






Preview text:
lOMoAR cPSD| 59078336
Chapter 1: The Fundametal Units of Life
- Cells come in a variety of shapes, sizes and function
- All living organisms are constructed from cells
- All parts of our body is make up of cells
- Living Cells all have a similar Basic Chemistry: •
All present-day Cells have apparently evolved from the same ancestral Cell •
Genes provide the instructions for Cell form, function, and Complex Behavior - Antonie van
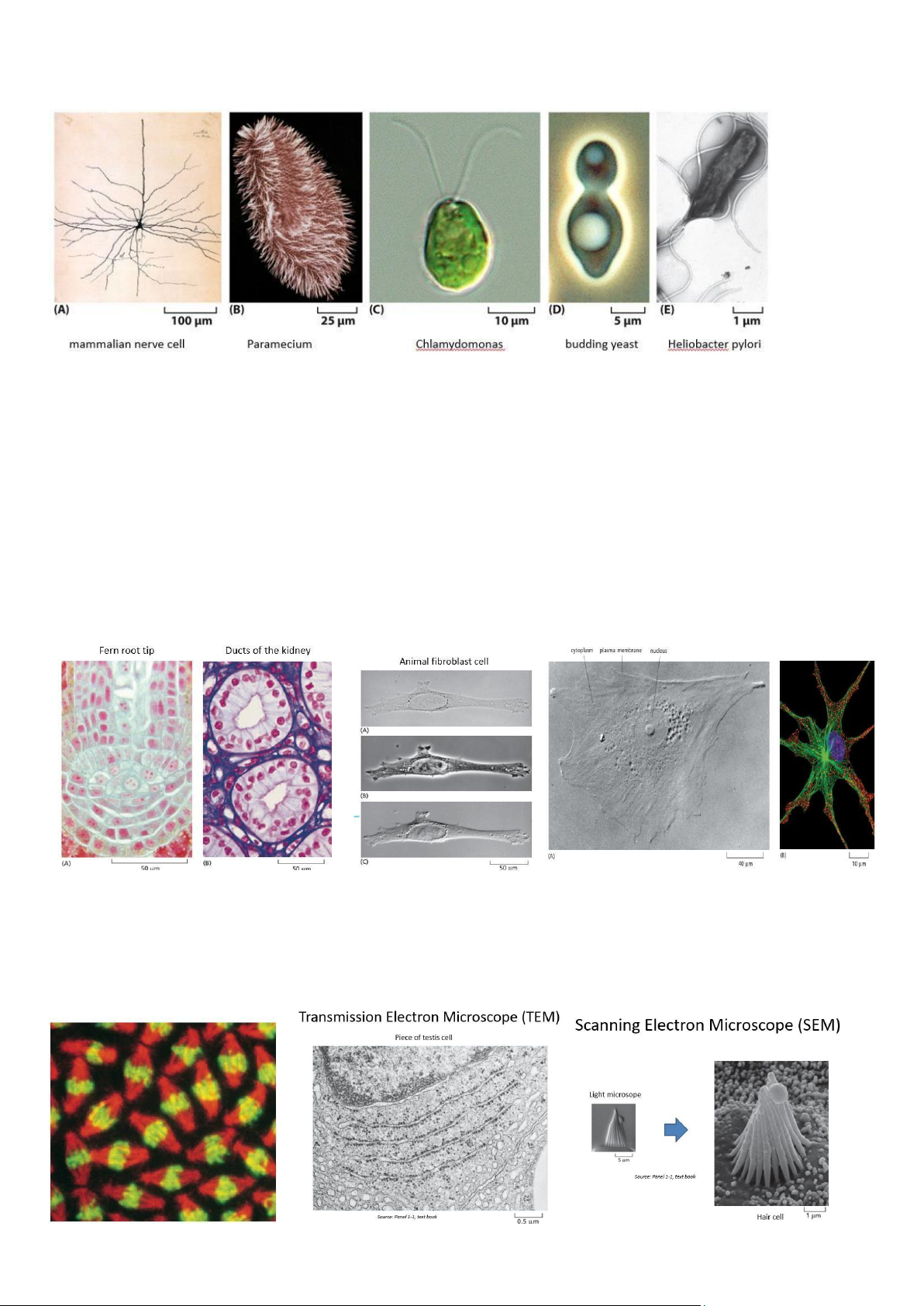
Leeuwenhoek (1675): was the first person to observe living cells Cells Under the Microscope:
- The invention of the light Microscope led to the discovery of Cells
- Light microscope: (Kính hiển vi quang học) •
Sử dụng ánh sáng để quan sát tế bào và mô. •
Độ phóng đại tối đa khoảng 1000 lần, độ phân giải khoảng 0,2 µm.
- Kính hiển vi huỳnh quang (Fluorescence Microscope) •
Dùng ánh sáng tử ngoại để kích thích các phân tử huỳnh quang trong mẫu. •
Giúp quan sát các protein hoặc cấu trúc đặc biệt trong tế bào bằng cách gắn kháng thể huỳnh quang.
- Kính hiển vi điện tử (Electron Microscope - EM) lOMoAR cPSD| 59078336 •
Độ phân giải cao hơn kính hiển vi quang học hàng nghìn lần, có thể nhìn thấy các bào quan và
cấu trúc chi tiết trong tế bào. • Hai loại chính:
o Transmission Electron Microscope (TEM): Quan sát cấu trúc bên trong tế bào. o
Scanning Electron Microscope (SEM): Quan sát bề mặt tế bào với hình ảnh 3D.
Chapter 2: Characteristics, Classification and Organells of the Cell - Basic Properties of Cells: •
Living is the most basic property of cells •
Cells are highly complex and organized •
Cells have a genetic program and the mean to use it •
Cell are capable of producing more of themselves •
Cell acquire and utilize energy •
Cells carry out a variety of chemical reactions •
Cells engage in mechanical activities •
Cell able to response to stimuli •
Cell are capable of self-regulation • Cell evolve •
Cell can grow and reproduce in culture for extented period (primary cell, cell line,..) -
Prokaryotes: the most diverse and numerous cells on earth • Small size • Having cell wall • No nucleus membrane • Fast division (~ 20 min) • Diverse characteristics in biology • Adapt diverse environments in the Earth • And used diverse materials as foods
- Prokaryotes also included Archaea: •
Archaea form other distinct group compared with Bacteria or Eukaryote •
Also present in hostile environments lOMoAR cPSD| 59078336 •
Many characteristics differ from bacteria: having intron, complex RNA polymerase - Eukaryotes:
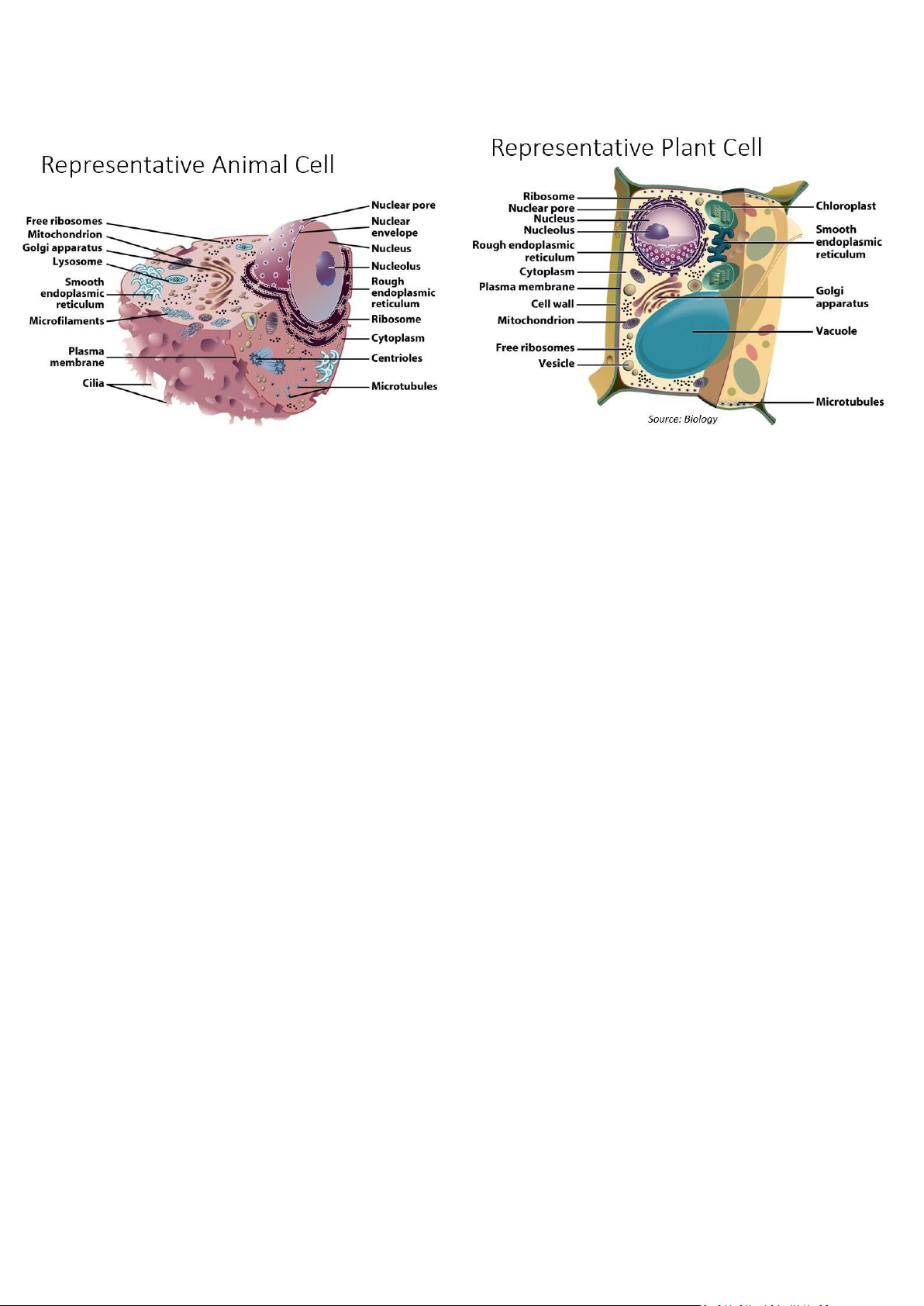
- Organelles: Cellular machinery • Two general kinds • Derived from membranes • Bacteria-like organelles
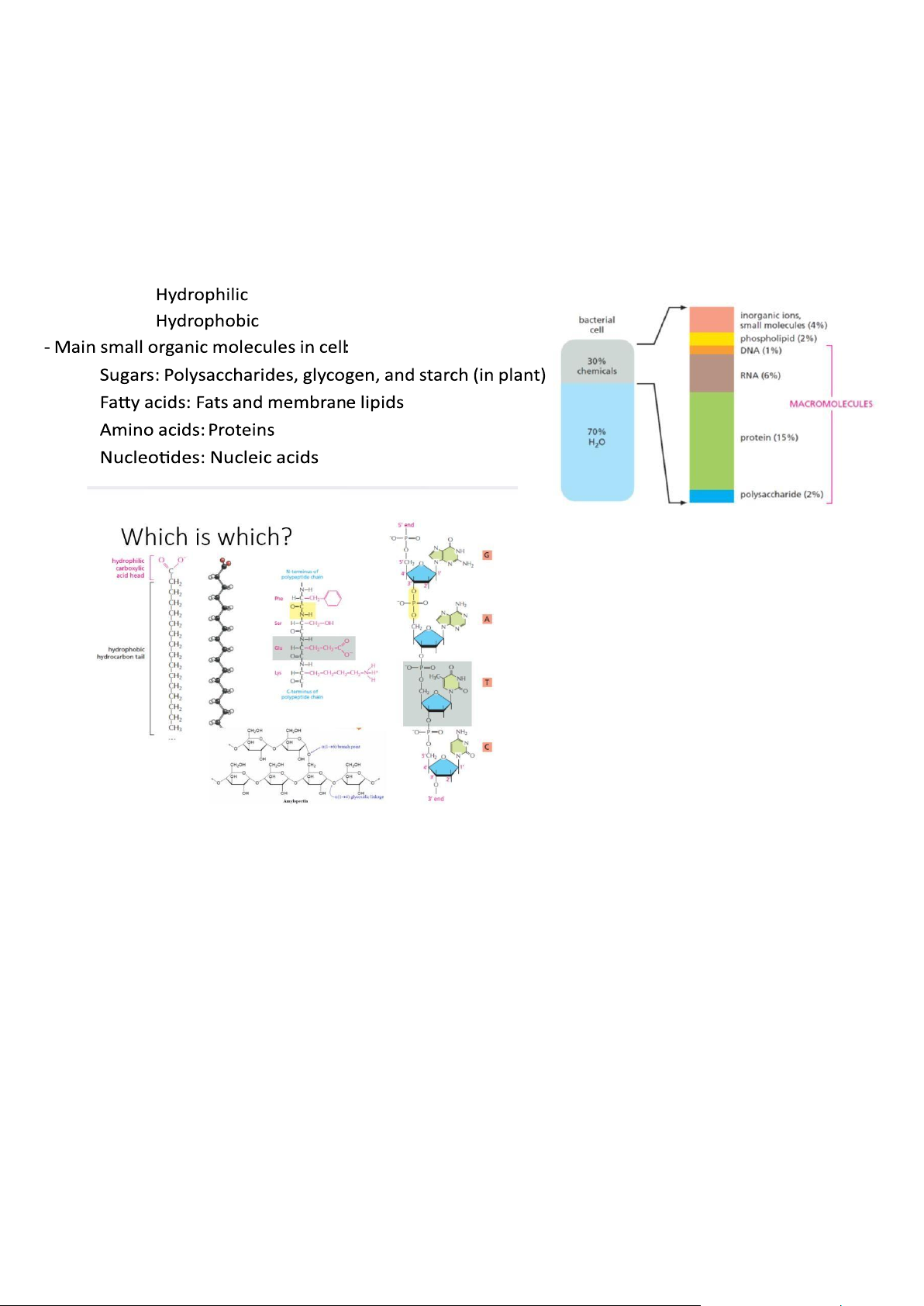
- Plasma Membrane: Contains cell contents, Double layer of phospholipids & proteins - Phospholipids:
+ Polar: Hydrophylic head Hydrophobic tail + Interacts with water
- Movement Across the Plasma Membrane: + A few molecules move freely •
Water, Carbon dioxide, Ammonia, Oxygen
+ Carrier proteins transport some molecules •
Proteins embedded in lipid bilayer •
Fluid mosaic model – describes fluid nature of a lipid bilayer with proteins - Membrane Proteins: 1. Channels or transporters
• Move molecules in one direction 2. Receptors
