




































Preview text:
Analytical Chemistry 1 Lecture 7
Complex Acid-Base Titration
Instructor:*Nguyen*Thao Trang Complex(Acid-base(titration
• (1)(two(acids(or(two(bases(of(different(strengths,
• (2)(an(acid(or(a(base(that(has(two(or(more(acidic(or(basic( functional(groups,(or •
(3)(an(amphiprotic substance,(which(is(capable(of(acting(as( both(an(acid(and(a(base. 2
Mixtures)of)Strong)and)Weak)Acids)or)Strong)and)Weak)Bases
• The(dissociation(constant(for(the(weak(acid(or(base(is(
somewhat(less(than(about(1.00(×10–4
Ex.*pH*of*0.1200*M*HCl and*0.0800*M*weak*acid*HA* (Ka=*1.00*×10–4) [H ](=( 3O+ CHCl +([A– – ](=(0.1200(+([A ]
If([A–](<<(0.1200(M,(([H ]( 0.1200( 3O+ ≈ M à pH(=(0.92 3
Mixtures of Strong and Weak Acids or Strong and Weak Bases
• The(dissociation(constant(for(the(weak(acid(or(base(is(
somewhat(less(than(about(1.00(×10–4. Check(assumption:
Ka=([H3O+]([A–]((/([HA](=(1.00(×10–4
[A–]((/([HA](=(Ka /([H3O+](=(1.00(×10–4( /(0.1200(=(8.33(×10–4(
[HA](=([A–]((/(8.33(×10–4(
CHA =([HA](+([A–]((=(0.0800(M(=({[A–]((/(8.33(×10–4(}(+([A–]((≈ (1.20×103()([A–](=(0.0800(M
à [A–](=(6.7(×10–5(M( <<(0.1200(M 4
Mixtures)of)Strong)& Weak)Acids)or)Strong)&)Weak)Bases
• When*25.00*mL*mixture*of*HCl*and*HA*is*titrated*with*a*strong* base:
– In)the)presence)of)5.00mL)OH- 0.1000)M: H+ +))))))))OH – ⇌ H O 2
5.00(x(0.1000(((((((((((((((((((((((((((((((((((((((mol
CHCl =((25.00(x(0.1200(- 5.00(x(0.1000)/(25.00(+(5.00)(=(0.0833(M
[H3O+]( =(CHCl +([A-](~(0.0833(M(à pH(=(1.08
We(can(check(if(the(assumption( is(still(valid:
[HA]( =(0.0800(x(25.00/30.00(=(0.0667(M
à [A-](=(8.0(x10-5 M(<<(0.0833(M 5
Mixtures)of)Strong)& Weak)Acids)or)Strong)&)Weak)Bases
– In)the)presence)of)29.00mL)OH- 0.1000)M: H+ +))))))))OH – ⇌ H O 2
29.00(x(0.1000(((((((((((((((((((((((((((((((((((((((mol
CHCl =((25.00(x(0.1200(- 29.00(x(0.1000)/(25.00(+(29.00)(=(1.85(x(10-3 M
[H3O+]( =(CHCl +([A-](~(1.85(x(10-3 M
We(can(check(if(the(assumption(is(still(valid:
CHA=(0.0800(x(25.00/54.00(=(3.7(x(10-2 M
à [A-](=(1.90(x10-3 M,(no(longer(<<(CHCl. 6
Mixtures)of)Strong)& Weak)Acids)or)Strong)&)Weak)Bases
– In)the)presence)of)29.00mL)OH- 0.1000)M:
[H3O+]( =(CHCl +([A-](=(1.85(x(10-3 +([A-]( (1)
CHA =([HA]( +([A-]( =(3.7(x(10-2 M( (2) [HA]( =([H3O+][A-]/K [H ][A a( =( 3O+ -]/(1.00( x(10-4 )( (3)
Substitute( (3)(into((2)(yields: [H ][A ]/K [A 3O+ - a( +( -]( =(3.7(x(10-2 M(
à [A-]( =(3.7(x(10-2 M(/([H3O+]( +(1.00(x(10-4 )( (4)
Substitute( (4)(into((1)(yields:([H ](
3O+ =( 3.03(x(10-3 M(à pH(=2.52 7
Mixtures)of)Strong)& Weak)Acids)or)Strong)&)Weak)Bases
• When*25.00*mL*mixture*of*HCl*and*HA*is*titrated*with*a*strong* base:
– When(the(amount(of(the(base( NaOH(=(amount(of(HCl(present:( the(
solution(is(identical(to(the(solution(containing(the(weak(acid(HA(and( NaCl.
– The(remainder( of(the(titration(curve( is(identical(to(that(for(a(diluted( HA.
