
Preview text:
International University, Vietnam National University - HCMC 1 CHEMISTRY LABORATORY REPORT
EXPERIMENT 3: REDOX TITRATION
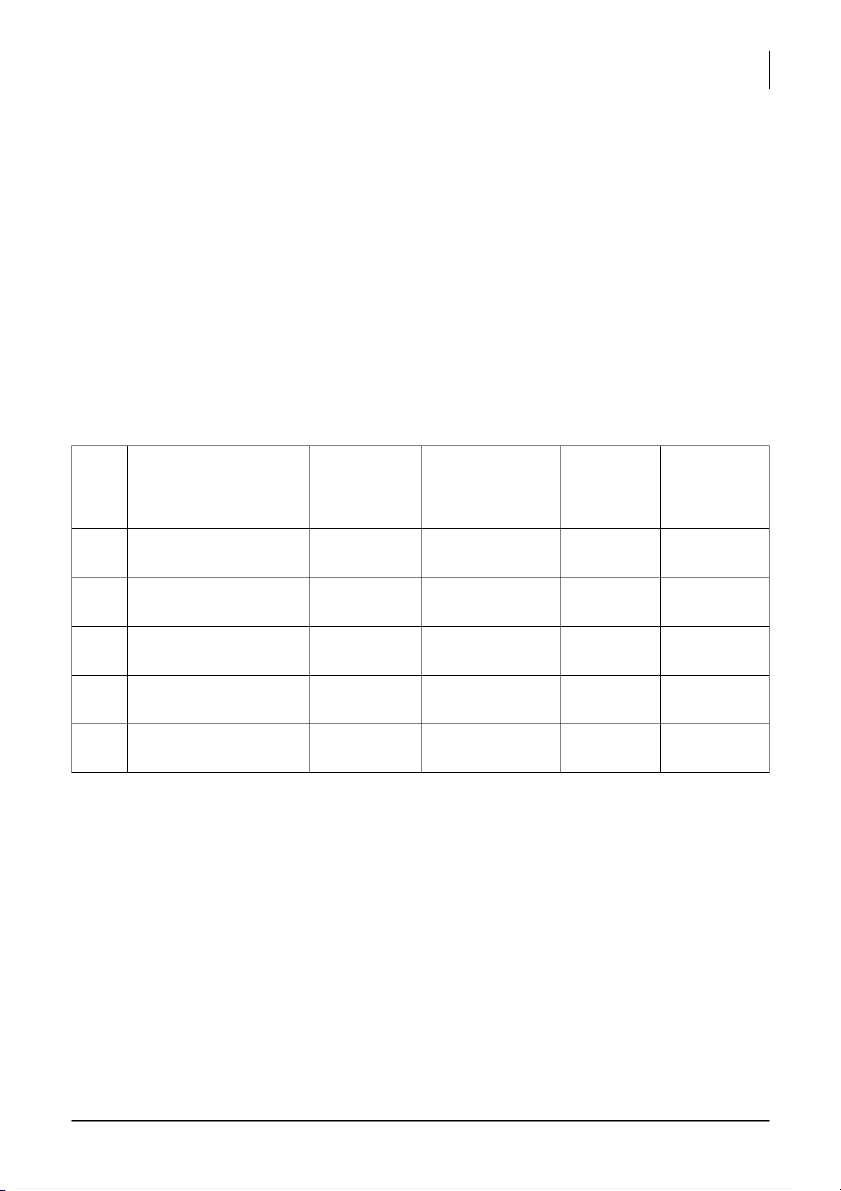
Group: _____________ Section: ______3________ Date: ____________ Group members: Seq. Full name Student ID % Signature Score contribution (total = 100%) 1 2 3 4 5 Total score:________/15__ Present well: + 1 point
Well reading buret (accuracy) + Clear picture: + 2 points Total = 15 points
The report must be typed and handed in together with the signed data sheet by the deadline
International University, Vietnam National University - HCMC 2 CHEMISTRY LABORATORY I. Introduction (1 pt)
In the experiment this week, we delved into the titration method, a widely used
technique in chemistry labs to determine the unknown concentration of a solution.
An oxidation-reduction reaction is a process where electrons are either fully or
partially transferred from one reactant to another. Oxidation is the half-reaction
in which a species loses electrons (or an increase in the oxidation number of an
atom). On the other hand, reduction is the half-reaction in which there is a gain
of electrons by a species (or a decrease in the oxidation number of an atom).
These types of reactions are also referred to as redox reactions.
The substance involved in the reaction that acts as an oxidizing agent is
considered to be reduced as it acquires electrons. Conversely, the material
undergoing oxidation is known as the reducing agent since it loses electrons.