• Recognize certain chemicals 3. Glycoproteins • Identify cell type 4. Enzymes
• Catalyze production of substances - Cell Walls: •
Found in plants, fungi, & many protists • Surrounds plasma membrane • Plants – mostly cellulose • Fungi – contain chitin
- Cytoplasm: Cytoplasm is stuffed with organelles and crowded by various molecules •
Viscous fluid containing organelles • components of cytoplasm •
Interconnected filaments & fibers lOMoAR cPSD| 59078336 • Fluid = cytosol • Organelles (not nucleus) •
storage substances - Cytoskeleton: • Filaments & fibers • Made of 3 fiber types • Microfilaments (8nm) •
Microtubules – protein subunit (25nm) •
Intermediate filaments – threadlike unit (10nm) • 3 functions: • mechanical support •
anchor organelles • help move substances - Cilia & Flagella: • Provide motility • Cilia • Short •
Used to move substances outside human cells • Flagella • Whip-like extensions •
Found on sperm cells • Basal bodies like centrioles • Structure: • Bundles of microtubules • With plasma membrane - Centrioles: •
Pairs of microtubular structures •
Play a role in cell division - Membranous Organelles: •
Functional components within cytoplasm • Bound by membranes - Nucleus: • Control center of cell • Double membrane • Contains • Chromosomes • Nucleolus - Nuclear Envelope: •
Separates nucleus from rest of cell • Double membrane •
Has pores - DNA – chromosome: • Hereditary material • Chromosomes • DNA • Proteins • Form for cell division • Chromatin - Nucleolus: • Most cells have 2 or more • Directs synthesis of rRNA •
Forms ribosomes - Endoplasmic Reticulum: •
Helps move substances within cells •
Network of interconnected membranes lOMoAR cPSD| 59078336 • Two types •
Rough endoplasmic reticulum •
Smooth endoplasmic reticulum - Rough Endoplasmic Reticulum: • Ribosomes attached to surface • Manufacture proteins •
Not all ribosomes attached to rough ER •
May modify proteins from ribosomes - Smooth Endoplasmic Reticulum: • No attached ribosomes •
Has enzymes that help build molecules • Carbohydrates • Lipids - Golgi Apparatus: •
Involved in synthesis of plant cell wall •
Packaging & shipping station of cell • Function: 1. Molecules come in vesicles 2.
Vesicles fuse with Golgi membrane 3.
Molecules may be modified by Golgi 4.
Molecules pinched-off in separate vesicle 5.
Vesicle leaves Golgi apparatus 6.
Vesicles may combine with plasma membrane to secrete contents - Lysosomes: • Contain digestive enzymes • Functions • Aid in cell renewal • Break down old cell parts • Digests invaders - Vacuoles: • Membrane bound storage sacs •
More common in plants than animals • Contents • Water • Food • wastes - Bacteria-Like Organelles: • Release & store energy • Types • Mitochondria (release energy) •
Chloroplasts (store energy) - Mitochondria: • Have their own DNA • Bound by double membrane •
Break down fuel molecules (cellular respiration) • Glucose • Fatty acids • Release energy • ATP •
Likely evolved from engulfed bacteria - Chloroplasts: •
Derived form photosynthetic bacteria •
Solar energy capturing organelle - Photosynthesis: lOMoAR cPSD| 59078336 •
Takes place in the chloroplast •
Makes cellular food – glucose •
Evolved from engulfed photosynthetic bacteria - Eukaryotic cells may have originated as predators.