– The(shape( of(a(titration(curve(varies( (depending( on(the(strength(of(the( weak(acid). 8
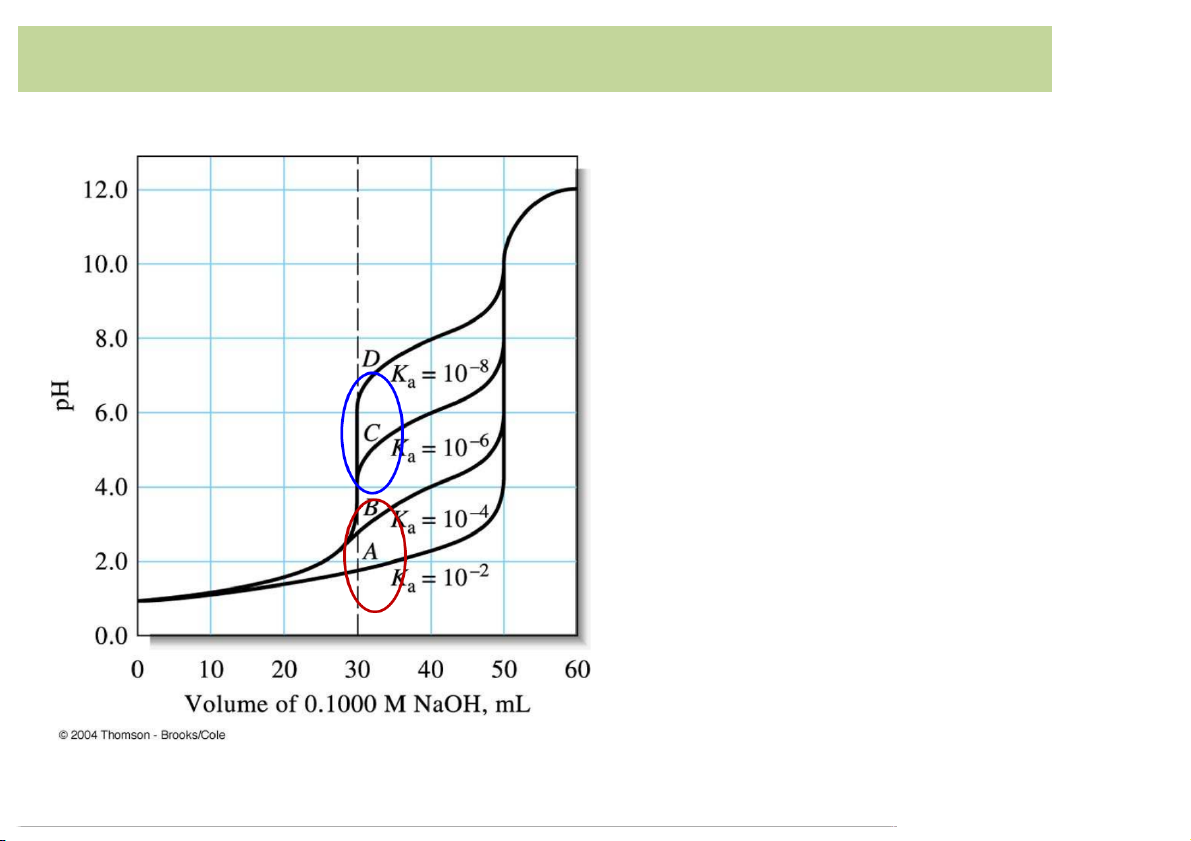
Mixtures)of)Strong)& Weak)Acids)or)Strong)&)Weak)Bases Curves for the titration of strong acid /weak acid mixture (with different Ka of the waek acid) with 0.1000 M NaOH. Two( Each titration is on 25.00 equivalence( mL of a solution that is points 0.1200 M in HCl and Small/nonexistent( 0.0800 M in HA. equivalence( point à The composition of a mixture of a strong acid with a weak acid can be determined if the Ka of the weak acid lies between 10-4 - 10-8. 9
Polyfunctional Acids and Bases
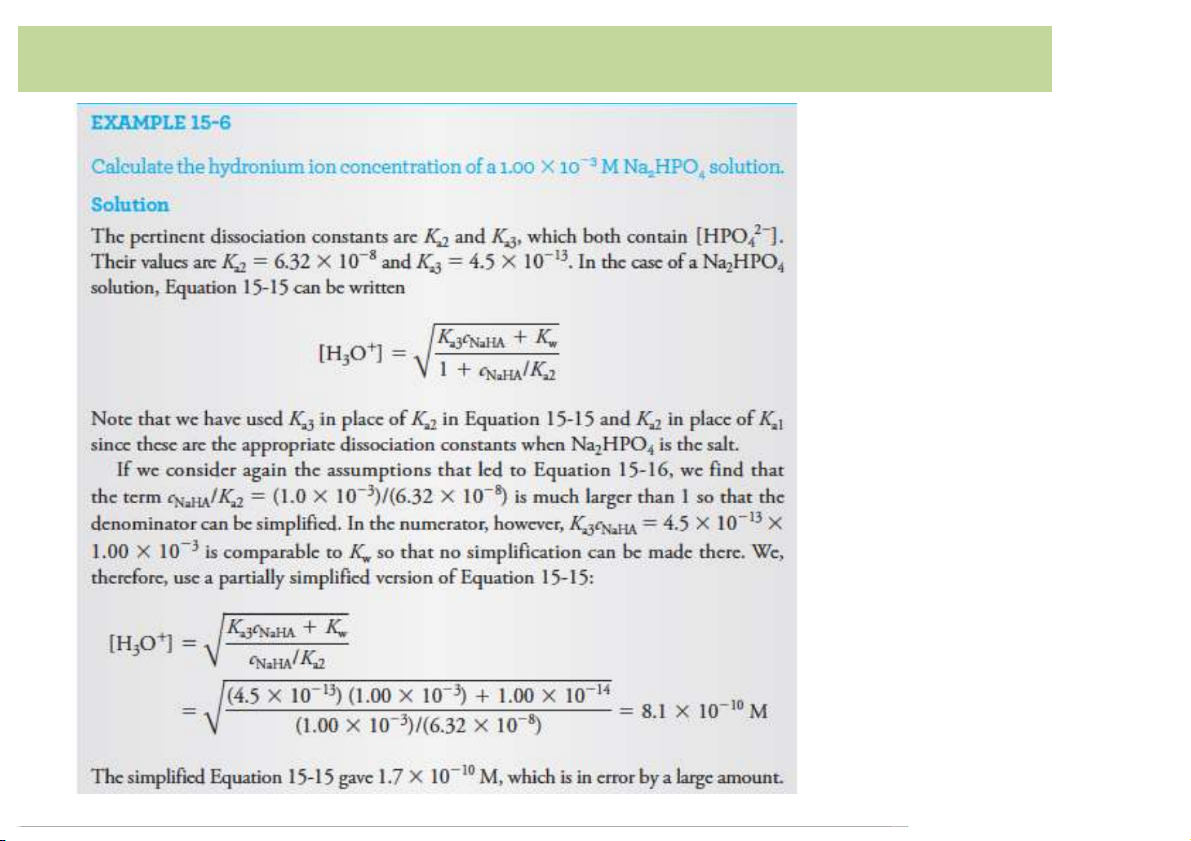
The)phosphoric)acid)system • H O ⇌ H – + – + 3PO4 +(H + H O ; K ][H O ]( /([H 2 2PO4 3 a1 =( [H2PO4 3 3PO4]( =(7.11 ×10–3( • H – 2( – 2( – + – 2PO4 +(H O ⇌ HPO + H O+ ; K ][H O ]( /([H 2 4 3 a2 =([HPO4 3 2PO4 ]( =(6.32×10–8( • HPO 2(– 3(– 3(– 2( – 4 +(H O ⇌ PO + H O+ ; K =([PO ][H O+]( /([HPO ]( 2 4 3 a3 4 3 4 =(4.5 ×10–13 with(K K K a1 >( a2 >( a3 10
Polyfunctional Acids and Bases
The)phosphoric)acid)system
Equilibrium(constant(Keq for(the(overall(reaction: H 2H 2– 3PO4 +( O ⇌ HPO + 2H O+ 2 4 3
àTwo(adjacent(stepwise(equilibria:(K K 2(– a1 a2 =([HPO4 ][H O+]2 /( 3 [H –8(
3PO4](=((7.11 ×10–3()× (6.32×10 )=(4.49×10–10( H 3H 3– 3PO4 +( O ⇌ PO + 3H O+ 2 4 3
à Three(adjacent(stepwise(equilibria: K 3(– a1Ka2Ka3 =([PO4 ](
[H O+]3/[H PO ](=((7.11 ×10–3()× (6.32×10–8 ( –
) × 4.5 ×10 13) =(2.0×10–22 3 3 4 11
Polyfunctional Acids and Bases