In redox titrations, the gram equivalent weight of the oxidizing agent must be
equivalent to the number of equivalents of the reducing agent:
GEWoxidizing = GEWreducing
Titration is a widely used quantitative chemical analysis method employed to
determine the concentration of an unknown solution. The concentration of the
unknown solution can be easily calculated using the following formula:
Voxidizing x Noxidizing = Vreducing x Nreducing
In this third experiment, we will perform a titration to standardize the KMnO4
solution using a standard solution of H2C2O4. Once the KMnO4 solution is
standardized, we will use it to determine the concentration of an unknown
oxalic acid solution and an unknown Fe2+ solution by recording and calculating the volume KMnO4 value. II. Experimental (1 pt)
(Describe what experiments you did in this report, write down each step of your experiments) III. Results and discussion
1. TITRATION OF KMnO4 SOLUTION WITH STANDARD H2C2O4 SOLUTION (3 pts)
Calculation: (1.5 pts)
Normality of the standard H2C2O4 solution, N(H2C2O4) = ___________________
The report must be typed and handed in together with the signed data sheet by the deadline
International University, Vietnam National University - HCMC 3 CHEMISTRY LABORATORY
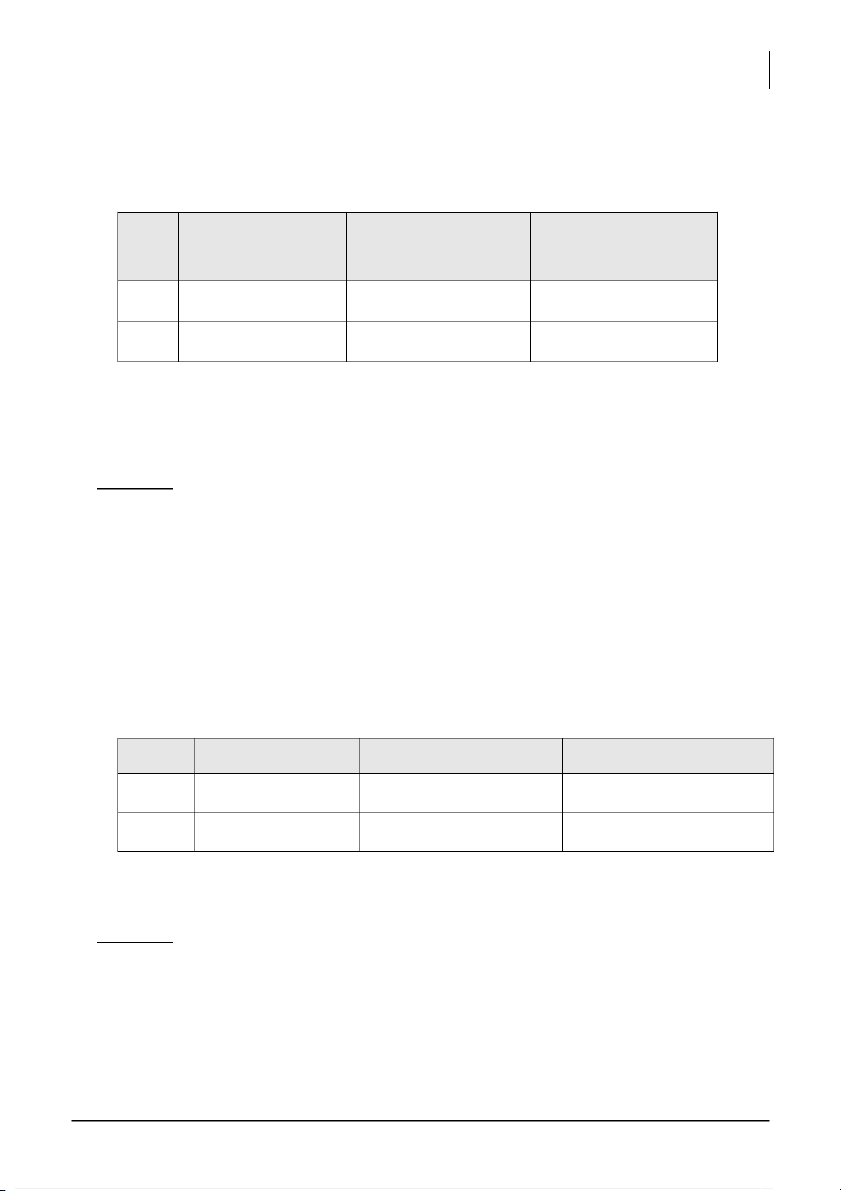
Volume of the standard H2C2O4 solution used, V(H2C2O4) = ___________________ Trial Burette reading (mL) Volume of KMnO4 (mL) Normality of KMnO4 (N) # 1 - 2 -
Average Normality of KMnO4 = ____________ ( ) Comment: (1.5 pts)
2. TITRATION OF UNKNOWN CONCENTRATION H2C2O4 SOLUTION WITH STANDARD KMnO4 SOLUTION (3 pts)