- Extract/isolate cell organelles: • Disruption of plasma membrane • Ultracentrifugation
Chemical Components of Cells
- Hydrogen Bonds Are Important Noncovalent Bonds For Many Biological Molecules • • • • • •
- Noncovalent Bonds Allow a Macromolecule to Bind Other Selected Molecules
- Both covalent bonds and noncovalent bonds are needed to form a macromolecular assembly such as a ribosome. lOMoAR cPSD| 59078336 Chapter 3: Bioenergetics
- Bioenergetics is the quantitative study of the energy transduction that occur in living cells and the chemical
processes underlying these transduction. - The Basic Concepts of Thermodynamics:
• The system: the portion of the universe with which we are concerned
• The surroundings: everything else
• Isolated system cannot exchange matter or energy
• Closed system can exchange energy
• Open system can exchange either or both
- Enthalpy (H) – the heat content of a system, reflects the number and kinds of chemical bonds in reactants and products
• ΔH is the change in heat of the system
• Exothermic reactions release heat ΔH < 0
• Endothermic reactions absorbs heat ΔH > 0
- Entropy (S) – a quantitative measure of disorder or randomness in the system
• An organized or ordered state is a low-entropy state
• A disordered state is a high-entropy state
• ΔS is the change in entropy of a system - The First Law of Thermodynamics:
• The total energy of the universe must be constant • Energy may be –
Converted from one form to another – Used to do work –
Moved within a system or between system and surroundings • But it cannot
be destroyed or created in any ordinary chemical process - The Second Law of Thermodynamics:
• In all natural processes, the entropy of the universe increases
• Living systems are highly complex and organized, do they violate the 2nd Law of Thermodynamics?
• Answer: Living organisms operate strictly within the 2nd Law of Thermodynamics
• Living organisms are OPEN system that exchange both matter and energy with surroundings
• Living systems preserve high complexity by obtaining energy from sun light and nutrients and return to
surrounding equal amount of energy in heat and entropy - Thermodynamics of Cells:
• Part of the energy that cells use is converted to heat and is released into the area around the cell
• While inside the cell becomes more ordered, the heat put into the area around the cell causes more
disorder – disorder is greater outside the cell than the order inside the cell
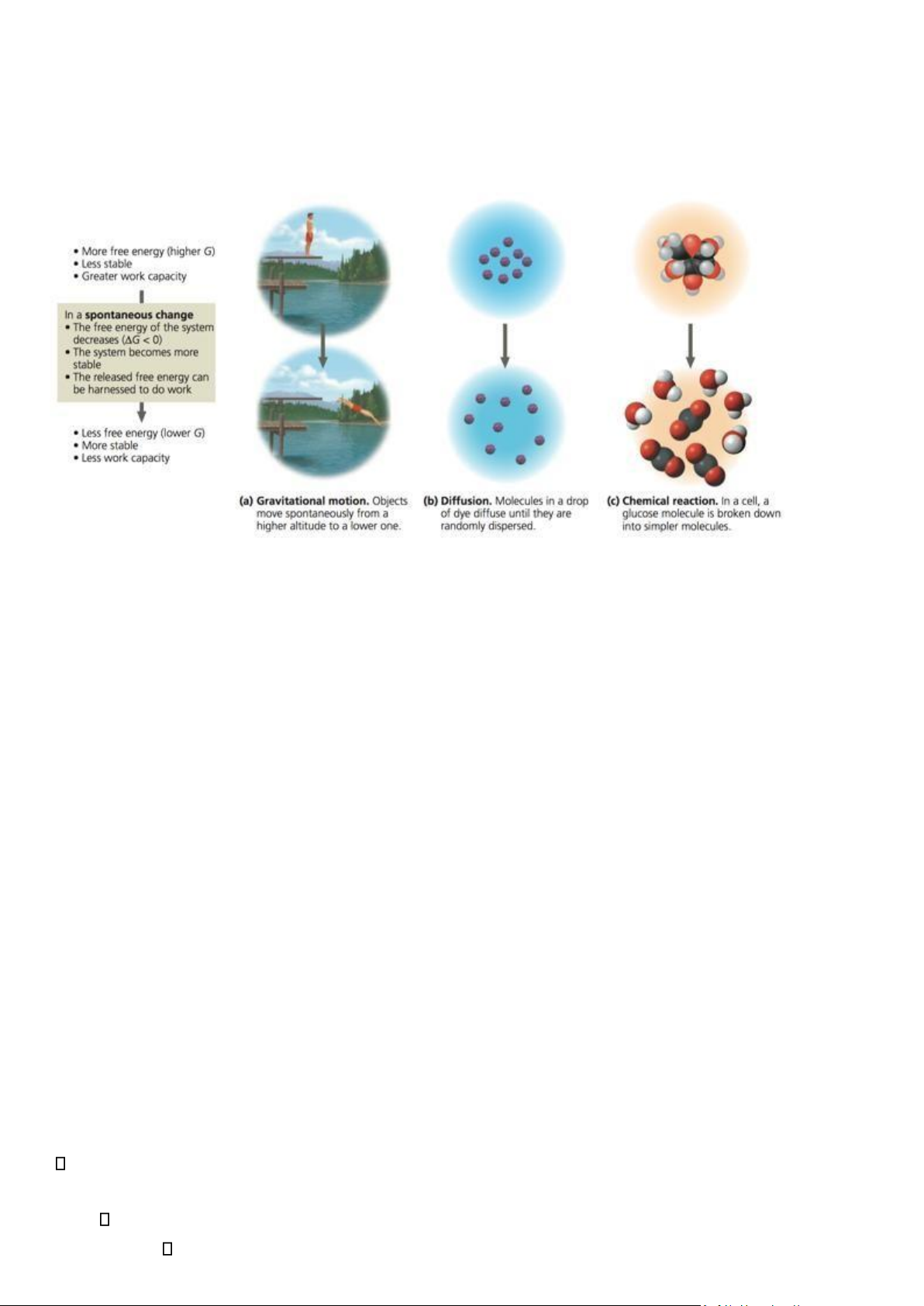
• Gibbs free energy = the amount of energy capable of doing work during reaction at constant Temperature and Pressure
• Exergonic reactions release energy ΔG<0
• Endergonic reactions require energy ΔG>0 lOMoAR cPSD| 59078336 G = H - TS
• For any process at constant P and T:
ΔG = ΔH - TΔS
• If ΔG < 0, reaction proceeds spontaneously - Exergonic reaction: energy
released, spontaneous - Endergonic reaction: energy required, nonspontaneous - Spontaneous Processes:
- Barriers to Chemical Reactions: •
Chemical reactions only proceed in the direction of the loss of free energy •
Molecules in stable states need to have an input of energy to cause them to go to a lower energy
state – activation energy, always positive - Activation Energy: •
In chemistry, molecules that decrease activation energy are catalyst such as platinum and zinc •
In cells the activation energy is reduced by a special protein - enzyme •
Enzymes link 1 or 2 molecules called substrates and hold them in a way that greatly decreases
the activation energy – transition state - Enzymes: Metabolic pathways •
series of enzyme-controlled reactions leading to formation of a product •
each new substrate is the product of the previous reaction Enzyme names commonly • Reflect the substrate • Have the suffix – ase •
sucrase, lactase, protease, lipase - Enzymes as Catalysts: •
Speed up reaction rates (x ~1014) •
Selective – usually 1 enzyme for 1 reaction •
Have a unique shape that contains the active site and only a particular substrate can fit – site where reaction takes place •
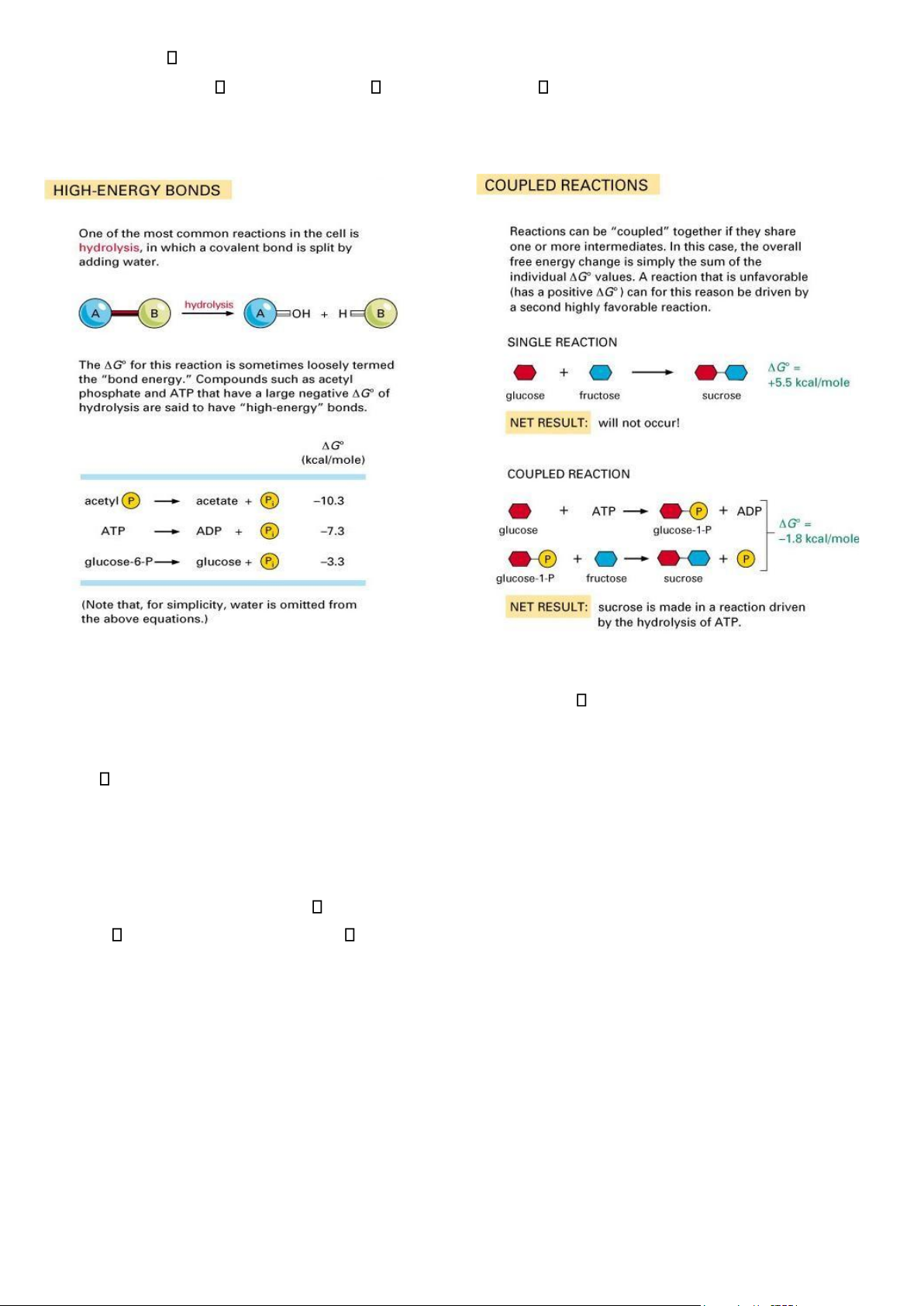
Remain unchanged and can be used over and over - Enzymatic Reactions are Coupled: •