The)carbon)dioxide/carbonic)acid)system • CO2((aq)(+(H O ⇌ H (1) 2 2CO3 Khyd =([H2CO ](/([CO 3 2(aq)](=(2.8 ×10–3( à [CO2(aq)](≫ [H2CO ]( 3 • H – 2CO +(H O ⇌ HCO + H O+ (2) 3 2 3 3 K – 1 =([HCO ]([H O+](/([H ](=(1.5×10–4( 3 3 2CO3
• HCO – +(H O ⇌ CO 2(– + H O+ (3) 3 2 3 3 K 2(– – 2 =([CO
][H O+](/([HCO ](=(4.69 ×10–11 3 3 3 12
Polyfunctional Acids and Bases
The)carbon)dioxide/carbonic)acid)system
Since([CO2(aq)](≫ [H2CO ](,(the(acidity(of(solutions(of(carbon( 3
dioxide(is(obtained(by(combining( 1)(&((2): • CO2(aq)(+(2H O ⇌ HCO – + H O+(((( 2 3 3 K – a1 =([HCO ]([H O+](/([CO × 4() 3 3 2(aq)]( (=((2.8 ×10–3 – ) × (1.5 10 =(4.2(×10–7( and
• HCO – +(H O ⇌ CO 2(– + H O+ 3 2 3 3 K 2(– – a2 =([CO
][H O+](/([HCO ](=(4.69 ×10–11 3 3 3 13
Polyfunctional Acids and Bases
Ex.)Calculate)the)pH)of)a)solution)that)is)0.02500M)CO2
The(mass(balance( for(CO2 containing(species: C ( – 2(– CO2 =([CO2 aq)]( +([H ]( +([HCO ]( +( 2CO [CO ](=(0.02500(M 3 3 3 Since([CO – 2( – 2(aq)]( ≫ [H ]( ,([HCO ],( ([CO ],( 2CO3 3 3 C ( CO2 ~([CO2 aq)]( =(0.02500(M
The(charge(balance( (equation(is: [H O+]( = [
( HCO –]( +(2 [CO 2(– ](+([OH – ] 3 3 3
Assume( [HCO –]( ≫ 2 [CO 2(– ](+([OH – ],(then:([H O+]( (~([HCO –]( 3 3 3 3 K – a1 =([HCO ]( [H O+]( /([CO 3 3 2(aq)]( =(4.2(×10–7(
à [H O+]( =(0.02500(× 4.2(×10–7( =(1.02(×10–4( M( 3 àpH(=(3.99 (Determine( [H ],( ([CO 2(– ],( 2CO
[OH – ](to(verify( the(assumption.) 3 3 14
Buffer involving polyprotic acids
- Buffer(composed(of(a(weak(diprotic(acid(H2A(and(its(salts( (NaHA(and(Na2A):
• 1st)system:(the(acid(H2A(and(its(conjugate(base(NaHA
– The(dissociation( of(HA- to(yield(A2- can(be(neglected.
