Preview text:
I. Introduction (1pt)
In chemical reactions, one or more chemical substances undergo a reaction
process leading to a chemical transformation of the initial substances into
different chemical compounds. The chemical products can contain one or a
combination of distinct chemical compounds. Chemical reactions may exhibit
various identifiable signs, such as changes in the state of matter from solid to
liquid, formation of precipitates, production of gases, alteration in color, or
generation of light and heat. Chemical reactions are commonly classified into
several types: combination, decomposition, single-replacement, double-
replacement, and combustion. To understand and study chemical substances, it
is necessary to conduct experiments and observe the reaction processes.
In the first laboratory lesson, we will learn about the tools, equipment, and their
proper usage to ensure safety during chemical experimentation. This initial
experiment consists of eight chemical reactions and a test involving an alcohol
lamp flame. The experiments in this lesson help us understand the different types
of reactions and their indicators in chemical reactions. Three objectives need to be accomplished:
Firstly, we follow the proper procedure for handling the chemical
substances in the experiment and ensure safety.
Next, we observe the indicators of chemical reactions, which may include
changes in color, formation of solids, precipitation, the presence of heat
and light, and various other phenomena. Then, we will classify the
chemical reactions into different types: combination, decomposition,
single-replacement, double-replacement, and combustion.
Lastly, we will balance the corresponding chemical equations for the observed reactions.
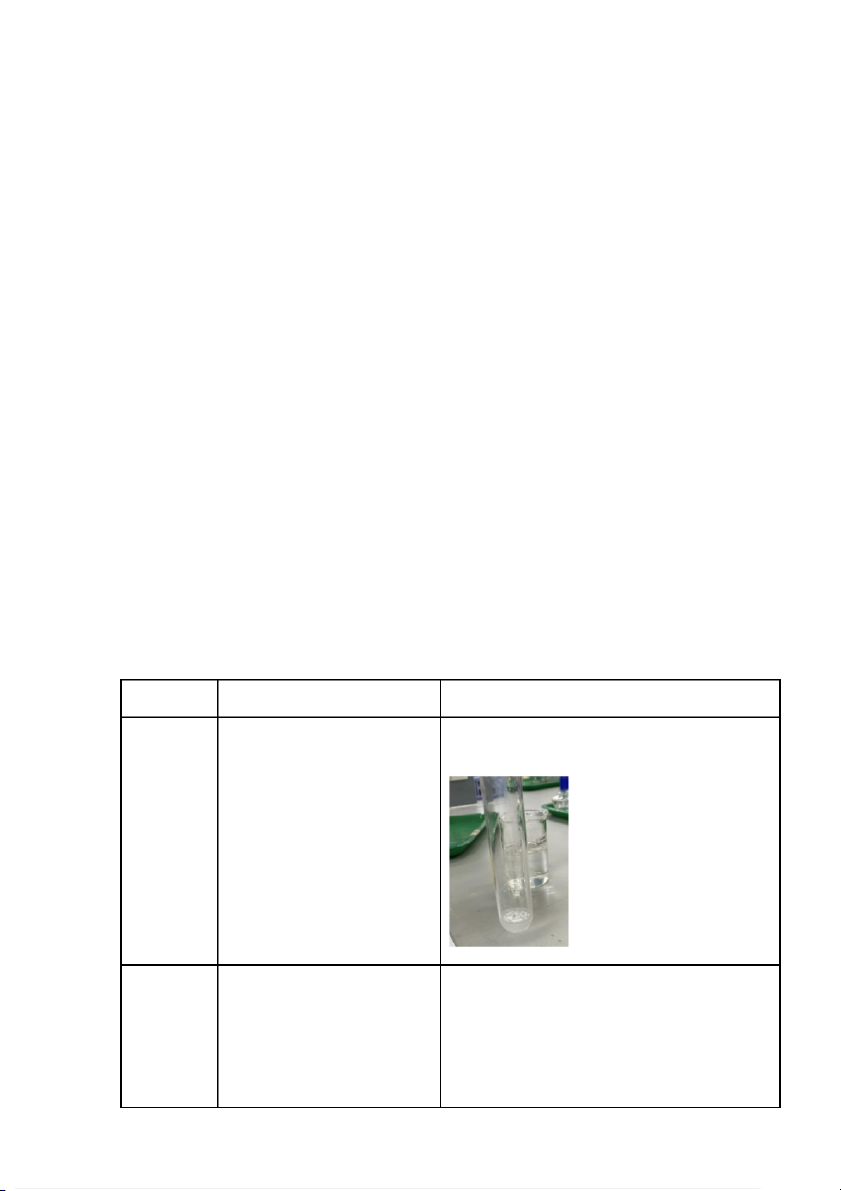
2. Reactions of silver halides (2 pts) Reaction Observation Chemical Equation 0.5M KCl The formation of white KCl + AgNO3 → AgCl +KNO ↓ 3 precipitate of the solution. + 0.1M AgNO3 0.5M KCl When AgNO3 was added,
KCl + AgNO3 + NH4OH → AgCl↓ + KNO3 the formation of white + 0.1M
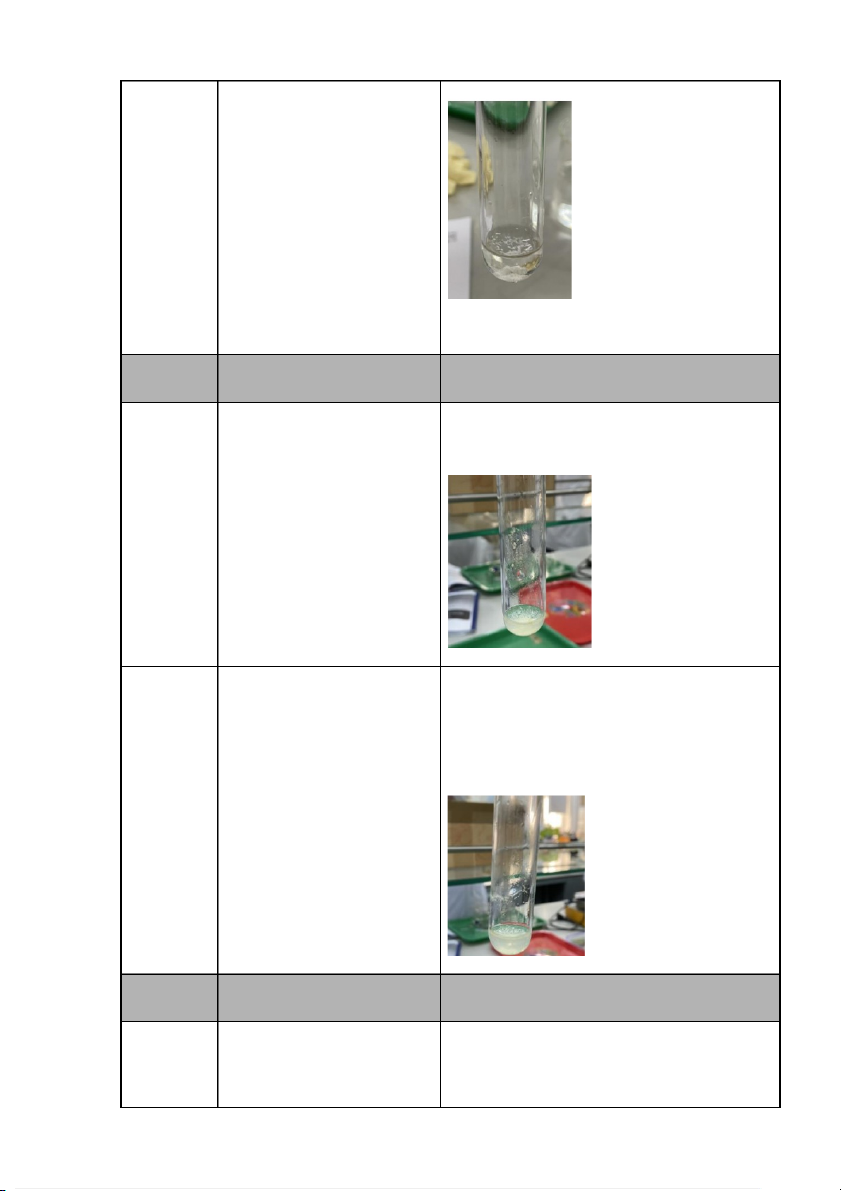
