



Preview text:
International University, Vietnam National University - HCMC 1 CHEMISTRY LABORATORY REPORT
EXPERIMENT 5: FACTORS AFFECTING REACTION RATE
Group: _____________ Section: ______5________ Date: ____________ Group members: Seq. Full name Student ID % Signature Score contribution (total = 100%) 1 2 3 4 5 Total score: ________/15__ Present well: + 1 point Clear picture: + 1 point Total = 15 points
The report must be typed and handed in together with the signed data sheet by the deadline
International University, Vietnam National University - HCMC 2 CHEMISTRY LABORATORY I. Introduction (1 pt)
(Introduce what experiments you do in this report) II. Experimental (1 pt)
(Describe what experiments you did in this report, write down each step of your experiments) III. Results and discussion
1. EFFECT OF CONCENTRATION ON REACTION TIME (5 pts) Reaction 1:
__________________________________________________ Reaction 2:
__________________________________________________
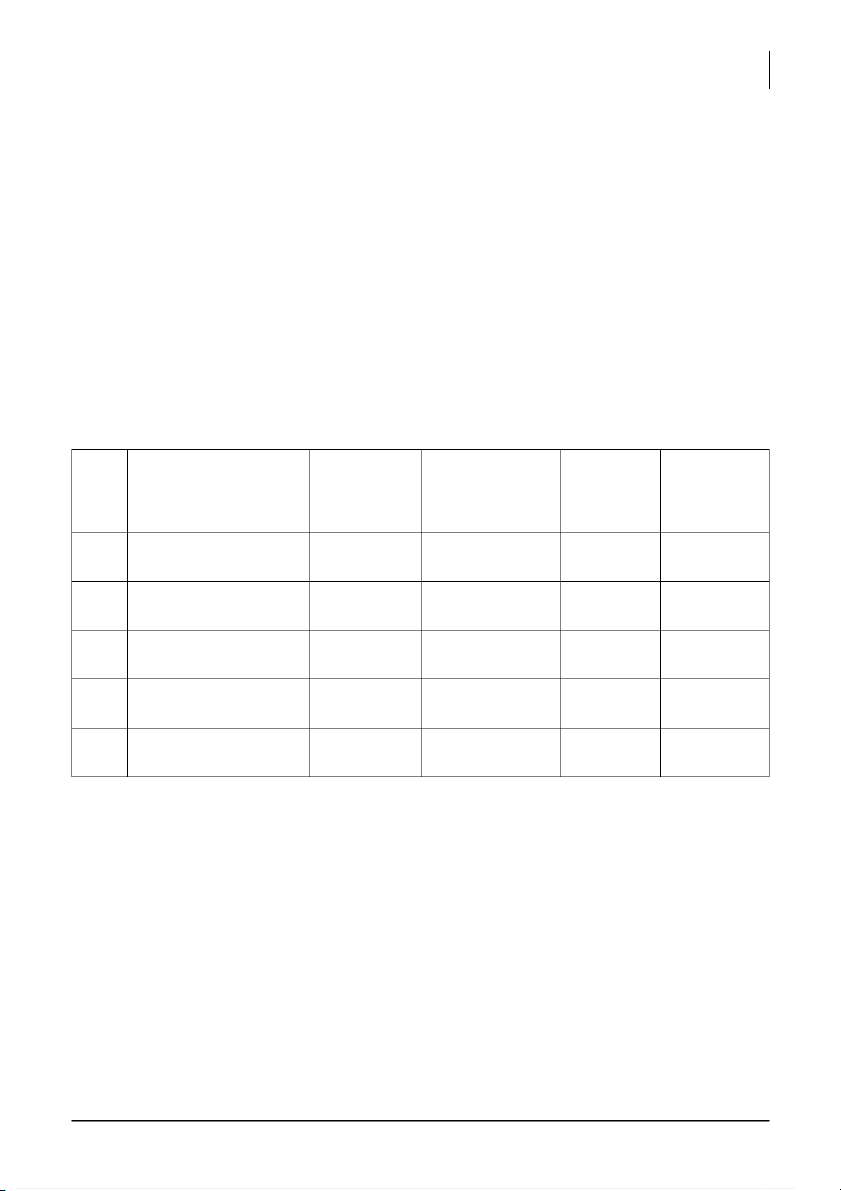
Calculate the initial concentrations of I- and S 2- 2O8 ions: Mixture # 5: (1 pt) [I-] = [S 2- 2O8 ] = Mixture Iodide ion Peroxydisulfate Time in seconds 1 0.08 0.04 2 0.068 0.04 3 0.056 0.04
The report must be typed and handed in together with the signed data sheet by the deadline
International University, Vietnam National University - HCMC 3 CHEMISTRY LABORATORY 4 0.044 0.04 5 0.032 0.04 6 0.02 0.04 Mixture Iodide ion Peroxydisulfate Time in seconds 7 0.08 0.034 8 0.08 0.028 9 0.08 0.022 10 0.08 0.016 11 0.08 0.01