– The(calculation(is(based( on(the(dissociation( of(H2A.(
• 2nd)system:(the(acid(HA- (or(NaHA)(and(its(conjugate(base( Na2A
– The(dissociation( of(HA- to(yield(A2- is(dominated.
– [H2A]( <<([HA-]( or([A2-]
• pH(of(system(2(>(pH(of(system(1(as(Ka( (H2A)>(Ka((HA-) 15
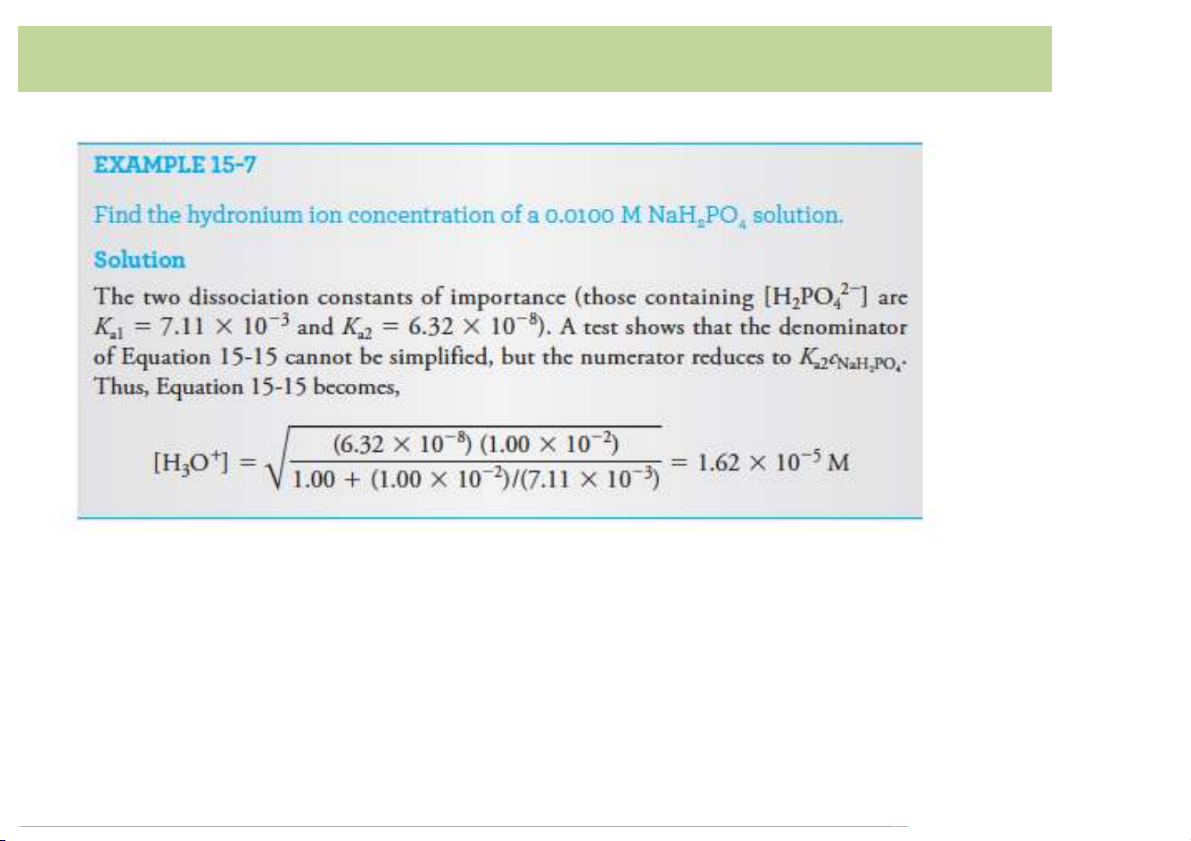
Buffer involving polyprotic acids
Ex.$Calculate$[H3O+]$of$$mixture$of$2.00$M$phosphoric$acid$H3PO4$+$1.50$M
potassium$dihydrogen phosphate$ H2PO – 4 • H O ⇌ H – 3PO4 +(H + H O+ 2 2PO4 3 K – + a1 =([H2PO4 ]( [H O ]( /([H 3 3PO4]( =(7.11 ×10–3( [H O+] =(K [H – 3 a1 × 3PO4]( /([H ]( 2PO4
=(7.11 ×10–3(× (2.00(/(1.50) =(9.48(×10–3(M • H – 2( – 2PO4 +(H O ⇌ HPO + H O+ 2 4 3 K 2( – – a2 =([HPO4 ][H O+]( /([H ]( =(6.32×10–8( 3 2PO4
Ka1 >>(Ka2:(Neglect(2nd dissociation •
Check(the(assumption:( [HPO 2(– – 4 ]=(K [H ]( / [H a2 2PO4 O+]( =1.00×10–5 M( 3 à valid! 16
Buffer involving polyprotic acids
Ex.$Calculate$[H O+]$of$$a$buffer$that$is$0.0500$M$is$KHP$and 3 0.150$M$in$K2P. • H2P(+(H2O(( ⇌ HP – + H O+ 3
Ka1 =([HP–]([H O+](/([H ]( 1.12 10–3 3 2P =( × • HP – +(H O ⇌ P2– + H O+ 2 3
Ka2 =([P2–]([H O+](/([HP – ](=(3.91 ×10–6 3
Assume([HP – ](~([KHP](=(0.0500(M;([P2–](~([K2P](=(0.150(M à [H O+]( =(1.30(×10–6 3 Check(the(assumption: [H P + 2 ]=([HP – ][H O ]/(K
6.00 10–5 M(<<([HP –],([P2–]( 3 a1 = × à valid! 17
pH of an amphiprotic salt NaHA
Calculate the pH of solutions of salts that have both acidic and
basic properties ~ amphiprotic salts formed during titration of
polyfunctional acids and bases. Ex: NaOH + H A ⇌ NaHA + H 2 2O
The pH of the solution is determined by: HA- + H2O ⇌ A2– + H O+ , K 3 a2 or HA- + H2O ⇌ H2A+ OH- , Kb2
The relative magnitudes of the Ka2 and Kb2 will determine if the solution is acidic or basic: [H + 2- 3O ] [A ] Ka2 = [HA-] K - w [H2A] OH ] Kb2 = = Ka1 [HA-] 18
