

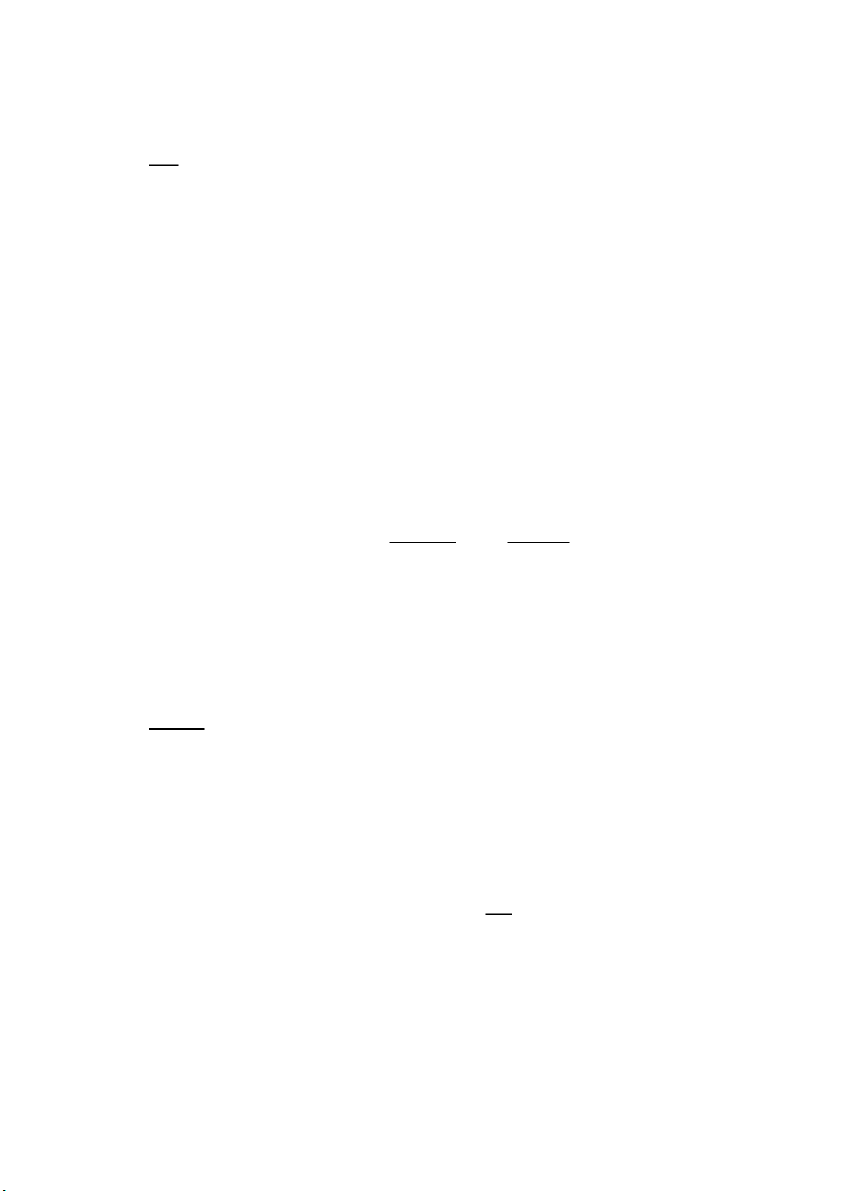













Preview text:
International University, Vietnam National University - HCMC CHEMISTRY LABORATORY REPORT
EXPERIMENT 2: pH AND BUFFERS Group: 04 Section: 02 Date: 07/03/2022 Group members: Seq. Full name Student ID % Signature Score contribution (total = 100%) 1 Khưu Đoàn Đức BEBEIU20038 25 Quang 2 Huỳnh Anh Quân BEBEIU20037 25 3 Nguyễn Tuyên BEBEIU20015 25 Hồng Hải 4 Lê Bùi Mai BEBEIU20235 25 Phương Total score: ________/100 CH012IU 1 S2_2021-22_G_17
International University, Vietnam National University - HCMC CHEMISTRY LABORATORY Contents I.
Introduction ................................................................................................................................... 3 1.
pH: .............................................................................................................................................. 3 2.
Buffer: ........................................................................................................................................ 3 3.
Dilution: ..................................................................................................................................... 4 4.
Objective: ................................................................................................................................... 4 II.
Experimental .............................................................................................................................. 4 1.
pH OF DEIONIZED WATER .................................................................................................. 4 2.
pH OF STRONG ACID ............................................................................................................. 5 3.
pH OF WEAK ACID ................................................................................................................. 5 4.
pH OF SALTS: .......................................................................................................................... 6 5.
pH OF BUFFERS ...................................................................................................................... 6 III.