Plotting the concentration of iodide ion versus time: [Note: X – axis: time; Y – axis: concentrations].
- Mixtures # 1-6: Graph (1 pt)
The order of reaction with respect to iodide ion? (0.5 pts) Comments: (0.5 pts)
- Mixtures # 1, 7, 8, 9, 10, and 11: Graph (1 pt)
The report must be typed and handed in together with the signed data sheet by the deadline
International University, Vietnam National University - HCMC 4 CHEMISTRY LABORATORY
The order of reaction with respect to peroxydisulfate ion? (0.5 pts) Comments: (0.5 pts)
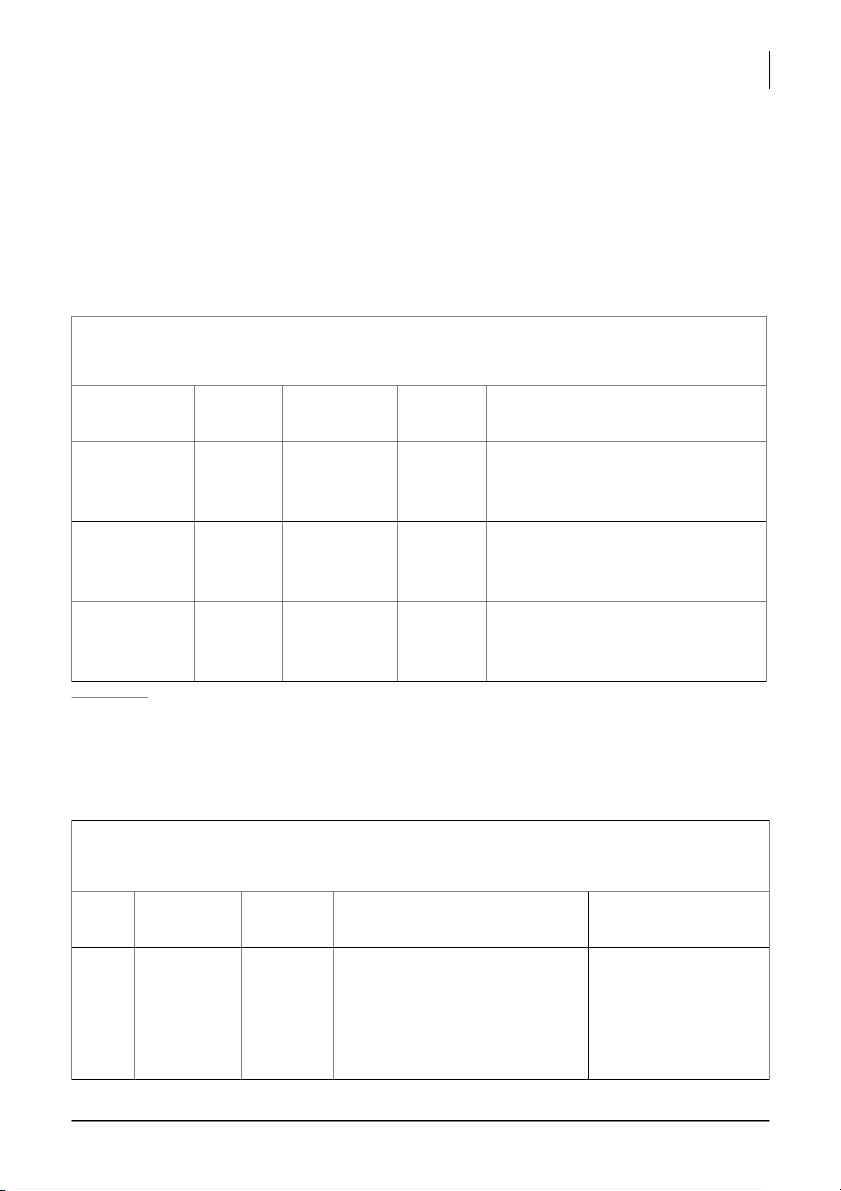
2. EFFECT OF TEMPERATURE ON THE REACTION RATE (2 pts) Reaction System: Description of Predicted Observation Reaction Explanation conditions outcome time Room (0.5 pts) temperature (0.5 pts) 500C 900C (0.5 pts) Comment: (1pt)
3. EFFECT OF A CATALYST ON THE REACTION RATE (3 pts)
Reaction System: of H2O2 with catalystes Trial Description Predicted Observation (Reaction rate) Explanation of conditions outcome 1 + MnCl2 Slow
Slow rate, insoluble in solution. MnCl2 is not a catalyst rate, small in this reaction so it bubble (78s) does not speed up of the reaction rate.
The report must be typed and handed in together with the signed data sheet by the deadline
International University, Vietnam National University - HCMC 5 CHEMISTRY LABORATORY 2 + MnO2 Fast Dramatically fast rate, big MnO2 is a good catalyst rate,
bubble, have black solution (0.1s) in this reaction by appears speeding up the black reaction rate. 3 + NaCl Slow