pH of an amphiprotic salt NaHA • Mass(balance( equation:
CNaHA =([HA –](+([H2A]( +(([A2–] (1) • Charge(balance( equation: [Na+](+([H [A ](+([OH 3O+](=( [HA –]( +(2( 2– –] Since([Na+]( =(C +([H ]((+(2([A ](+([OH NaHA à CNaHA( 3O+]( =([HA – 2– –] (2) (2)(– (1)(yields:([H –
3O+]( =([A2–](+( [OH ]( - [H2A]( (3)
Ka1 =([H3O+] [HA –](/ [H2A]( à [H A]( 2 =([H ]( 3O+]( [HA – / Ka1
Ka2 =([H3O+]( [A 2–](/([HA –]à [A 2–](= K ]( a2 [HA – /[H3O+](
Kw =([H3O+]([OH–]( à [OH–]( =(Kw / [H3O+]( From((3): [H ](/[H + + 3O+]( =(Ka2 [A 2– 3O+]( +(Kw /[H3O ]( - [H O 3 ]([HA –](/(Ka1 (–]("#K à [H w 3O+]( = Ka2HA ( $#"#[HA –]/Ka1 19
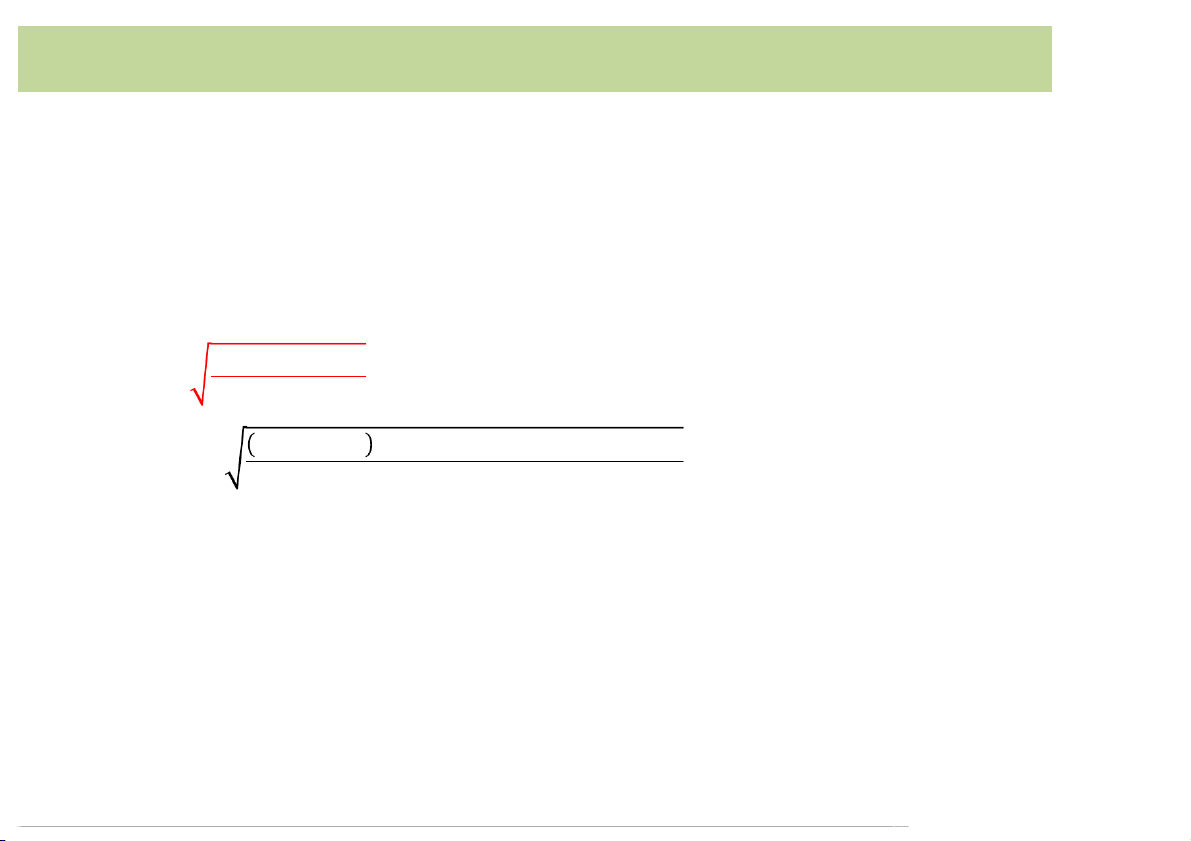
pH of an amphiprotic salt NaHA
pH(of(NaHA(is(determined(by(assuming([HA –](~ CNaHA [H3O+](= Ka2CNaHA(("#Kw $#"#CNaHA/Ka1 When(C ≫ ≫ NaHA/Ka1 1(and(Ka2CNaHA Kw : [H3O+](= Ka1Ka2 20
pH of an amphiprotic salt NaHA 21
pH of an amphiprotic salt NaHA 22
Titration curves for polyfunctional acids
• Compounds with two or more acid functional groups yield
multiple end points in a titration curve.
• If the ratio Ka1/Ka2 > 103, the theoretical titration curve for the
polyprotic acids can be calculated as that for the monoprotic acids. •
2 usable end points appear if the ratio of dissociation
constants > 104 and if the weaker acid or base has K K a or b > 10-8 23
Titration curves for polyfunctional acids
Ex.(Construct(the(titration(curve(for(the(titration(of(25.00(mL(of(
0.1000(M(maleic(acid((HOOC-CH=CH-COOH,(Ka1 =((1.3(x(10-2;(Ka2 =((
5.9(x(10-7)(with(0.1000(M(NaOH.
Ka1/Ka2 >(103,(the(titration(curve( can(be(calculated(as(for(simple(monoprotic(
weak(acids (except( for(the(1st(equivalence( point). Titration(reactions: H2A +(NaOH ⇌ NaHA +(H2O((1st)
NaHA +(NaOH ⇌ Na2A +(H2O(((2nd) 24
