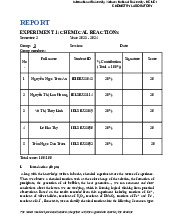
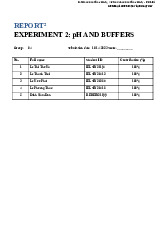
Preview text:
lOMoARcPSD|364 906 32
International University, Vietnam National University - HCMC CHEMISTRY LABORATORY
KEY TO PRE-LAB OF EXPERIMENT 3 – REDOX TITRATION WITH KMnO4
1A. What is an oxidation-reduction reaction? What are the two half reactions of an
oxidation-reduction reaction? (5 pts)
- Oxidation-reduction reaction is a chemical reaction that involves the transfer of
electrons between two species. The oxidation states of atoms are changed. (2.5 pts)
- The two half reactions of an oxidation-reduction reaction are oxidation and reduction:
- Oxidation: A loss of electrons by a species (an increase of oxidation number of an atom)
- Reduction: A gain of electrons by a species (a decrease of oxidation number of an atom) (2.5 pts)
1B. In a redox reaction, what are the oxidizing agent and reducing agent? (5 pts)
- An oxidizing agent is a substance that causes oxidation by accepting electrons;
therefore, it gets reduced. (2.5 pts)
- A reducing agent is a substance that causes reduction by losing electrons; therefore it gets oxidized. (2.5 pts)
2. Complete and balance the following equation: (5 pts)
2 KMnO4 + 5 H2C2O4 + 3 H2SO4 → 10 CO2 + K2SO4 + 2 MnSO4 + 8 H2O
(correct equation, 1.25 pts; balanced equation, 1.25 pts)
List out the oxidizing species and the reducing species in the equation:
Oxidizing species: KMnO4 (1.25 pts) Reducing species: H2C2O4 (1.25 pts) lOMoARcPSD|364 906 32
1 3A. In an oxidation-reduction reaction, the species that gains electrons is said to be: (2.5 pts) a) an oxidizing agent b) a reducing agent c) reduced d) oxidized
e) a and c are correct (2.5 pts)
f) b and d are correct g) none of the above
3B. For dark colored solution, the volume of such solution is to be read at: (2.5 pts)
a) the bottom of the meniscus
b) the top of the meniscus (2.5 pts)
c) either at the bottom or the top of the meniscus d) all of the above
4. Consider the following equation between iron and oxygen:
4 Fe + 3 O2 → 2 Fe2O3 Oxidation number: 0 0 +3 −2
Please choose the correct statement:
a) Iron is the oxidizing species.
b) Oxygen is the reducing species.
c) Iron is oxidized. (2.5 pts) d) Iron is reduced.