Calculation: (1.5 pts)
Normality of the standard KMnO4 solution, N(KMnO4) = ___________________
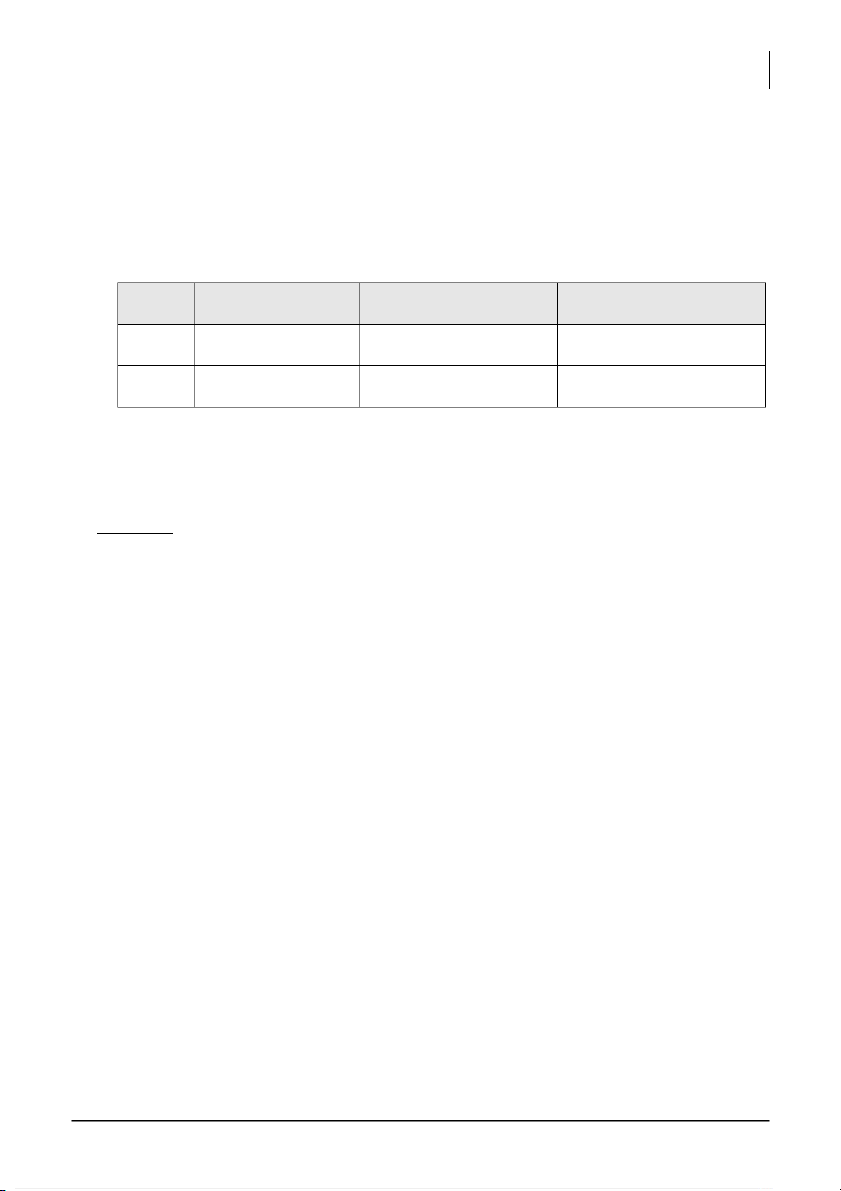
Volume of the unknown H2C2O4 solution used, V(H2C2O4) = ___________________ Trial # Burette reading (mL) Volume of KMnO4 (mL) Normality of H2C2O4 (N) 1 - 2 -
Average Normality of H2C2O4 =_______ ( ) Comment: (1.5 pts)
3. TITRATION OF UNKNOWN CONCENTRATION FeSO4 SOLUTION WITH STANDARD KMnO4 SOLUTION (3 pts)
The report must be typed and handed in together with the signed data sheet by the deadline
International University, Vietnam National University - HCMC 4 CHEMISTRY LABORATORY
Calculation: (1.5 pts)
Normality of the standard KMnO4 solution, N(KMnO4) =___________________
Volume of the unknown FeSO4 solution used, V(FeSO4)= ___________________ Trial # Burette reading(mL) Volume of KMnO4 (mL) Normality of FeSO4(N) 1 - 2 -
Average Normality of FeSO4 =_______ ( )
Comment: (1.5 pts) IV. Conclusions (1 pt)
In this experiment, we can observe that titration is crucial for calculating the volume and
standardization of the substance being sought when it reaches the endpoint, as well as
the rate of change at the initial point.
However, there may be some errors during the process. There are several factors that
contribute to the existence of errors, such as the concentration of the reacting
substance and the reaction temperature. These are two of the factors that are
frequently varied, leading to changes in the reaction rate.
The report must be typed and handed in together with the signed data sheet by the deadline