Even though enzymes are good catalysts, they are unable to perform reactions that are thermodynamically unfavorable •
Enzyme reactions are coupled to harvest the energy and heat from a favorable reaction to drive an unfavorable reaction
- G – Change in Free Energy and couple reaction:
• Value of G is only important when the system undergoes a change •
G is the measure of the amount of disorder when a reaction takes place – - G occur spontaneously lOMoAR cPSD| 59078336 – + G are unfavorable
• Need to link a - G reaction with a + G so that the overall G is negative
- Couple reaction: Enzyme catalyzed reactions capture the energy released from the oxidation of glucose in a
chemically useful form rather than as heat - Concentration of Reactants:
• The amount of reactants in the reaction mix is important for the G
• In a reversible reaction, i.e., can go from A to B and from B to A, when there is more A present, the
tendency will be to go from A to B rather than B to A •
G° or standard free-energy change – depends on intrinsic characters of the reacting molecules
• Equilibrium – forward and reverse reactions proceed at exactly equals rates so that no net chemical change occurs
- Equilibrium: Equilibrium constant (K) – number that characterizes the equilibrium state for a reversible
chemical reaction; given by the ratio of the forward and reverse rate constants of the reaction
- Sequential Reactions: Most of the G° values are known for the reactions of the cells and so we can determine
overall G for a pathway – add up the G for each step - Activated Carriers:
• Energy released by catabolism is stored in the chemical bonds of carrier molecules
• The energy can be moved around the cell to where it is needed
• Carrier molecules in the cell are ATP, NADH and NADPH - ATP Serves in a Cellular Energy Cycle:
• ATP is the energy currency of cells
• Phototrophs transform light energy into the chemical energy of ATP
• In heterotrophs, catabolism produces ATP, which drives activities of cells
• ATP cycle carries energy from photosynthesis or catabolism to the energy-requiring processes of cells -
Sunlight – Ultimate Energy Source:
• All organisms live on the organic molecules that are made by photosynthetic organisms
• Photosynthesis traps the energy of the sun in the chemical bonds of sugars which can be turned into
nucleotides, amino acids and fatty acids • 2 steps lOMoAR cPSD| 59078336
– Energy stored in ATP and NADPH, release O2
– ATP and NADPH drive carbon fixation → H2O and CO2 from air and make sugars
- Metabolism: 2 opposing pathways make up metabolism
• Catabolism – process of obtaining energy and building blocks from ‘food’ molecules
• Anabolism – process of using energy and building blocks to create the macromolecules that make up the cell
- Anabolism and Catabolism Are Not Mutually Exclusive:
• Catabolic pathways converge to a few end products
• Anabolic pathways diverge to synthesize many biomolecules
• Some pathways serve both in catabolism and anabolism
• Such pathways are amphibolic
Chapter 4: Protein Structure and Function - Several protein functions:
Enzymes: Catalyze covalent bond breakage or formation (DNA polymerase, protein kinase, pepsin, ribulose bisphosphate carboxylase)
Structural proteins: Provide mechanical support to cells and tissues (collagen, elastin, tubulin, actin, keratin)
Transport protein: Carry small molecules or ions (hemoglobin, serum albumin)
Motor proteins: Generate movement in cells and tissues (Myosin in skeletal muscle, kinesin, dynein)
Storage proteins: Store amino acids or ions (ferritin, ovalbumin in egg, casein in milk)
Signal proteins: Carry extracellular signals from cell to cell (insulin, nerve growth factor, epidermal growth factor, netrin)
Receptor proteins: Detect signals and transmit them to the cell's response machinery. (Rhodopsin, acetylcholine,
insulin receptor, adrenergic receptor)
Gene regulatory proteins: Bind to DNA to switch genes on or off. (lactose repressor, homeodomain proteins)
Special-purpose proteins: Highly variable. (Monellin, antifreeze protein, green fluorescent proteins) 1. The