Results and discussion................................................................................................................ 7 1.
pH OF DEIONIZED WATER .................................................................................................. 7 2.
pH OF STRONG ACID ........................................................................................................... 10 3.
pH OF WEAK ACID ............................................................................................................... 11 4.
pH OF SALTS .......................................................................................................................... 15 5.
pH OF BUFFERS .................................................................................................................... 17 IV.
Conclusions .............................................................................................................................. 22
V. Acknowledgement ........................................................................................................................ 22 CH012IU 2 S2_2021-22_G_17
International University, Vietnam National University - HCMC CHEMISTRY LABORATORY I. Introduction 1. pH:
pH (potential of hydrogen or power of hydrogen) is a scale used to describe the
acidity or basicity of an aqueous solution. Acidic solutions (containing a larger
concentration of H+ ions) have a lower pH than basic or alkaline solutions.
Based on Arrhenius’s theory, an acid is a material that dissociates in water to form
hydronium ions (H3O+), while a base is a chemical that dissociates in water to
generate hydroxide (OH-) ions.
On the other hand, according to Lewis-Brønsted theory, an acid is a proton donor,
whereas a base is a proton acceptor. An acid's H+ combines with water to generate
H3O+ (a hydronium ion) in an aqueous solution, whereas a base accepts a proton
from water to form OH-. (a hydroxide ion).
- Acid: HA (aq) + H2O ⇌ H3O+ (aq) + A- (aq) Ka
- Base: A - (aq) + H2O ⇌ HA (aq) + OH- (aq) Kb - Formula: 끫歼 �끫歶3끫殄+ − �[끫歨 ]
[끫歶끫歨][끫殄끫歶−] 끫 殜 = 끫歼끫 殞 = [끫歶끫歨] �[끫歨−]�
pKa = -log(Ka) pKb = -log(Kb) pH = - log[H3O+ ] - Therefore:
• Acidic solution: pH < 7 or [H3O+] > [OH-]
• Basic solution: pH > 7 or [H3O+] < [OH-]
• Neutral solution: pH = 7 or [H3O+] = [OH-] 2. Buffer:
A buffer is a mixture of a weak acid or weak base and its weak conjugate acid or
weak base. Buffers have the ability to resist a considerable change in pH when H+
or OH- is added. Because the weak base, A-, reacts with added H+ and the weak
acid, HA, reacts with added OH-.
The Henderson-Hasselbach equation can be used to calculate pH changes in buffer solutions: [끫歨−]
pH = 끫殺끫歼끫 殜 + lg ( ). [끫歶끫歨] CH012IU 3 S2_2021-22_G_17
International University, Vietnam National University - HCMC CHEMISTRY LABORATORY 3. Dilution:
Dilution is a method of lowering the concentration of a solution by adding a solvent
to it. The moles of solute in the diluted solution is the same as the moles of solute in the initial solution.
On the other hand, we can dilute the solution by the concentration formula: Mi x Vi = Mf x Vf where:
- Mi is the initial solution concentration (M)
- Vi is the initial solution volume needed for dilution (mL)
- Mf is the final solution concentration after dilution (M)
- Vf is the final solution volume after dilution (mL) 4. Objective:
- To have knowledge about the definition of pH, buffer and how to dilute the
solution. To understand the difference between strong and weak acids is also a purpose of this lab.
- To apply the formula to measure the pH value and the volume of chemicals to dilute it correctly.
- To calculate, prepare, and test a buffer solution's buffering ability.
- There are five tests to practice in this lab, including: • pH of deionized water • pH of strong acid • pH of weak acid • pH of salts • pH of buffers II. Experimental 1. pH OF DEIONIZED WATER
- Step 1: Approximately 50 mL distilled water is measured by cylinder, then pour it into a beaker.
- Step 2: Stir rod is used to stir continuously in 20 seconds, then a pH meter is used to record the pH
- Step 3: Keep doing step 2 until there is no significant change in pH value.
In our procedure, we repeat the second step for 9 times in total. CH012IU 4 S2_2021-22_G_17
International University, Vietnam National University - HCMC CHEMISTRY LABORATORY 2. pH OF STRONG ACID
We have two main sections for the second experiment. - For the 1st section:
• Step 1: Approximately 10 mL 0.1M HCl is taken along with
approximately 20 mL 0.1M NaOH
• Step 2: 100 mL 0.01M NaOH solution is prepared by mixing 10 mL 0.1M NaOH and 90 mL H2O
Note: We should use the pipette to drip the substances to cylinder to have the
accurate volume and pour them into volumetric flask for the mixing step. - For the 2nd section:
• Step 1: 10 mL 0.1M HCl is taken by pipette, dripped to the volumetric
flask containing 100 mL 0.01M NaOH solution and the pH is recorded by the pH meter
• Step 2: 90 mL distilled water is added to the solution. The solution is
stirred by the stir rod and the pH is recorded by the pH meter
• Step 3: 10 mL 0.1M NaOH is added to the solution. The solution is stirred
by the stir rod and the pH is recorded by the pH meter
• Step 4: 90 mL 0.01M NaOH is added to the solution. The solution is
stirred by the stir rod and the pH is recorded by the pH meter 3. pH OF WEAK ACID
We have two main sections for the third experiment.