Titration curves for polyfunctional acids
Ex. Construct the titration curve for the titration of 25.00 mL of
0.1000 M maleic acid (HOOC-CH=CH-COOH, Ka1 = 1.3 x 10-2; K = a2
5.9 x 10-7) with 0.1000 M NaOH.
Ka1/Ka2 > 103, the titration curve can be calculated as for simple monoprotic
weak acids (except for the 1st equivalence point). • Before the titration:
The 1st dissociation dominates and the 2nd dissociation is negligible: à [H [A 3O+] ~ [HA –] ; 2–] ~ 0 Mass balance:
CH2A = [H2A] + [HA –] + [A2–] ~ [H2A] + [HA –] = [H2A] + [H ] 3O+ à [H2A] = 0.1000 - [H3O+] Ka1 = [HA –] [H = 3O+] / [H2A]
[H3O+]2 /(0.1000 - [H3O+]) = 1.3 x 10-2
à [H3O+] = 3.01 x 10-2 M à pH = 1.52 25
Titration curves for polyfunctional acids •
Start the titration VOH- = 5.00 mL : buffer region H st 2A + NaOH ⇌ NaHA + H2O (1 )
NaHA + NaOH ⇌ Na nd 2A + H2O (2 )
Buffer mainly consists: H2A and HA – (1st dissociation dominates and the 2nd dissociation is negligible):
CNaHA ~ [HA –] = (5.00 mL)(0.1000 M)/(25.00 + 5.00 mL) = 1.67 x 10-2 M
[H2A] = (25.00 - 5.00 mL)(0.1000 M)/ (25.00 + 5.00 mL) = 6.67 x 10-2 M
Ka1 = [HA –] [H3O+] / [H2A] = 1.3 x 10-2
à [H3O+] = 5.2 x 10-2 M , which is not ≪ CH2A and CHA– Then,
[H2A] = CH2A - [H3O+] + [OH-] ~ CH2A - [H3O+] = 6.67 x 10-2 - [H3O+] ;
[HA –] = CNaHA + [H3O+] - [OH-] ~ CNaHA - [H3O+] = 1.67 x 10-2 +[H3O+] . à [H3O+] = 1.81 x 10-2 M à pH = 1.74
Adding more OH-, the pH is calculated in a similar way! 26
Titration curves for polyfunctional acids •
Right before the equivalence point, VOH- = 24.90 mL H2A +(NaOH ⇌ NaHA +(H2O((1st)
NaHA +(NaOH ⇌ Na2A +(H2O(((2nd)
[H2A] is so small, comparable to [A2–], the 2nd dissociation should be considered:
CNaHA ~ [HA –] = (24.90 mL)(0.1000 M)/(25.00 + 24.90 mL) = 4.99 x 10-2 M