shape and structure of proteins:
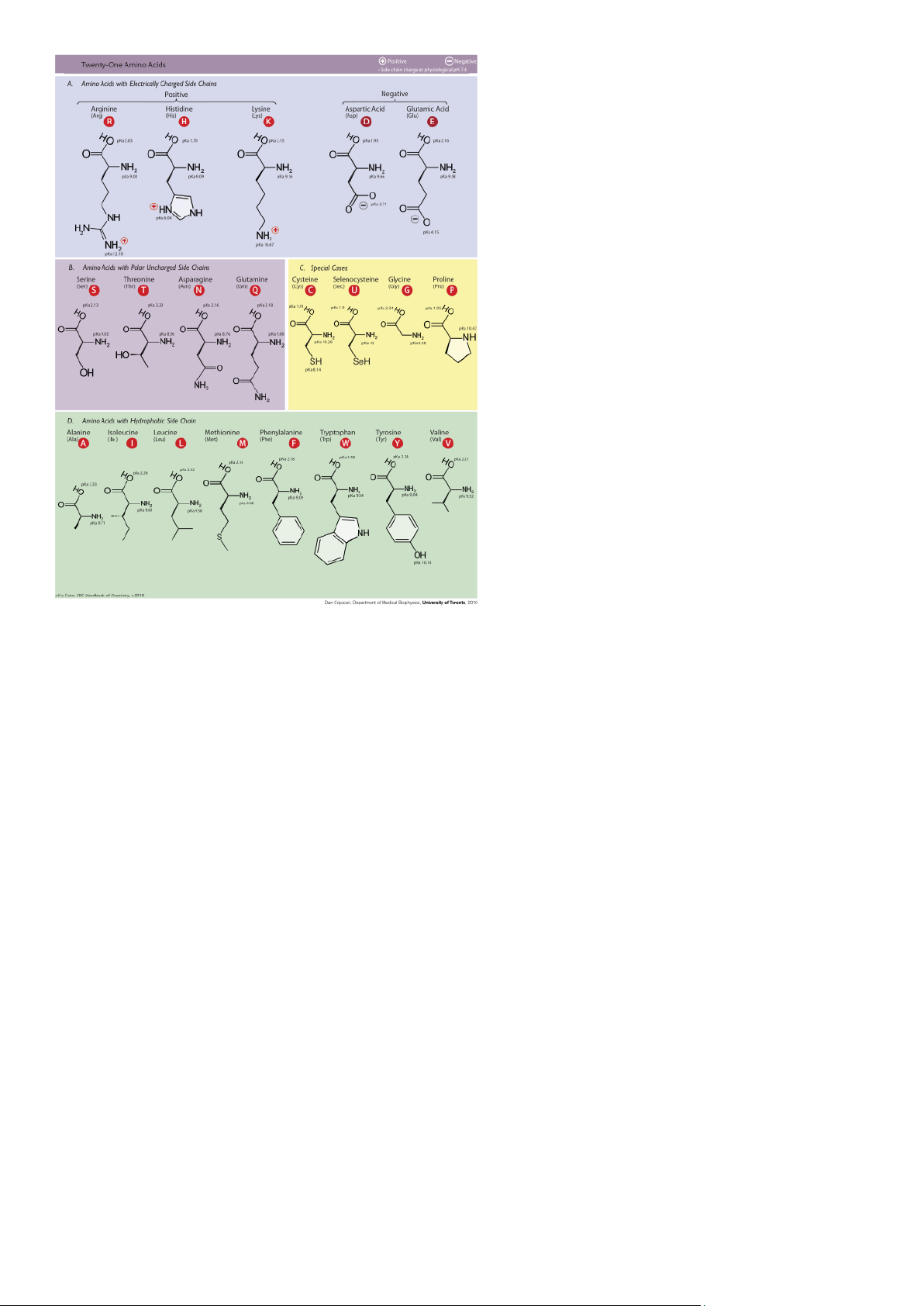
- The Shape Of A Protein Is Specified By Its Amino Acid Sequence • Subunits of proteins
• 20 major types of amino acids
• Side groups of amino acids dictate protein structure (non-polar, polar, and charged subgroups) - When
amino acids combine in a condensation reaction, the amide bond that is formed between them is called a peptide bond. lOMoAR cPSD| 59078336 lOMoAR cPSD| 59078336 -
All 3 types of noncovalent bonds help a protein fold properly
- Together, multiple weak bonds cooperate to produce a strong bonding arrangement
- The polypeptide chain folds in 3-D to maximize weak interactions
- Hydrogen bonds play a major role in holding different regions together
- Hydrogen bonds within a protein molecule help stabilize its folded shape
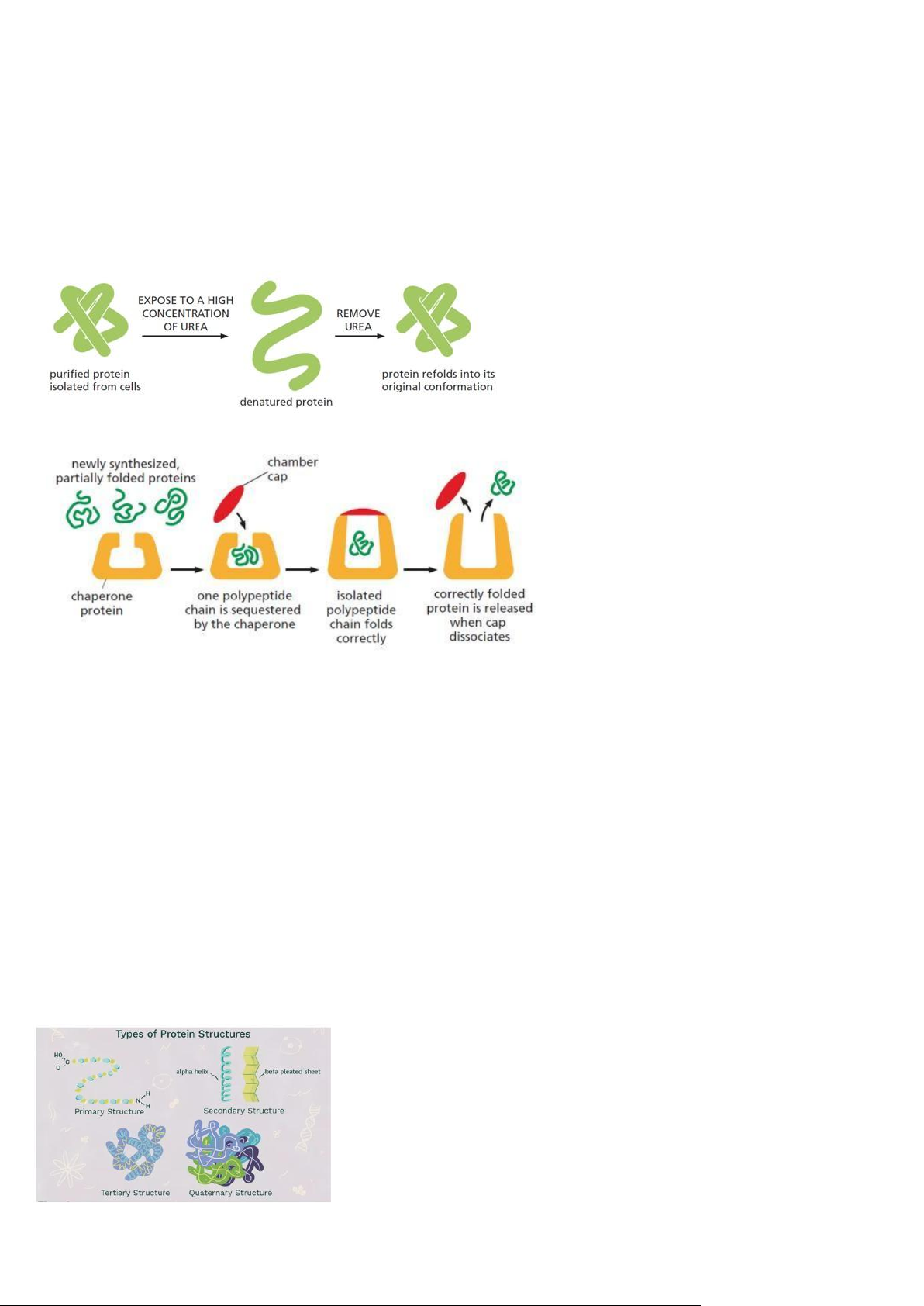
- Proteins Fold Into A Conformation Of Lowest Energy
- Denatured proteins can often recover their natural shapes
- Other chaperone proteins act as isolation chambers that help a polypeptide fold:
- Protein Come In A Wide Variety Of Complicated Shapes: (50 – 2000 aa) filaments, sheets, rings, or spheres
- The α helix (keratin- móng tay) and the β Sheet (silk – tơ) are common folding patterns - Helices form
readily in Biological Structures:
A hydrogen bond is made every fourth amino acid
Many membrane-bound proteins cross the lipid bilayer as an α helix.
Intertwined α helices can form a stiff coiled-coil.
- β Sheets form rigid Structures at the core of Many proteins β Sheets properties
- Extraordinary tensile strength of silk fibers
- Formation of amyloid fibers-insoluble protein associated with neurodegenerative disorders (Alzheimer, prion diseases) - In bacteriology:
+ Formation of biofilms of infectious bacteria
+ Formation of filaments into the air -
Levels Of Protein Organization:
- Many proteins also contain unstructured regions:
- Intrinsically disordered sequences lOMoAR cPSD| 59078336 -
- Unstructured polypeptide chain - Lack of definite structure
Bending and flexing continuously
- Function: Increasing the frequency of encounters between the domains by providing its flexibility -
Few of the Many Possible Polypeptide Chains Wil Be Useful
- Peptide with n aa can have 20n proteins
- Evolution, selection - Only useful proteins were favored
- Proteins can be classified into Families: Amino acid sequence and 3D conformation closely related from protein family members
- If a binding site recognizes the surface of a second protein, the tight binding of two folded polypeptide
chains at this site will create a larger protein.
- A dimer: Two identical, folded polypeptide chains form a symmetrical complex of two protein subunits.
- Proteins can assemble into Filaments, Sheets, or Spheres
- Some types of proteins have elongated fibrous shapes: Fibrous protein, Globular Protein - Extracellular
proteins are often stabilized by covalent cross-linkages 2. How proteins work:
- All Proteins Bind to Other Molecules:
- Biological properties = its physical interaction with other molecules.
- Each protein molecule can bind to just one or a few molecules out of the many thousands of different
molecules it encounters --> shows great specificity - Example:
+ Antibodies >< viruses or bacteria as part of the body’s defenses
+ Enzyme hexokinase + glucose and ATP to catalyze a reaction between them
+ Ligand (from the Latin ligare, “to bind”): an ion, a small organic molecule, or a macromolecule
+ Formation of a set of weak, noncovalent interactions-hydrogen bonds, electrostatic attractions, and van
der Waals attractions-plus favorable hydrophobic forces
→ Fit very closely to the protein, matching it like a hand in a glove, key and lock
- There are Billions of different antibodies, each with a different Binding Site -
Enzymes Are Powerful and Highly Specific Catalysts:
- Enzymes act as catalysts that permit cells to make or break covalent bonds at will
- Enzymes + Substrate → Speed up reaction to million and more - Lysozyme Illustrates How an Enzyme Works:
- Lysozyme—an enzyme that acts as a natural antibiotic in egg white, saliva, tears, and other secretions.