- For the 1st section, prepare: • Solution A: 0.1M CH3COOH
• Solution B: 0.01M CH3COOH by diluting solution A 10 times
• Solution C: 0.001M CH3COOH by diluting solution B 10 times or solution A 100 times
Note: We should use the pipette to drip the substances to cylinder to have the
accurate volume and pour them into volumetric flask for the mixing step.
- For the 2nd section, we record the pH and Ka of three solutions by taking 20 mL of each CH012IU 5 S2_2021-22_G_17
International University, Vietnam National University - HCMC CHEMISTRY LABORATORY 4. pH OF SALTS:
We have two main sections for the fourth experiment.
- For the 1st section, prepare: • Solution A: 0.1M NaCl • Solution B: 0.1M CH3COONa • Solution C: 0.1M NH4Cl
- For the 2nd section, we record the pH and Ka of three solutions by taking 20 mL of each 5. pH OF BUFFERS
We have main sections for the last experiment.
- For the 1st section, we prepare 4 solutions in four beakers:
• Beaker 1: Approximately 50 mL 0.1M CH3COOH
• Beaker 2: Approximately 50 mL 0.1M CH3COONa
• Beaker 3: Approximately 40 mL 0.1M HCl
• Beaker 4: Approximately 40 mL 0.1M NaOH
- For the 2nd section, we have 4 main steps:
• Step 1: 10 mL 0.1M CH3COOH and 40 mL 0.1M CH3COONa are mixed
to have buffer A, then the pH is record twice by the pH meter.
• Step 2: The solution is divided equally into buffer A1 and buffer A2, then
the pH is recorded again.
• Step 3: 10 drops 0.1M HCl is added to A1 solution by dropper and 10
drops 0.1 NaOH is added to A2 solution by dropper.
• Step 4: More drops 0.1M HCl is added to A1 solution and more drops 0.1
NaOH is added to A2 solution to change the pH by one unit from the start.
The VHCl in drops are recorded for both.
- For the 3rd section, we have 4 main steps:
• Step 1: 40 mL 0.1M CH3COOH and 10 mL 0.1M CH3COONa are mixed
to have buffer B, then the pH is record twice by the pH meter. CH012IU 6 S2_2021-22_G_17
International University, Vietnam National University - HCMC CHEMISTRY LABORATORY
• Step 2: The solution is divided equally into buffer B1 and buffer B2, then
the pH is recorded again.