AgCl↓ + 2NH4OH → 2H2O + Ag(NH3)2Cl precipitate but then AgNO3 + 2M dissolved partly when NH NH 4OH 4OH was added. 0.5M KBr The formation of light- KBr + AgNO3 → KNO3+ AgBr ↓ yellow precipitate of the + 0.1M solution. AgNO3 0.5M KBr When AgNO3 was added, KBr + AgNO3 → KNO3+ AgBr ↓ the formation of light-yellow + 0.1M
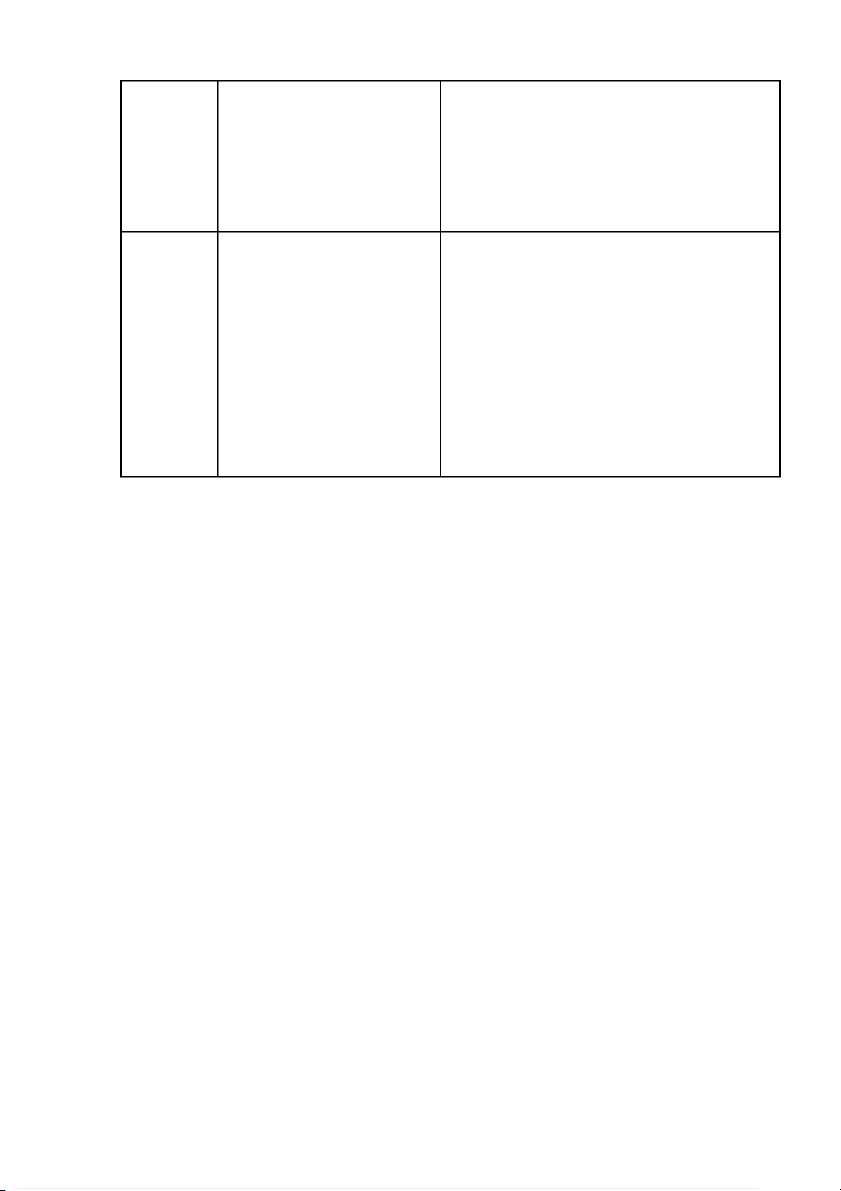
AgBr + 2NH4OH → 2H2O + Ag(NH3)2Br precipitate but then AgNO3 dissolved partly when + 2M NH4OH is added. NH4OH 0.5M KI + 0.1M AgNO3 0.5M KI + 0.1M AgNO3 + 2M NH4OH Comments: -
Ag is a metal that can combine with halogen groups (Cl,F,Br,I) to form
precipitates with different colors. -
Chemical reaction experiments between halogen ion groups and Ag ions:
Reaction 1: Reaction between KCl and AgNO3 creates a white precipitate of AgCl.
Reaction 2: The reaction between KBr and AgNO3 creates a pale yellow precipitate of AgBr. -
When adding NH4OH solution and the above reactions, these precipitates may be partially dissolved.
These reactions are of the double-replacement type.