- Lysozyme severs the polysaccharide chains that form the cell walls of bacteria.
- Lysozyme is a relatively small and stable protein, which can be isolated easily in large quantities ->
intensively studied, and it was the first enzyme whose structure was worked out in atomic detail by X- ray crystallography. - Many Drugs Inhibit Enzymes:
- Many of the drugs we take to treat or prevent illness work by blocking the activity of a particular enzyme. - Example:
+ Cholesterol-lowering statins inhibit HMG-CoA reductase, an enzyme involved in the
synthesis of cholesterol by the liver.
+ Anticancer drug Gleevec® was designed to specifically inhibit an enzyme whose aberrant behavior is
required for the growth of a type of cancer called chronic myeloid leukemia - Tightly Bound Small Molecules
Add Extra Functions to Proteins 3. How protein are controlled:
- The Catalytic Activities of Enzymes Are Often Regulated by Other Molecules
- Active site, regulatory site - Feedback control lOMoAR cPSD| 59078336 -
- Complex biochemical pathways
- Concentration of subtrates and enzymes (gene expression)
- Feedback inhibition can work almost instantaneously and is rapidly reversed when product levels fall.
Negative regulation: it prevents an enzyme from acting
- Positive regulation: stimulated by a regulatory molecule rather than being suppressed. Positive
regulation occurs when a product in one branch of the metabolic maze stimulates the activity of an enzyme in another pathway
- Allosteric Enzymes Have Two or More Binding Sites That Influence One Another:
- Allosteric: binding of a regulatory molecule at one site on a protein, activity at another site on the protein is altered
- Phosphorylation Can Control Protein Activity by Causing a Conformational Change
- Covalent Modifications Also Control the Location and Interaction of Proteins
- GTP-Binding Proteins Are Also Regulated by the Cyclic Gain and Loss of a Phosphate Group
- Proteins Often Form Large Complexes That Function as Protein Machines 4. How proteins are studies:
- Proteins can be purified from cells or Tissues
- The basis of the electrophoresis technique (Gel electrophoresis)
- Electric field is applied to a solution containing protein molecules
- The molecules will migrate in a direction and at a speed that reflects their size and net charge
- Two-dimensional gel electrophoresis can be applied when
- Too many proteins are present in the sample
- The proteins are very similar in their migration rate - Procedure