[H2A] = (25.00 – 24.90 mL)(0.1000 M)/ (25.00 + 24.90 mL) = 2.00 x 10-4 M
Mass balance: CH2A + CNaHA = [H2A] + [HA –] + [A2–] (a ; ) Charge balance: [Na+] + [H [A 3O+] = [HA –] + 2 2– - ] + [OH ] (b)
Close to Ve, the solution mainly consists of HA – (OH- can be negelected), from (b): [Na+ – +
] ~ CNaHA ~ [HA ] + 2[A2–] - [H3O ] (c) Substitute (c) into (a): [H [A 3O+] = CH2A + 2–] - [H2A] = C + K H2A
a2 [HA –] / [H3O+] + [H3O+] [HA –] / Ka1 à [H3O+] = 1.014 x 10-4 M à pH = 3.99 27
Titration curves for polyfunctional acids •
At)the)1st equivalence) point,) VOH- =)25.00)mL H2A +(NaOH ⇌ NaHA +(H2O((1st)
NaHA +(NaOH ⇌ Na2A +(H2O(((2nd) C [
NaHA( ~(( HA –]( =(((25.00(mL)(0.1000(M)/(25.00( +(25.00(mL)(=( 5.00(x(10-2(M [H3O+]( = Ka2C (( NaHA "#Kw ( $#"#[HA –]/Ka1 ) $.00(x(10−$+ à [H3O+]( = 5.'(x(10−( (5.00(x(10−2 (+ =(7.80(x(10-5(M
$#"(5.'(x(10−()($.-(x(10−.) à pH(=(4.11 28
Titration curves for polyfunctional acids •
Just)after)the)equivalence) point,) VOH- =)25.01)mL H2A +(NaOH ⇌ NaHA +(H2O((1st)
NaHA +(NaOH ⇌ Na2A +(H2O(((2nd)
nH2A( =(nNaHA((in(1)(=((25.00(mL)(0.1000(M)=(2.500(mmol
nOH- excess(=((25.01(- 25.00)(0.1000(M)=( 0.001000(mmol nNaHA((in(2)(=( N
n a2A(=(nOH- excess(=(0.001000(mmol
à nNaHA(excess(=(nNaHA((in(1)(- nNaHA((in(2)(=(2.499(mmol
à cNaHA(excess(=(((2.499(mmol)/(25.00(+(25.01(mL)(=(4.997(x(10-2(M
cNa2A((=(((0.001(mmol)/(25.00( +(25.01(mL)(=(2.000(x(10-5(M 29
Titration curves for polyfunctional acids •
Just)after)the)equivalence) point,) VOH- =)25.01)mL H2A +(NaOH ⇌ NaHA +(H2O((1st)
NaHA +(NaOH ⇌ Na2A +(H2O(((2nd)
The(solution(is(mainly(composed(of(NaHA)and)Na2A ,( Mass(balance:( C [H A 2– Na2A +( C =( NaHA( 2 ]( +([HA –](+( [A
](=(4.997(x(10-2(+ 2.000(x(10-5( =(4.999(x(10-2(M Charge(balance:( [Na+]+[H ]+2[A [OH 3O+]( =([HA – 2–]( +( -]à OH- can(be(negelected.
