe skin burns and eye damage. Shall not be classified as a
respiratory or skin sensitiser. 4. Suggested questions
1. What is the objective of today lab work?
To examine the effects of concentration, temperature, and catalysts on reactions rates.
2. What is the rate of a chemical reaction?
The rate of reaction refers to the speed at which the products are formed from the reactants in a
chemical reaction. It gives some insight into the time frame under which a reaction can be completed.
3. How can the rate of a reaction be determined?
To measure reaction rates, chemists initiate the reaction, measure the concentration of the
reactant or product at different times as the reaction progresses, perhaps plot the concentration as
a function of time on a graph, and then calculate the change in the concentration per unit time.
4. What is the unit expression of reaction rate?
Reaction rates are usually expressed as the concentration of reactant consumed or the
concentration of product formed per unit time. The units are thus moles per liter per unit time, written as M/s, M/min, or M/h.
5. Please list out factors that can affect the rate of a reaction?
The concentration of the reactants. The more concentrated the faster the rate.
Temperature. Usually reactions speed up with increasing temperature.
Physical state of reactants. Powders react faster than blocks - greater surface area and since the
reaction occurs at the surface we get a faster rate.
The presence (and concentration/physical form) of a catalyst (or inhibitor). A catalyst speeds up a
reaction, an inhibitor slows it down.
Light of a particular wavelength may also speed up a reaction
6. How does temperature affect the raction rate?
Raising the temperature of a chemical reaction results in a higher reaction rate. When the
reactant particles are heated, they move faster and faster, resulting in a greater frequency of collisions.
7. How does the concentration of reactants affect the reaction rate?
If the concentration of reactants is increased, there are more reactant particles moving together.
There will be more collisions and so the reaction rate is increased. The higher the concentration
of reactants, the faster the rate of a reaction will be.
8. What is a catalyst? Is it consumed during the reaction?
A catalyst is a substance that speeds up a chemical reaction, or lowers the temperature or
pressure needed to start one, without itself being consumed during the reaction.
9. In part 1, what is the role of starch? Please explain.
Starch is a catalyst for (NH4)2S2O
8 and Na2S2O3 to react. Starch solution as a reaction medium is
proved to be a very efficient catalyst. The most important characteristics of starch as a catalyst
include its bio-degradability, non-toxic nature.
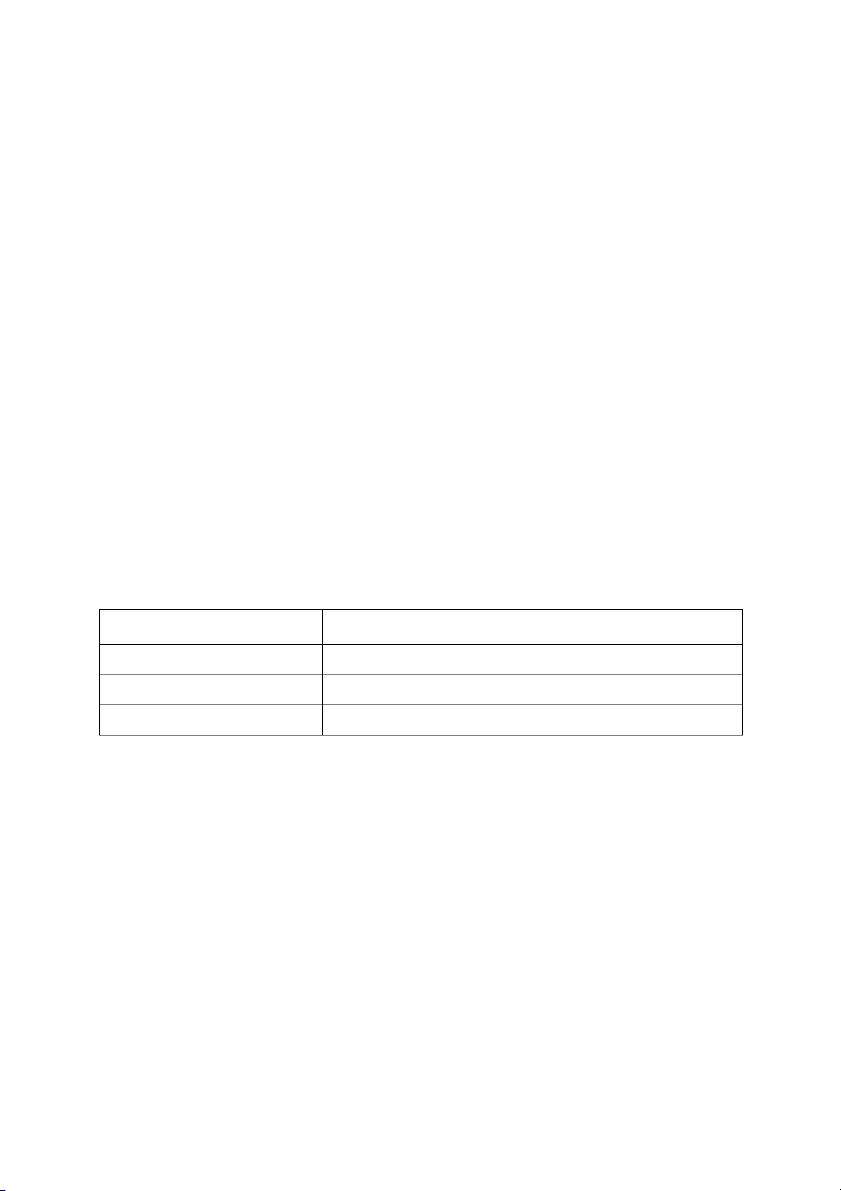
10. In part 2, please predict the outcome of the experiment. Description of conditions Predicted outcome Room temperature There is no phenomenon 500C Releasing gas 900C Realeasing more gas