- First, native proteins are separated based on isoelectric focusing.
- Second, proteins are subjected to SDS-PAGE in a direction perpendicular to that used in the first step - Isoelectric focusing
- At isoelectric point: protein has no net charge → not move in an electric field
- Proteins are electrophoresed in a narrow tube of polyacrylamide gel in which a pH gradient is established
- Each protein moves to a point in the pH gradient that corresponds to its isoelectric point and stays there. - Protein sequencing - X-ray crystallography
- Protein structure prediction
Chapter 5: DNA and Chromosomes
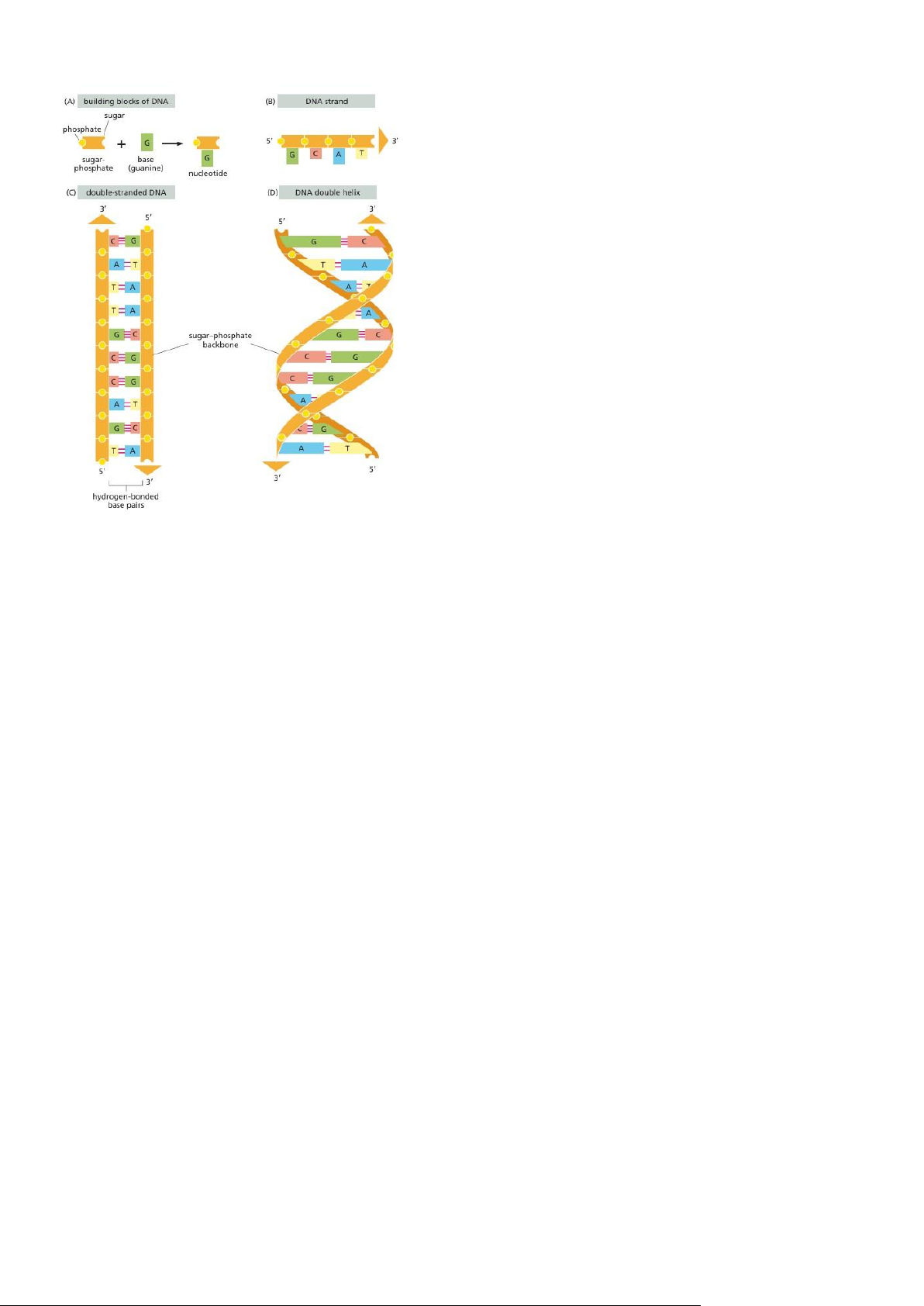
- A DNA molecule Consists of Two Complementary Chains of Nucleotides: - DNA is made of four nucleotide building blocks. lOMoAR cPSD| 59078336 - lOMoAR cPSD| 59078336
The Structure of DNA Provides a mechanism for heredity
- Eukaryotic DNA Is Packaged into Multiple Chromosomes
- Chromosomes Contain Long Strings of Genes
- Chromosomes Specialized DNA Sequences Are Required for DNA Replication and Chromosome Segregation
- Interphase Chromosomes Are Not Randomly Distributed Within the Nucleus
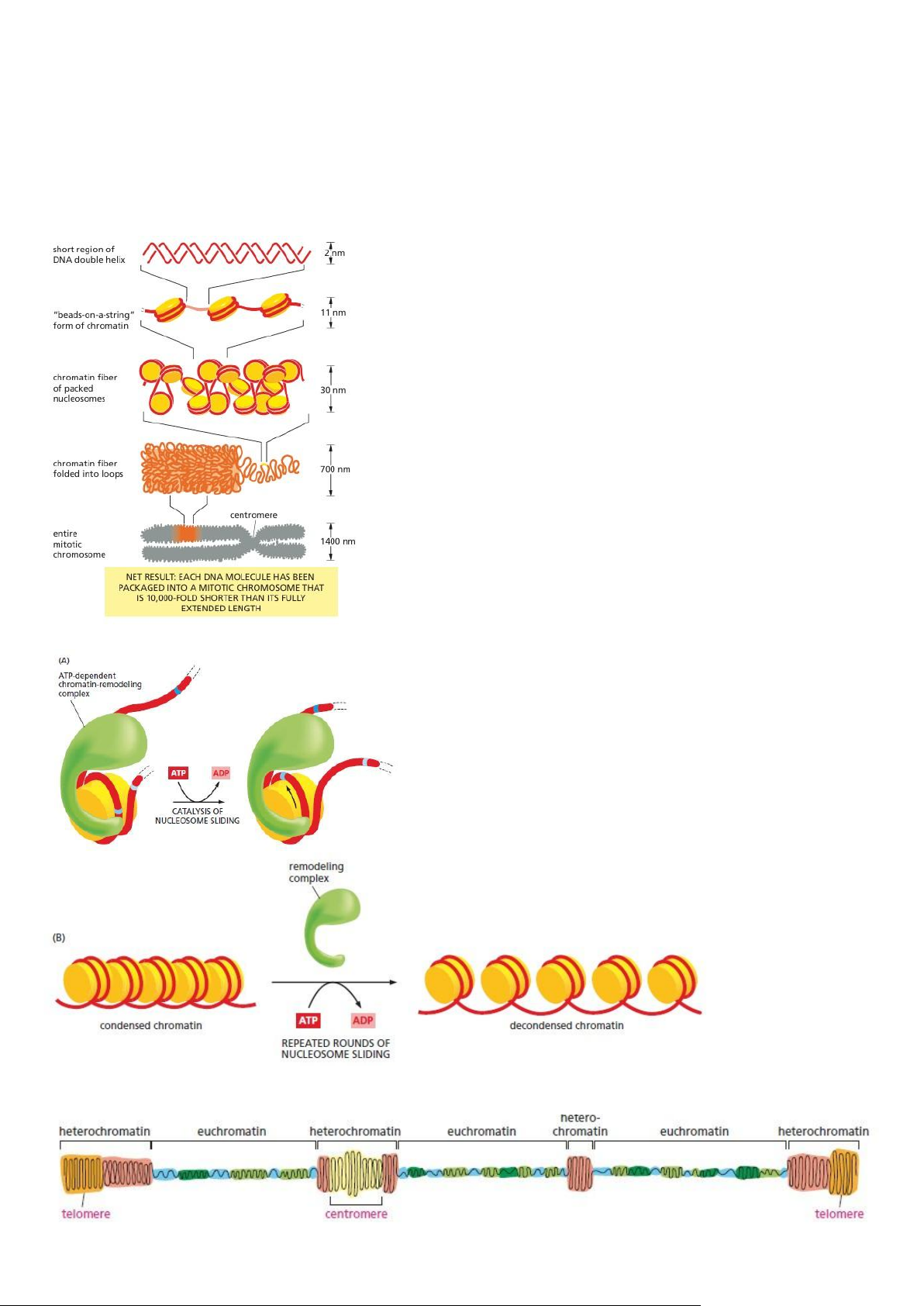
- The DNA in Chromosomes Is Always Highly Condensed
- Nucleosomes Are the Basic Units of Eukaryotic Chromosome Structure - Chromosome Packing Occurs on Multiple Levels
- Changes in Nucleosome Structure Allow Access to DNA
- Interphase Chromosomes Contain Both Condensed and More Extended Forms of Chromatin
The structure of chromatin varies along a single interphase chromosome
Chapter 6: DNA Replication, Repair, and Recombination lOMoAR cPSD| 59078336 - 1.DNA replication:
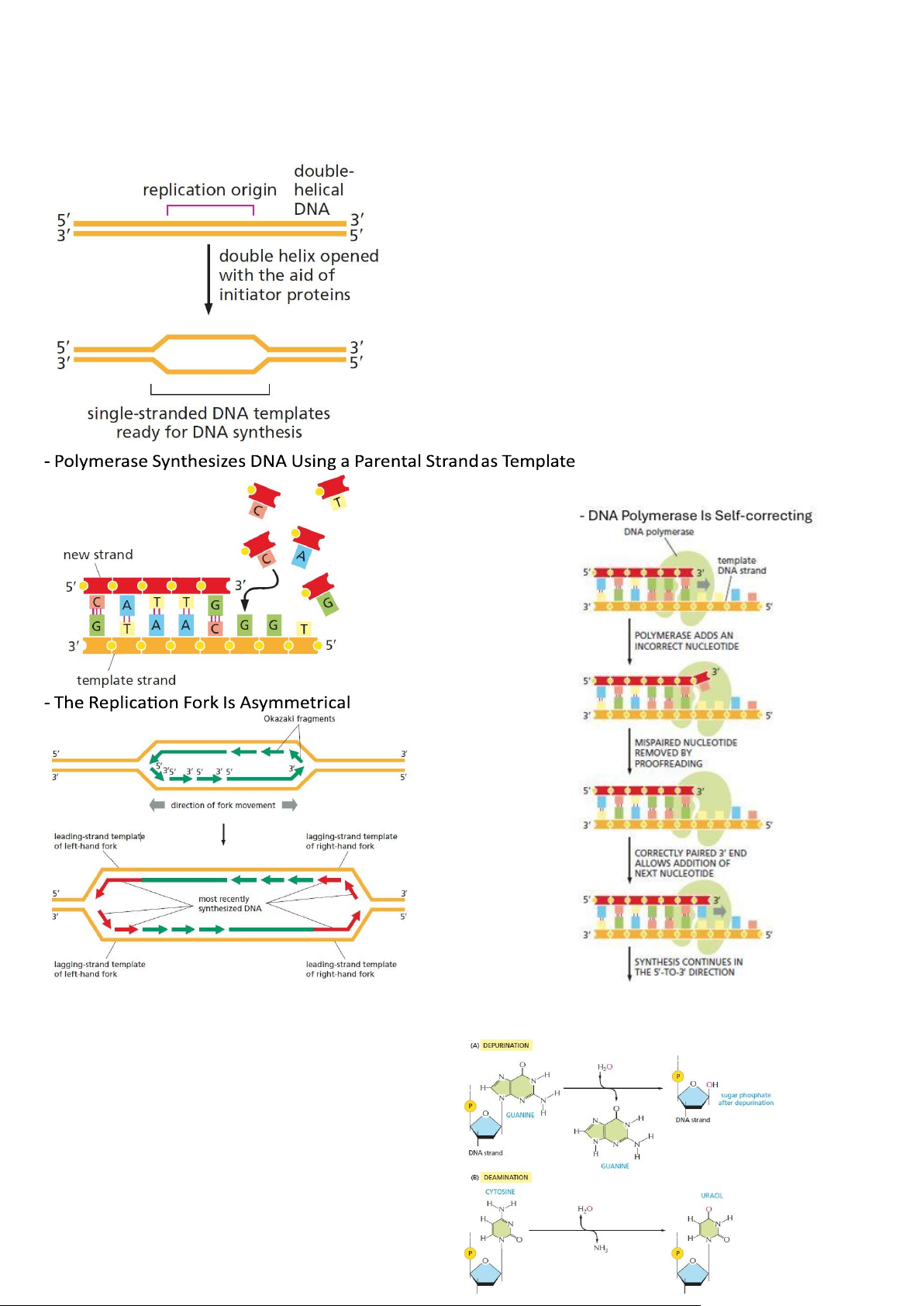
- Base-Pairing Enables DNA Replication
- DNA Synthesis Begins at Replication Origins: 2 rep forks for each origin
- Short Lengths of RNA Act as Primers for DNA Synthesis
- DNA topoisomerases relieve the tension that builds up in front of a replication fork - Telomerase Replicates
the Ends of Eukaryotic Chromosomes 2. DNA repair:
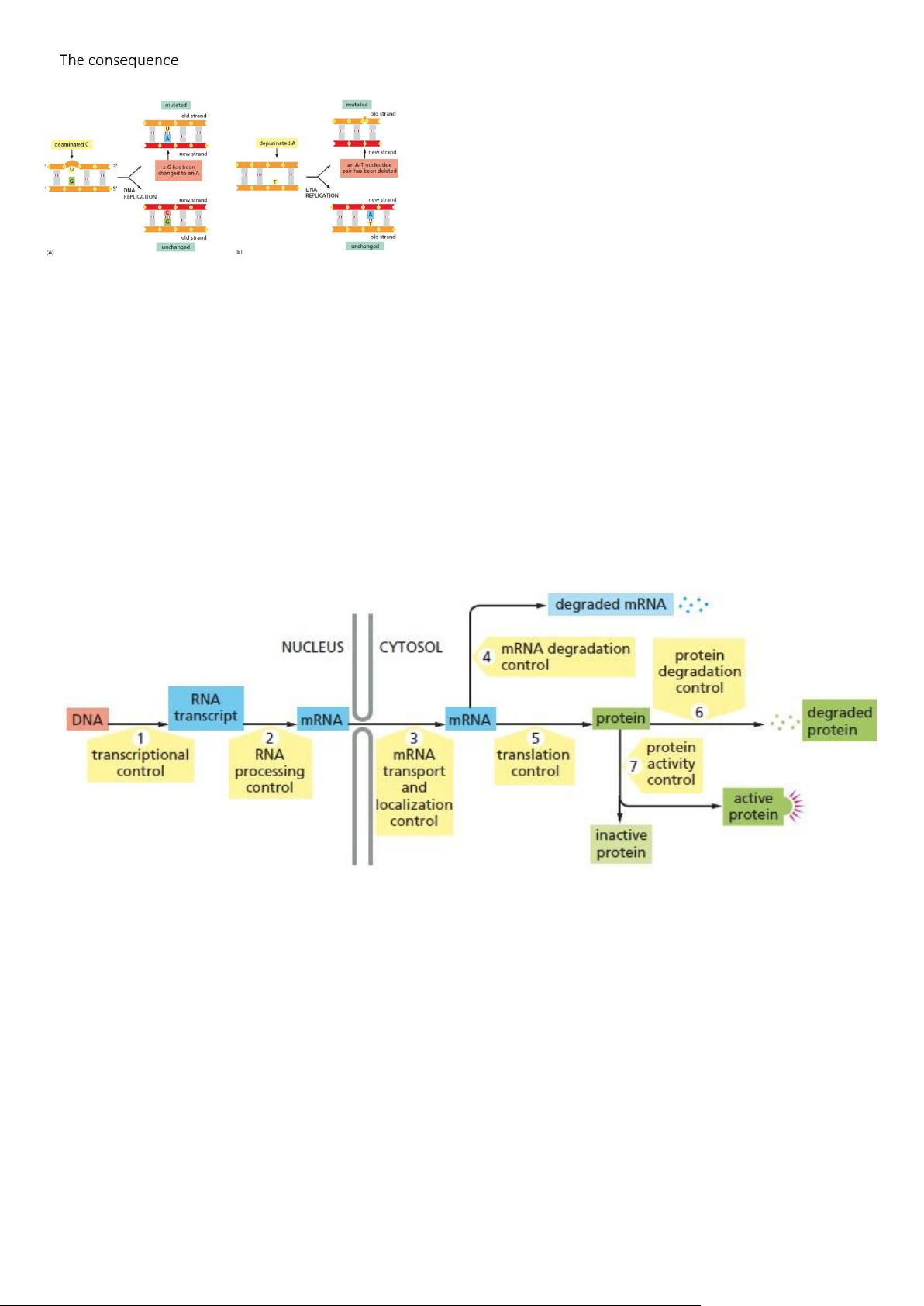
- DNA Damage Occurs Continually in Cells: lOMoAR cPSD| 59078336
Cells Possess a Variety of Mechanisms for Repairing DNA:
• Repair machinery using double helix (complementary) and homologous of chromosome pair of cell
• Step 1: Regconize and remove the damaged bases (varied due to the damage types) • Step 2: Dna polymerase • Step 3: DNA ligase
- Double-Strand DNA Breaks Require a Different Strategy for Repair