• Step 3: 10 drops 0.1M HCl is added to B1 solution by dropper and 10
drops 0.1 NaOH is added to B2 solution by dropper.
• Step 4: More drops 0.1M HCl is added to B1 solution and more drops 0.1
NaOH is added to B2 solution to change the pH by one unit from the start.
The VHCl in drops are recorded for both. III. Results and discussion 1. pH OF DEIONIZED WATER Observed pH Time 1st 2nd Explanation (second) (Group 2) (Group 4)
Because the deionized water has 9.76 interacted with oxygen while
being stirred, the pH of the water drops after being given a continuous stir every 20 0 7.51 seconds. Using the following equation, we can see what
happens throughout this process: CO2 + H2O ⇌ H2CO 3 With the appearance of weak 20 9.78 7.50 acid H2CO3, pH is decrease CH012IU 7 S2_2021-22_G_17
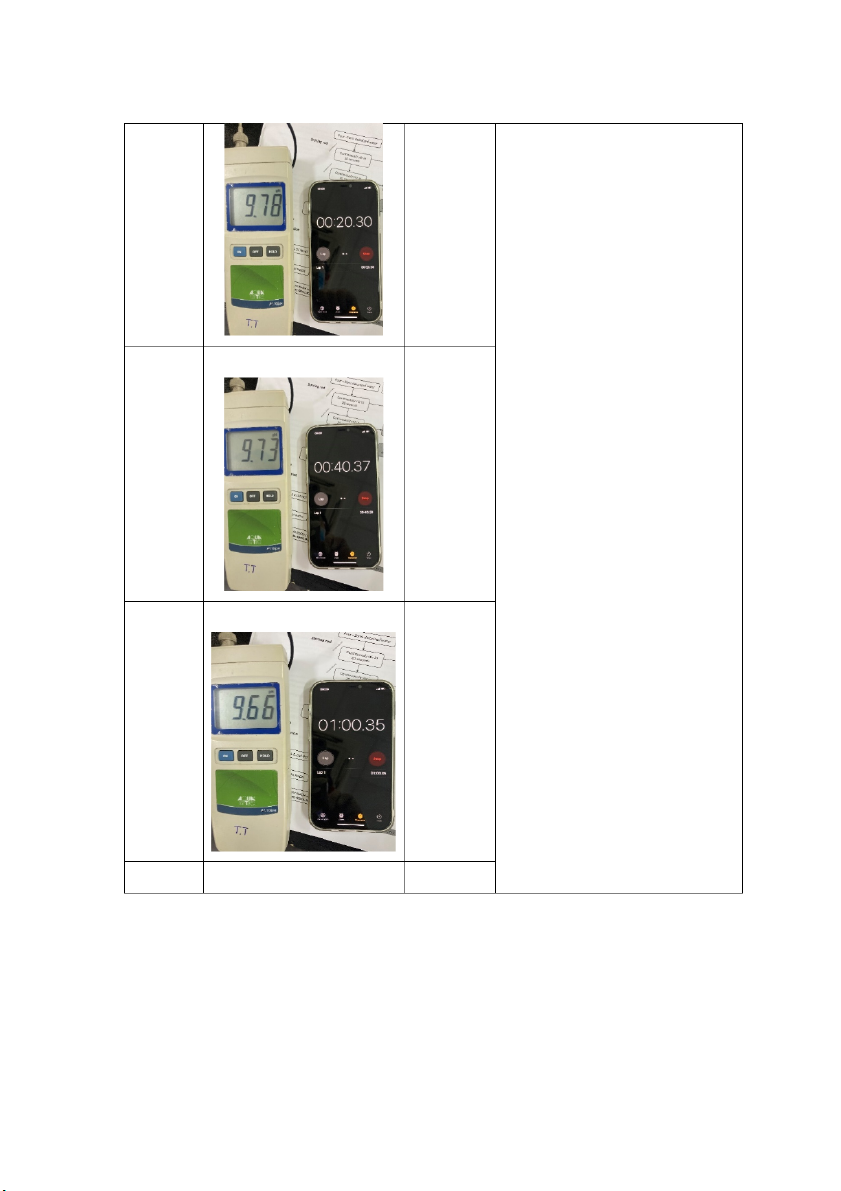
International University, Vietnam National University - HCMC CHEMISTRY LABORATORY 9.73 40 7.47 9.66 60 7.45 80 9.61 7.44 CH012IU 8 S2_2021-22_G_17
International University, Vietnam National University - HCMC CHEMISTRY LABORATORY 9.57 100 7.46 9.54 120 7.55 Comments:
• There is a considerable disparity in measurement results between the two groups.
This dissimilarity arises from differences in the equipment used to measure the pH
value and the technique utilized in the experiment.
• Despite the disparity in values, the statistics of the groups 2 , as the explanation demonstrates. CH012IU 9 S2_2021-22_G_17
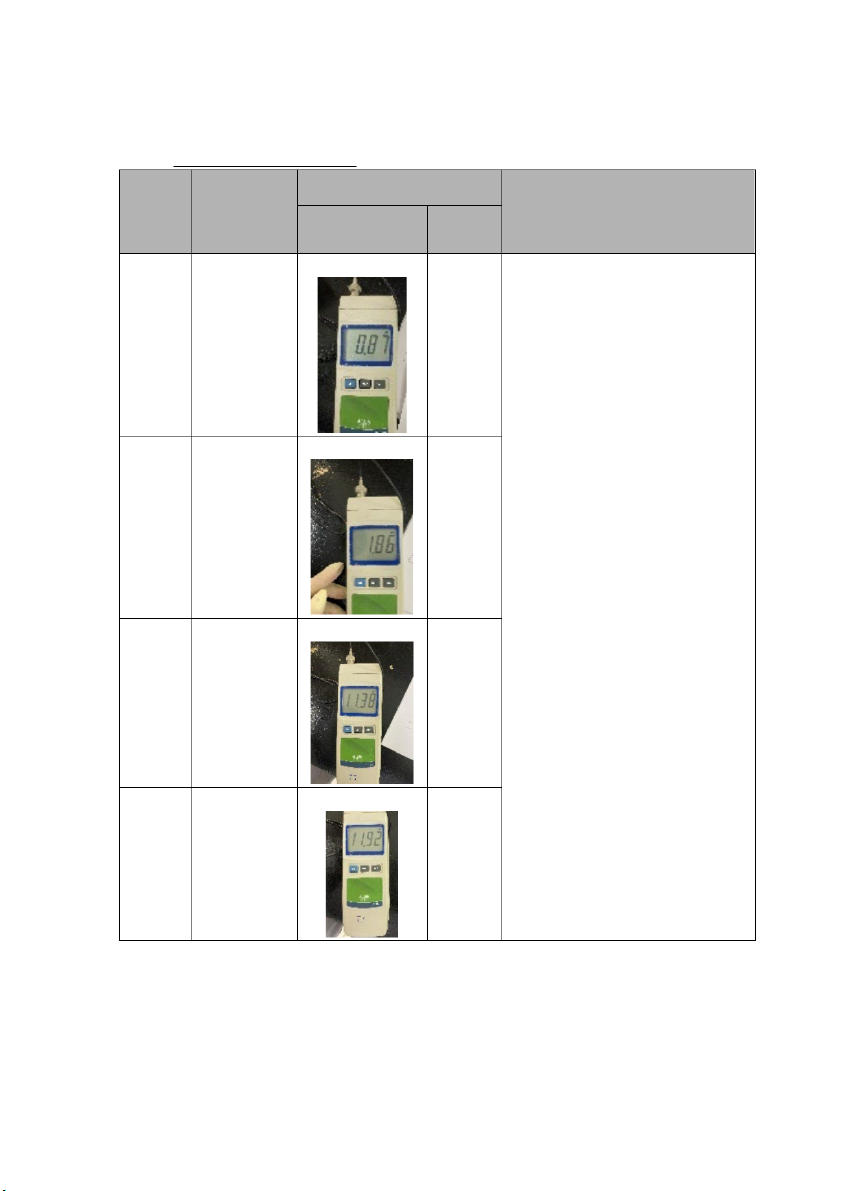
International University, Vietnam National University - HCMC CHEMISTRY LABORATORY 2. pH OF STRONG ACID Measured pH Solutio Theoretical Comments/ Explanation n pH 1st 2nd (Group 2) (Group 4) 1 0.87 1.64
- The theoretical pH is given by the formula: 10 mL pH = -log[H+] of
- After determining that the pH 0.1M is around 1, 90 mL is HCl progressively added to the
solution and there is a modest rise in pH because we have 2 1.87 2.49 already diluted the solution, 10ml of 0.1M NaOH is added, Add 90
and the pH is recorded, pH rises mL of distille
significantly because a strong d