[Na+](=nOH-/V( =((25.01mL)((0.1000(M)/(25.00(+(25.01(mL)((=(5.001(x(10-2(M
Solving(([H3O+](from(the(mass(and(charge(balance(eq.( : [H3O+] =([A2–]( - [H2A]( +(C +(C Na2A NaHA - [Na+] =((K / +( a2 [HA –]( [H3O+]( [H ](
3O+]( [HA – / Ka1((+(CNa2A +(CNaHA - [Na+] Substitute( the(values(of(K +
a1(,(Ka2(,(CNa2A +(CNaHA and([Na ]: à [H -5( 3O+] =(7.60((x(10 M( à pH(=(4.12 30
Titration curves for polyfunctional acids •
Second)buffer)region,) VOH- =)25.50)mL) H2A +(NaOH ⇌ NaHA +(H2O((1st)
NaHA +(NaOH ⇌ Na2A +(H2O(((2nd)
Buffer( mainly(consists:(HA – and(A2 – (the(reaction( HA – +(H H 2O(⇌ 2A(+(OH – can( be(neglected )
nOH- excess(=((25.50(- 25.00)(0.1000(M)=( 0.05000(mmol
nNaHA((in(2)(=(nNa2A(=(nOH- excess(=(0.005000(mmol à nNaHA(excess(=( N
n aHA((in(1)(- nNaHA((in(2)(=(0.04500(mmol
à cNaHA(excess(=(((0.04500(mmol)/(25.00(+(25.50(mL)(=(8.911(x(10-4(M
cNa2A((=(((0.005(mmol)/(25.00( +(25.50(mL)(=(9.901(x(10-5(M Ka2(=([H ][ ](=5.9( 3O+ A2 – ]/ [HA – x(10-7(M
à [H3O+] =2.89(x(10-5(M(,(which(is(≪ CA2 – and(CHA– à pH(=(4.54(
Adding(more(OH-,(the(pH(is(calculated(in(a(similar(way! 31
Titration curves for polyfunctional acids •
Right)before)the)2nd equivalence) point,) VOH- =)49.90)mL and) more H2A +(NaOH ⇌ NaHA +(H2O((1st)
NaHA +(NaOH ⇌ Na2A +(H2O(((2nd) [A2–] [ / HA –](becomes( large!( C (
NaHA(=( 25.00(mL)(0.1000(M)(– (24.90(mL)(0.1000(M)/(25.00( +(49.90(mL)( =(1.335(x(10-4(M
CA2– =((24.90)(0.1000(M)/(25.00(+(49.90(mL)(=(3.324(x(10-2(M
The(primary(equilibrium( now(is: A2– +((((((((((H2O((⇌ HA – +((((OH –
(3.324(x(10-2(– x)((((((((((((((((((((((((((((((x(((((((((((((x
Kb1 =Kw/Ka2=[HA –].[OH –]/ [A2–]( =(1.335(x(10-4(+(x)x/(3.324(x(10-2(– x)=1.69(x10-8
à x(=([OH –](=(4.10(x(10-6 M(à pOH((=(5.39 à pH(=(8.61 32
Titration curves for polyfunctional acids •
At)the)2nd equivalence)point,) VOH- =)50.00)mL H2A +(NaOH ⇌ NaHA +(H2O((1st)
NaHA +(NaOH ⇌ Na2A +(H2O(((2nd)
All(HA – reacts(completely(with(NaOH(to(produce( A2–
CA2– =((25.00)(0.1000(M)/(25.00(+(50.00(mL)(=(3.333(x(10-2(M
The(primary(equilibrium( now(is: A2– +((((((((((H2O((⇌ HA – +((((OH –
(3.333(x(10-2(– x)((((((((((((((((((((((((((((((x(((((((((((((x K 2–
b1 =Kw/Ka2=[HA –].[OH –]/[A
]( =x2/(3.333(x(10-2(– x)=1.69(x(10-8
à x(=([OH –](=(2.37(x(10-5 M(à pOH((=(4.62 à pH(=(9.38 33
Titration curves for polyfunctional acids •
At)just) beyond)the)2nd equivalence)point,) VOH- =)50.01)mL H2A +(NaOH ⇌ NaHA +(H2O((1st)
NaHA +(NaOH ⇌ Na2A +(H2O(((2nd)
CA2– =((25.00)(0.1000(M)/(25.00(+(50.01(mL)(=(3.332889 x(10-2(M
[OH –]excess =((50.00(- 50.01(mL)/(25.00(+(50.01(m ) L (=(1.333(x(10-5(M
The(primary(equilibrium( now(is: A2– +((((((((((H2O((⇌ HA – +((((OH –
(3.332889 x(10-2(– x)((((((((((((((((((((((((((x(((((((((((((x K 2– -5( b1=Kw/Ka2=[HA –].[OH –]/[A
]( =x (x(+(1.333(x(10 )/(3.332889 x(10-2(– x) à x(=(1.807(x(10-5(M
à [OH –](=(1.807(x(10-5(+(1.333(x(10-5((=(3.140(x(10-5( à pOH((=(4.50( à pH(=(9.50 34
Titration curves for polyfunctional acids •
At)beyond) the)2nd equivalence) point,) VOH- =)51.00)mL H2A +(NaOH ⇌ NaHA +(H2O((1st)
NaHA +(NaOH ⇌ Na2A +(H2O(((2nd)
CA2– =((25.00)(0.1000(M)/(25.00(+(51.00(mL)(=(3.289(x(10-2(M
[OH –]excess =((51.00(- 50.00(mL)(0.1000)/(25.00(+(51.00(mL)(=(1.316(x(10-3(M
The(primary(equilibrium( now(is: A2– +((((((((((H2O((⇌ HA – +((((OH –
(3.289(x(10-2(– x)((((((((((((((((((((((((((((((x(((((((((((((x K 2– -3( b1=Kw/Ka2=[HA –].[OH –]/[A
]( =x (x(+(1.316(x(10 )/(3.289(x(10-2(– x) à x((=(1.807(x(10-5(
à [OH –](=(1.807(x(10-5(+(1.316(x(10-3((~ 1.316(x(10-3( M à pOH((=(2.88 à pH(=(11.12 35