- Failure to Repair DNA Damage Can Have Severe Consequences for a Cell or Organism:
Chapter 8: Control of Gene Expression:
- The Different Cell Types of a Multicellular Organism Contain the Same DNA
- A cell can change the expression of its genes in response to external signals
- Gene Expression Can Be Regulated at Various Steps from DNA to RNA to Protein
- Transcription Regulators Bind to Regulatory DNA Sequences
- Transcriptional Switches Allow Cells to Respond to Changes in Their Environment
- Repressors Turn Genes Off and Activators Turn Them On
- An Activator and a Repressor Control the Lac Operon
- Eukaryotic Transcription Regulators Control Gene Expression from a Distance
- Eukaryotic Transcription Regulators Help Initiate Transcription by Recruiting Chromatin-Modifying Proteins
- The Molecular Mechanisms That Create Specialized Cell Types lOMoAR cPSD| 59078336 -
- Eukaryotic Genes Are Controlled by Combinations of Transcription Regulators
- The Expression of Different Genes Can Be Coordinated by a Single Protein
- Combinatorial Control Can Also Generate Different Cell Types
- Specialized Cell Types Can Be Experimentally Reprogrammed to Become Pluripotent Stem Cells
- The Formation of an Entire Organ Can Be Triggered by a Single Transcription Regulator - Evolution also altered gene expression lOMoAR cPSD| 59078336 -
Epigenetic Mechanisms Allow Differentiated Cells to Maintain Their Identity: Cell Memory
- Each mRNA Controls Its Own Degradation and Translation
- Regulatory RNAs Control the Expression of Thousands of Genes
- MicroRNAs Direct the Destruction of Target mRNAs. (In human, miRNAs regulate for 1/3 protein encoded genes)
- Small Interfering RNAs Are Produced From Double-Stranded, Foreign RNAs to Protect Cells From Infections
- Thousands of Long Noncoding RNAs May Also Regulate Mammalian Gene Activity • 200 bp in length • ~ 8000 in human genomes
• Not very clear in function
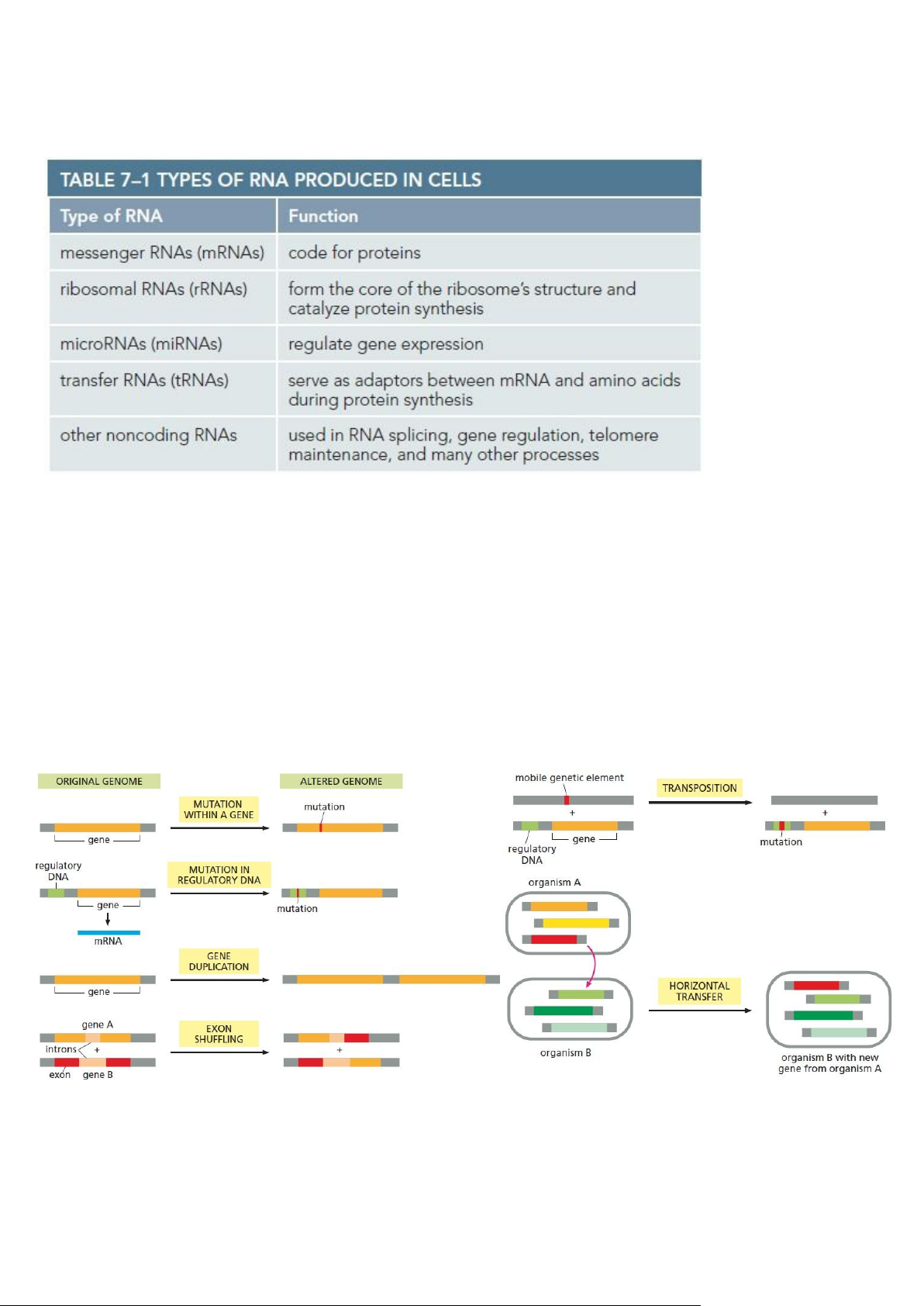
Chapter 9: How Genes and Genomes Evolve
- Small differences in DNA sequence account for differences in appearance between one individual and the next
• GENERATING GENETIC VARIATION
• RECONSTRUCTING LIFE’S FAMILY TREE • TRANSPOSONS AND VIRUSES
• EXAMINING THE HUMAN GENOME
- In Sexually Reproducing Organisms, Only Changes to the Germ Line Are Passed On To Progeny. (Germline
cells and somatic germ cell cells have fundamentally different functions) - Mutations in germ-line cells and
somatic cells have different consequences