base (NaOH) is added. Finally, water we add 90ml of 0.01M NaOH
and repeat the measurement four times. 7 11.38 7.46 Add 10 mL of 0.1M NaOH 12 11.92 10.78 Add 90 mL of 0.01M NaOH Comments:
10 mL of 0.1M HCl: pH = -log[H+] = -log[0.1] = 1 CH012IU 10 S2_2021-22_G_17
International University, Vietnam National University - HCMC CHEMISTRY LABORATORY
Add 90 mL of distilled water: C1 x V1 = C2 x V2 10 x 0.1 = 100 x C2 C2 = 0.01 M => pH = -log[0.01] = 2
Add 10 mL of 0.1M NaOH: nNaOH = nHCl => pH =7
Add 90 mL of 0.01M NaOH: C2 x V2 = C3 x V3 10 x 0.1 = 100 x C2 C2 = 0.01 M
=> pOH = -log[0.01] = 2 => pH = 14 – pOH = 14-2 = 12
The result in the third step of group 2 is not the same as the result of group 4 and
theoretical result can be explained as when we add NaOH to the solution, we do not wait
for a moment to stabilize the value of pH, which is a systematic error. 3. pH OF WEAK ACID Measured pH Solution 1st 2nd Ka Comments/ Explanation (Group 4) (Group 2) The pH of acidic solution is always lower than 7.0. Our 2.49
recorded pH value of acetic acid
is in the range from about 3.0 to
5.0 and it is correct compare to the theory.
Ka is the equilibrium constant for 0.1M 1.078 chemical reactions in aqueous acetic 3.65
× 10−4 solution that involve weak acids. acid
The extent of acid dissociation is
predicted by using the this Ka value. Formula to calculate Ka: [끫歶 끫歼 3끫殄+][끫歨−] 끫 殜 = [끫歶끫歨] CH012IU 11 S2_2021-22_G_17
International University, Vietnam National University - HCMC CHEMISTRY LABORATORY Where: - HA is the undissociated acid. 3.20 - A- is the conjugate base of acid.
Step to solve Ka when pH value of the solution is given: 0.01M - Step 1: An ICE table for 4.250 acetic 4.34 chemical reaction is set up. × 10−5 acid - Step 2: The equation for
pH: [끫歶3끫殄+] = 10−끫殺끫歶 is used to evaluate the concentration of H+. - Step 3: The concentration of the other products and reactants are calculated by using the concentration of H3O+.
- Step 4: Plug all the value of 5.30 the concentration of reactants and products that we found in step 3 into the equation for Ka and solve it.