Insoluble in solution, colorless NaCl is not a catalyst rate, (61s) for this process so it insoluble does not change the reaction rate. 4 + CaCl2 Slow Slow rate, have small bubbly CaCl2 is not a catalyst rate, (28s) in this reaction so it colorless does not speed up the in solution reaction rate.
The report must be typed and handed in together with the signed data sheet by the deadline
International University, Vietnam National University - HCMC 6 CHEMISTRY LABORATORY 5 + Zn slow slow rate, have light-gray Zn is not a catalyst in rate, small solution (39s) this process so it does bubbly not speed up the reaction rate. 6 + KNO3 x x x 7 + Fe(NO3)3 Fast Fast rate, appears bubbly, Fe(NO3)3 is a good rate, dark-brown in solution (2.4s) catalyst in this reaction bubbly, by speeding up the dark- reaction rate. brown in solution
The order of catalyst activity: (0.5 pts)
MnO2 > Fe(NO3)3, > CaCl2 > Zn > NaCl > MnCl2
The report must be typed and handed in together with the signed data sheet by the deadline
International University, Vietnam National University - HCMC 7 CHEMISTRY LABORATORY Comments:
A catalyst is a substance that speeds up a chemical reaction without being consumed in the process.
Its mass remains unchanged throughout the entire reaction.
A catalyst facilitates or accelerates a reaction without adversely impacting it by lowering the energy
required for bond breaking and formation. By reducing the energy barrier, the catalyst enables the
reaction to proceed more rapidly, ultimately requiring less energy in the process. IV. Conclusions (1 pt)
(conclude all your performance in this report)
The report must be typed and handed in together with the signed data sheet by the deadline