Acetic acid is a weak acid with 0.001M 2.52 Ka approximately equal 1.8 × acetic 5.90 × 10−8 10−5. acid Calculation of Ka: • 0.1M CH3COOH:
끫歬끫歶3끫歬끫殄끫殄끫歶 + 끫歶2끫殄 ⇆ 끫歶3끫殄+ + 끫
Initial concentration: 0.1 0 0 (M)
Change in concentration: -x x x (M) CH012IU 12 S2_2021-22_G_17
International University, Vietnam National University - HCMC CHEMISTRY LABORATORY
Equilibrium concentration: 0.1 – x x x (M)
According to the measurement, pH of 0.1M acetic acid = 2.49
We have 끫殺끫歶 = −끫殲끫殲[끫歶3끫殄+] = 2.49
→ [끫歶3끫殄] = 10−2.49 = 3.23 × 10−3 = 끫毊 [끫歶 (3.23 끫 × 毊2 끫歼
3끫殄+][끫歬끫歶3끫歬끫殄끫殄−] 10−3)2 끫 殜 = = [끫歬끫歶3끫歬끫殄 0. 끫 1 殄 − 끫 끫 歶 毊 = ]
0.1 − 3.23 × 10−3 = 1.078 × 10−4 • 0.01M CH3COOH:
끫歬끫歶3끫歬끫殄끫殄끫歶 + 끫歶2끫殄 ⇆ 끫歶3끫殄+ + 끫
Initial concentration: 0.01 0 0 (M)
Change in concentration: -x x x (M)
Equilibrium concentration: 0.01 – x x x (M)
According to the measurement, pH of 0.01M acetic acid = 3.20
We have 끫殺끫歶 = −끫殲끫殲[끫歶3끫殄+] = 3.20
→ [끫歶3끫殄] = 10−3.20 = 6.31 × 10−4 = 끫毊 [끫歶 (6.31끫 × 毊2 끫歼
3끫殄+][끫歬끫歶3끫歬끫殄끫殄−]10−4)2 끫 殜 = = [끫歬끫歶3끫歬끫殄 0. 끫 01殄 −끫歶 끫 ] 毊 =
0.1 − 6.31 × 10−4 = 4.250 × 10−5 • 0.001M CH3COOH:
끫歬끫歶3끫歬끫殄끫殄끫歶 + 끫歶2끫殄 ⇆ 끫歶3끫殄+ + 끫
Initial concentration: 0.001 0 0 (M)
Change in concentration: -x x x (M)
Equilibrium concentration: 0.001 – x x x (M)
According to the measurement, pH of 0.001M acetic acid = 5.30
We have 끫殺끫歶 = −끫殲끫殲[끫歶3끫殄+] = 5.30
→ [끫歶3끫殄] = 10−5.30 = 5.01 × 10−6 = 끫毊 [끫歶 (5.0 끫 1 毊2 끫歼
3끫殄+][끫歬끫歶3끫歬끫殄끫殄−] × 10−6)2 끫 殜 = = [끫歬끫歶3끫歬끫殄 0. 끫 00殄 1 끫 − 歶 끫 ] 毊 =
0.1 − 5.01 × 10−6 = 2.52 × 10−8 CH012IU 13 S2_2021-22_G_17
International University, Vietnam National University - HCMC CHEMISTRY LABORATORY Comments:
• The solution is acidic. Therefore, as the solution is diluted, the pH value of the solution increase.
• As the solution is diluted, the Ka value decreases, indicating that the lower the molar
concentration of the solution is, the less acid dissociation happens.
• Theoretically, the pH of 0.1M acetic acid is 1 according to the formula 끫殺끫歶 =
−끫殲끫殲끫殲[끫歶3끫殄+]. However, the recorded pH value of 0.1M acidic acid is 2.49 whic
slightly higher than the calculated result. The difference between the measured and
theoretical result can be found in the 2 remaining cases. This problem proves that
only a small amount of acidic acid dissociates and little H3O+ are formed. Small
amount of acid dissociation indicates weak acids. Therefore, acetic acid is the weak acid. CH012IU 14 S2_2021-22_G_17
International University, Vietnam National University - HCMC CHEMISTRY LABORATORY 4. pH OF SALTS Measured pH Predicte Solution 1st 2nd Explanation d pH (Group 4) (Group 2) Since NaCl is a salt made of ion Na+ which is strongly 6.89 basic ion and Cl- which is
strongly acidic ion, NaCl is a
neural salt. Therefore, the pH
value is predicted to be equal 7.0. 0.1M 7 6.99 NaCl Since CH3COONa is a salt made of ion Na+ which is strongly basic ion and CH3COO- which is weakly acidic ion, CH3COONa is a basic salt. Therefore, the pH
value is predicted to be larger than 7.0. 7.25 Since NH4Cl is a salt made of ion NH4+ which is weakly basic ion and Cl- which is 0.1M
strongly acidic ion, NH4Cl is a CH3COO >7 (8.47) 7.20
acidic salt. Therefore, the pH Na
value is predicted to be smaller than 7.0. 0.1M 6.35 <7 (2.88) 6.50 NH4Cl CH012IU 15 S2_2021-22_G_17
International University, Vietnam National University - HCMC CHEMISTRY LABORATORY Calculation: • pH of CH3COOH:
끫歬끫歶3끫歬끫殄끫殄끫歬끫歬 ⇆ 끫歬끫歶3끫歬끫殄끫殄− + 끫歬 0.1 0.1 (M)
We have: 끫歼끫殞(끫歬끫歶3끫歬끫殄끫殄−) = 5.71 × 10−10
끫歬끫歶3끫歬끫殄끫殄− + 끫歶2끫殄 ⇆ 끫歬끫歶3끫歬끫殄끫
Initial concentration: 0.1 0 0 (M)
Change in concentration: -x x x (M)
Equilibrium concentration: 0.1 – x x x (M)
[끫歬끫歶3끫歬끫殄끫殄끫 끫 歶 毊2][끫殄끫歶−]
Kb(끫歬끫歶3끫歬끫殄끫殄−) = = [끫歬끫歶3끫歬끫殄 0. 끫 1 殄−
− 끫 ]毊 = 5.71 × 10−10
→ 끫毊 = 7.56 × 10−6 = [끫殄끫歶−]
끫歶끫殲끫歶끫歶끫歶끫歶끫歶, [끫歶+] × [끫殄끫歶−] = 10−14
→ [끫歶+] = 1.32 × 10−9
Therefore, 끫殺끫歶 = −끫殲끫殲[끫歶+] = 8.47 • pH NH4Cl: 끫歬끫歶 + 4끫歬끫殲 ⇆
끫歬끫殲− + 끫歬끫歶4 0.1 0.1 (M)
We have: 끫歼끫殜(끫歬끫歶3) = 1.8 × 10−5 끫歬끫 + 歶 + 끫歶
4 2끫殄 ⇆ 끫歬끫歶3 + 끫歶3끫殄+
Initial concentration: 0.1 0 0 (M)
Change in concentration: -x x x (M) CH012IU 16 S2_2021-22_G_17
International University, Vietnam National University - HCMC CHEMISTRY LABORATORY
Equilibrium concentration: 0.1 – x x x (M) [끫歬끫歶 2 3][끫歶 끫 3 毊 끫殄+] Ka(끫歬끫歶3) = = [끫歬끫
+] 歶4 0.1 − 끫毊 = 1.8 × 10−5
→ 끫毊 = 1.333 × 10−3 = [끫歶3끫殄+]
Therefore, 끫殺끫歶 = −끫殲끫殲[끫歶3끫殄+] = 2.88 Comments:
• The recorded pH value of three solutions in this experiment is the same as our
initial prediction which is the pH value of NaCl (neural salt) = 7.0, CH3COONa
(basic salt) >7.0, and NH4Cl (acidic salt) <7.0.
• However, the measured results are not exactly the same with the calculated results.
That there are some errors in our measuring performance might be the cause of
this issue. Another possible explanation for this problem is that we leave the
chemicals in the outside environment for a long time, causing the quality of the chemicals to deteriorate. 5. pH OF BUFFERS Volume Measured pH Volume (mL) 0.1M Calculated Buffer (mL) 0.1M [Acid] [Base] 1st 2nd CH3COO pH CH (Group (Group 3COONa H 4) 2) 10.0 40.0 A 4.5×10-6 0.08 5.35 5.22 7.6 40.0 10.0 B 7.2×10-5 0.02 4.15 3.80 4.55 Calculation pH: • Buffer A - Total volume:
Vtotal = VCH3COOH + VCH3COOHNa = 10 + 40 = 50 (mL)
- Using the formula: M끫 殬 × V끫 殬 = M끫 殦 × V 끫殦
o The final solution concentration of CH3COOH: CH012IU 17 S2_2021-22_G_17
International University, Vietnam National University - HCMC CHEMISTRY LABORATORY 10 × 0,1 MCH = 0.02 (M) 3COOH끫 殦 = 50
o The final solution concentration of CH3COONa: 40 × 0,1 MCH = 0.08 (M) 3COONa끫 殦 = 50 CH COONa + − 3 → Na + CH COO 3 0.08 → 0.08 (M) CH + − 3COOH + H2O ⇆ H O + CH COO 3 3
Initial concentration: 0.02 0 0.08 (M) Reacted concentration: x x x (M)
Final concentration: 0.02 - x x 0.08 + x (M) - We have:
pKa (CH3COOH) = 4.75 = -log (Ka) → Ka = 10−4.75 ≈ 1.8 × 10−5 - Also: [H3O+][CH3COO−] x(0.08 + x) Ka = = = 1.8 × 10−5 [CH3COOH] 0.02 − x
→ x = 4.5 × 10−6(M) = [H3O+] = [Acid]
→ x + 0.08 = 0.08 + 4.5 × 10−6 = 0.08 M = [CH COO− 3 ] = [Base] [CH −5
3COOH] = 0.02 − x = 0.02 − 7.2 × 10 ≈ 0.02 (M) [CH − 3COO ] → pH = pKa + log ≈ 5.35 [CH3COOH] • Buffer B - Total volume:
Vtotal = VCH3COOH + VCH3COOHNa = 40 + 10 = 50 (mL)
- Using the formula: M끫 殬 × V끫 殬 = M끫 끫 殦 殦 × V
o The final solution concentration of CH3COOH: 40 × 0,1 MCH = 0.08 (M) 3COOH끫 殦 = 50
o The final solution concentration of CH3COONa: CH012IU 18 S2_2021-22_G_17
International University, Vietnam National University - HCMC CHEMISTRY LABORATORY 10 × 0,1 MCH = 0.02 (M) 3COONa끫 殦 = 50 CH COONa + − 3 → Na + CH COO 3 0.02 → 0.08 (M) CH + − 3COOH + H2O ⇆ H O + CH COO 3 3
Initial concentration: 0.08 0 0.02 (M) Reacted concentration: x x x (M)
Final concentration: 0.08 - x x 0.02 + x (M) - We have:
pKa (CH3COOH) = 4.75 = -log (Ka) → Ka = 10−4.75 ≈ 1.8 × 10−5 - Also: [H3O+][CH3COO−] x(0.02 + x) Ka = = = 1.8 × 10−5 [CH3COOH] 0.08 − x
→ x = 7.2 × 10−5(M) = [H3O+] = [Acid]
→ x + 0.02 = 0.02 + 7.2 × 10−5 = 0.02 (M) = [CH COO− 3 ] = [Base] [CH −5
3COOH] = 0.08 − x = 0.08 − 7.2 × 10 ≈ 0.08 (M) [CH − 3COO ] → pH = pKa + log ≈ 4.15 [CH3COOH] Comments:
• The measured pH in buffer B is lower than the calculated pH value, this might be
the method errors when our group mixing the solution.
• There is also difference in the measured pH between 2 groups since the might also
due to the difference in performance of each group.
Part I: Addition of 10 drops 0.1 M HCl CH012IU 19 S2_2021-22_G_17
International University, Vietnam National University - HCMC CHEMISTRY LABORATORY pH pH from after Total volume HCl the
adding (drops) to change Buffer Comments/ Explanation start, 10 pH by one unit pHo drops (pHo-1) HCl 300
When HCl is added to the buffer
solution, the pH value decreases slowly. A 5.21 5.07 CH COOH 3 (aq) + H O 2 ⇆ CH3COO−(aq) + H3O+ (aq) CH3COONa(aq) → CH3COO−(aq) + Na+(aq)
It can be seen from the chemical 170
equation that CH3COOH dissociates
partially to produce CH3COO− and H + 3O . C H COONa 3 is entirely ionized in water solution. B 3.79 3.75 If we add little volume of
If we add HCl to the solution, hydrogen
ions will combine with ethanoate anions,
producing a little more undissociated
ethanoic; thus, the pH will not change
significantly since there is no rise in
hydrogen ion concentration.
Part II: Addition of 10 drops 0.1 M NaOH pH from Total volume NaOH pH after Buffer the start,
(drops) to change pH by Comments/ Explanation adding 10 pHo one unit (pHo+1) CH012IU 20 S2_2021-22_G_17
International University, Vietnam National University - HCMC CHEMISTRY LABORATORY drops NaOH 260 When HCl is added to the buffer solution, the pH value increases very slowly. A 5.19 5.19 If strong base is added to the buffer compound, the hydroxide ions will be neutralized by the hydrogen ions, making it 370 resisting the change in pH B 3.77 3.74 Comments:
• Although our group successfully changed the pH level by one unit, when we
compare the number of drops we used with other group, it show significant increase
in number. To answer to this difference, our group predicted three reasons:
1. Because of the way we mix the chemicals and dilute the solution.
2. The chemical’s quality is reduced because we let it outside for a long time when we do the test.
3. The buffering ability is relatively high; thus, it resists the change in pH, which
is why we had to use a large volume of acid or base to make it change by one unit. CH012IU 21 S2_2021-22_G_17
International University, Vietnam National University - HCMC CHEMISTRY LABORATORY IV. Conclusions
In this experiment, we had a chance to practice with acids, bases, and salt solutions.
Besides that, we have learned how to measure pH, dilute the solution correctly,
generate buffer solutions, evaluate the efficacy of those buffers, and distinguish
between acids of varying concentrations.
However, there are several mistakes we need to discuss after this lab:
• Because of the pH meter and the way we collect the data, our record’s
values are approximate. The exchange values between two groups have a
slight difference due to the chemicals and practical skills when we
compare the result during the time we finish this report. V. Acknowledgement
Thanks to Mr. Le Nguyen Thien Phuc for helping g
roup 4 found out the problem in
test 5 and suggested the solution to fix it. Group 4 also appreciated our TAs for
supporting us in chemical preparation, monitoring as well as reminding us
throughout the process of the experiment. CH012IU 22 S2_2021-22_G_17
International University, Vietnam National University - HCMC CHEMISTRY LABORATORY I. References:
1. https://chem.libretexts.org/Bookshelves/Physical_and_Theoretical_Chemistry_Textboo
k_Maps/Supplemental_Modules_(Physical_and_Theoretical_Chemistry)/Acids_and_Bas
es/Ionization_Constants/Calculating_A_Ka_Value_From_A_Measured_Ph CH012IU 23 S2_2021-22_G_17




